Short Session High Intensity Interval Training and Treadmill Assessment in Aged Mice
Summary
Short session (≤10 min) high intensity interval training (HIIT) is emerging as an alternative to longer exercise modalities, yet the shorter variants are rarely modeled in animal studies. Here, we describe a 10 min, 3 day a week, uphill treadmill HIIT protocol that enhances physical performance in male and female aged mice.
Abstract
High intensity interval training (HIIT) is emerging as a therapeutic approach to prevent, delay, or ameliorate frailty. In particular short session HIIT, with regimens less than or equal to 10 min is of particular interest as several human studies feature routines as short as a few minutes a couple times a week. However, there is a paucity of animal studies that model the impacts of short session HIIT. Here, we describe a methodology for an individually tailored and progressive short session HIIT regimen of 10 min given 3 days a week for aged mice using an inclined treadmill. Our methodology also includes protocols for treadmill assessment. Mice are initially acclimatized to the treadmill and then given baseline flat and uphill treadmill assessments. Exercise sessions begin with a 3 min warm-up, then three intervals of 1 min at a fast pace, followed by 1 min at an active recovery pace. Following these intervals, the mice are given a final segment that starts at the fast pace and accelerates for 1 min. The HIIT protocol is individually tailored as the speed and intensity for each mouse are determined based upon initial anaerobic assessment scores. Additionally, we detail the conditions for increasing or decreasing the intensity for individual mice depending on performance. Finally, intensity is increased for all mice every two weeks. We previously reported in this protocol enhanced physical performance in aged male mice and here show it also increases treadmill performance in aged female mice. Advantages of our protocol include low administration time (about 15 min per 6 mice, 3 days a week), strategy for individualizing for mice to better model prescribed exercise, and a modular design that allows for the addition or removal of the number and length of intervals to titrate exercise benefits.
Introduction
Regular exercise is effective at preventing or delaying many age-associated diseases such as sarcopenia and frailty1,2,3,4. However, less than 15% of those 65 and older meet recommendations of 150 min a week of moderate intensity exercise plus strength
training5,6. As the lack of time and lengthy sessions are common barriers to exercise, high intensity interval training (HIIT) is emerging as an alternative to traditional regimens. HIIT features multiple short bursts of intense activity that are interspersed with brief periods of active recovery, and there has been recent interest in identifying the shortest regimens that still yield beneficial outcomes. Such studies include 3 day a week regimens featuring total session times of 4 min7, 2-3 min8, 1.5 min9, a single min10, and even 40 s11.
Likewise, there has been substantial interest in HIIT animal models. A majority of studies used mice12,13,14,15,16,17,18,19,20,21 or rats22,23,24,25 and were performed using a treadmill, although a few others used swimming protocols26,27,28. A majority of these studies use VO2max to set the initial intensity of the exercise13,14,19,21,24. Additionally, although an often described benefit of HIIT is having shorter regimens, almost all of these identified studies feature regimens that last 30 min or longer11,12,13,14,15,18,19,21, with the exception of one with a slightly longer than 10 min regimen20, and another with 19 min across three different intensities16. To our knowledge, there are no reported animal studies that examine a 10 min or less HIIT regimen, or tailor the regimen to individual animals, with the exception of our published study17 that serves as the basis for this protocol.
Here, we describe a protocol for HIIT in aged mice designed to model individualized, short session (≤10 min) variants used recently in human studies7,8,9,10,11. The method includes a 10 min regimen on an inclined (25°) treadmill with a 3 min warm up, and four 1 min intervals at high intensity, interspersed with three 1 min active recovery segments. Advantages of the protocol include greater clinical relevance as it features strategies for tailoring intensity to individual animals, setting intensities that are not based on VO2max, thereby avoiding the need for metabolic treadmills, and modular design whereby the number of intervals and timings are easily adjustable. Additionally, within this protocol we provide instructions for two strategies for treadmill assessment, which include flat continuous and uphill interval, to examine endurance. Using these methods, we extend our previous findings that short session HIIT increased functional capacity in aged male mice17, and now demonstrate HIIT increases treadmill performance in aged female mice.
Protocol
All studies and experimental protocols were approved by and in compliance with guidelines of the University at Buffalo and VA Western New York Animal Care and Use Committees.
1. Experiment Setup and General Advice
NOTE: A total of twenty-four female mice on a C57BL/6J background were used in this protocol starting at 23 months of age. The mice carried a conditional SIRTloxp-exon4-loxp mutation29, however, this was not induced in this experiment.
- Ensure that mice receive permanent identifiers such as ear tags, RFID chips, or tail tattoos.
NOTE: It is recommended to also use temporary markings (e.g., permanent marker to mark tails) for quick identification during assessment periods. - Clean the treadmill with 0.25-0.5% bleach (v/v) or 70% (v/v) ethanol at the end of the day or to remove feces or urine between experimental trials. Dry solutions completely before initiating a new trial.
NOTE: Ethanol may increase wear to the treadmill belt. It is recommended that the treadmill be cleaned between each run if working with group-housed male mice in order to minimize in cage fighting. It is recommended to perform treadmill assessments at the same time of day at each time point in longitudinal studies30,31,32, and if other assessments are being performed, the order should remain the same. The investigator running the experiment should also be blind to the group designations of the mice.
2. Acclimatizing Mice to Treadmill Apparatus
NOTE: Initiate acclimatization of mice 1 month prior to baseline experiments.
- Set up the initial training program using the treadmill software (v3.4.7) in Manual mode (Figure 1 and Table 1).
- Open the treadmill software. Then, click on the file and open the experiment.
- Input the values indicated in Table 1 on the Manual tab (Figure 1) under Acclimation.
- Input the session number as 1, the number of active channels as 1 to 6 depending on the number of mice, the number of visits to the grid as 10, and the number of shocks as 20.
NOTE: Shock intensity for this initial run is set at level 1 (0.46 mA).
- Set the inclination of the treadmill to 0° (flat).
- Hold mice by the tail when placing in the treadmill and place mice directly on the belt to avoid starting mice on the shock grid.
- Use a brush or tongue depressor to keep mice away from the shock grid when training or assessment begins. Nudge mice to begin running to avoid unintended shocks.
- After each mouse is given the initial training program, repeat the program as above with the shock intensity increased to level 2 (0.59 mA) with at least 15 min between trainings.
- One to two days after the initial training program administer the uphill treadmill acclimatization.
- Open the treadmill software. Then click on the file and then open the experiment.
- Input the values on the Basic tab as indicated in Table 2 under Acclimation.
- Under Shock Detection, input the session number as 1, the number of active channels as 1 to 6 depending on the number of mice, the number of visits to the grid as 10, and the number of shocks as 20.
- Click on the Profile Mode tab (Figure 2) and for Step 1, input a start speed of 0 m/min and an end speed of 5 m/min with a period of 5 s.
- Add a warm up step 2 with a start speed of 5 m/min and end speed of 5 m/min for 30 seconds. Add a transition step that starts at 5 m/min and an end speed of 6 m/min for 5 s.
- Add a test speed step that starts at 6 m/min and ends at 6 m/min for 20 s. Add a transition step that starts at 6 m/min and ends at 5 m/min.
- Add a recovery interval that starts at 5 m/min and ends at 5 m/min for 20 s (Figure 2).
- Repeat step 2.6.4 to add test speed steps as indicated in Table 2 under acclimation.
- Two weeks prior to baseline assessments, continue acclimatization by providing two treadmill flat aerobic capacity assessments as described in section 3, given on consecutive days.
- One to two days after the second endurance training, provide two treadmill uphill anaerobic capacity assessments as described in section 4, given on the same day with a minimum of 30 min between trainings.
3. Flat Continuous Treadmill Assessment
- Create a treadmill program as described in step 2.1, using values indicated in Table 1 under Assessment. Under Shock Detection, set the session number as 1, the number of active channels as 1 to 6 depending on the number of mice, the number of visits to the grid as 10, and the number of shocks as 20.
- Monitor the mice throughout the trial and remove mice from the instrument that reach the endpoint criteria.
NOTE: This step is to avoid mice inadvertently touching the shock grid of neighboring mice and affecting data. Parameters include the total time on belt, the distance travelled, and the shocks to visits ratio. It is recommended to perform two assessments at each time point, separated by at least one day between assessments.
4. Uphill Interval Treadmill Assessment
- Create a treadmill program as described in step 2.6, using values indicated in Table 2 under Assessment. Under Shock Detection, set the session number as 1, the number of active channels as 1 to 6 depending on the number of mice, the number of visits to the grid as 5, and the number of shocks as 10.
- Set the interval field in the treadmill program to 0.5.
NOTE: This allows for data points to be collected every second instead of every minute, which aids in identifying the speed at endpoint for each mouse. The program will automatically stop after the 50 m/min stage due to software limitations. Parameters include the time on belt, the distance travelled, and the test speed of the last successfully completed stage before endpoint. The latter is used for determining baseline intensity for the HIIT regimen described in step 5. It is recommended to perform two assessments per time point, separated by at least 30 min.
5. Short session High Intensity Interval Training
- Set the treadmill uphill (25°) and remove the plastic cover.
- Determine the intensity for each mouse. Use the speed of the last successfully completed stage (Step 4.5) and find the intensity group and corresponding Base, Sprint, and Dash speeds using Table 3.
- Open the treadmill software and click on file to create a new program.
- Click on the Profile Mode tab and for Step 1, input a start speed of 0 m/min and an end speed as indicated under Base speed as indicated in Table 3 with a period of 5 s.
- Add a warm up step 2 with a start speed at Base speed and end speed at Base speed for 180 s.
- Input 3 intervals each with 1) a transition step that goes from Base 세스 Sprint for 5 s, 2) an step at Sprint speed for 60s, 3) a transition step that goes from “sprint” to Base over 5 s, and 4) a step for a recovery interval at Base speed for 60 s.
- Add a transition step that goes from Base 세스 Sprint speed over 5 s and a final step that goes from Sprint 세스 Dash speed over 60 s.
NOTE: Programs can be saved, modified, and reloaded.
- Perform exercise three days a week with at least one day of rest between sessions (i.e., Monday, Wednesday, Friday).
- Rewrite exercise programs to increase Base, Sprint, and Dash speeds 1 m/min every two weeks.
NOTE: No electric shocks are given during the exercise. - Motivate mice to run using a makeup brush (recommended) or tongue depressor to lightly motivate mice that fall near or on the shock grid. If a mouse does not respond retry in 5 s, then 10 s, and then retry every 30 s or during a recovery interval until the end of the session.
NOTE: consider removing mice from the study that do not run on the treadmill at any intensity or are unable to complete the first three intervals at the intensity defined for the lowest intensity group (Table 3) - Move mice that cannot be motivated to complete the first three intervals in two consecutive exercise sessions to lower intensity groups (Table 3).
- Move mice that do not require motivation in two consecutive exercise sessions to higher intensity groups (Table 3).
- Take sedentary control mice in cages place beside the treadmill as it runs. Alternatively, sedentary controls can be placed in the lanes of a non-moving treadmill for 10 min.
Representative Results
A total of twenty-five female mice were bred and aged in house. The C57BL/6J background mice carried a SIRT1loxp-exon4-loxp mutation29; however, this conditional knockout was not induced and therefore all mice exhibited full length Sirtuin1 (data not shown). At 24 months of age, mice were assessed for treadmill endurance and uphill sprint capacity prior to and after the administration of two months of HIIT exercise (n = 14), or remaining cage sedentary (n = 11). Our data show all 14 mice in the HIIT group increased treadmill time on belt compared to 7 of 11 of the SED mice (Figure 4A, paired student's T-Test p < 0.0001 for HIIT and p < 0.14 for SED). In total HIIT mice exhibited greater improvement in time on belt based on the better of two trials (HIIT: 18.2 ± 10.5 min to 31.8 ± 13.7 min, delta 13.6 ± 7.5 min versus SED: 19.9 ± 10.0 min to 25.3 ± 7.3 min, delta 5.3 ± 11.4 min, unpaired student's T-test p < 0.0391).
Additionally, we detected greater increase in uphill treadmill capacity as 12 of 14 HIIT mice increased maximal speed while we observed decline in 8 of 11 SED mice (Figure 4B, paired student's T-Test p < 0.0022 for HIIT and p < 0.85 for SED). HIIT mice also demonstrated greater improvement in maximal speed compared to SED mice based on the better of two trials (HIIT: 14.6 ± 4.3 m/min to 17.6 ± 5.5 m/min, delta 3.0 ± 3.0 m/min versus SED: 16.9 ± 3.8 m/min to 16.5 ± 5.0 m/min, delta 0.5 ± 5.0 m/min, unpaired student's T-test p = 0.0441). Shock tolerance was similar between the two groups of mice (Shock to grid visit ratio baseline – SED: 1.2 ± 0.1 versus HIIT: 1.4 ± 0.4, p=0.24; endpoint – SED: 1.2 ± 0.4 versus HIIT: 1.3 ± 0.4, p=0.46).
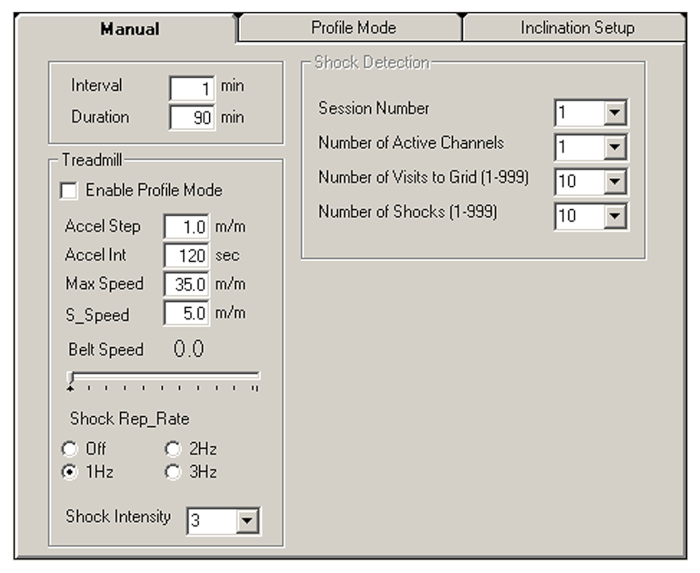
Figure 1: Treadmill software manual mode parameters for treadmill endurance assessment. The treadmill software manual mode allows adjustment for protocols involving continuous belt acceleration. Please click here to view a larger version of this figure.
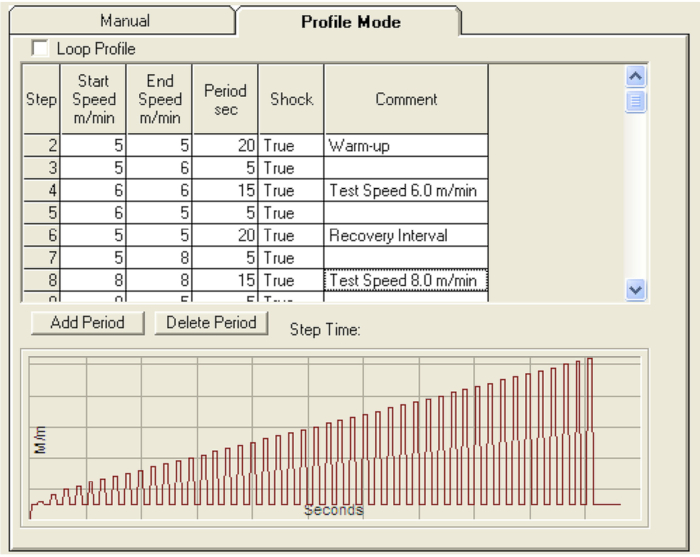
Figure 2: Treadmill software profile mode program parameters for uphill sprint assessment. The treadmill software profile mode, used in conjunction with the options on the manual mode tab (Figure 1), allows the ability to create protocols with custom speed intervals. Please click here to view a larger version of this figure.
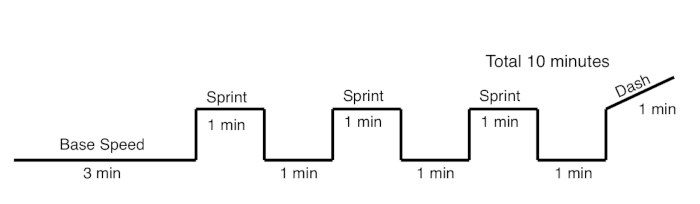
Figure 3: High Intensity Interval Training regimen schematic. Base, Sprint, and Dash speeds are indicated in Table 3. Speeds increase by 1 m/min every two weeks. Please click here to view a larger version of this figure.
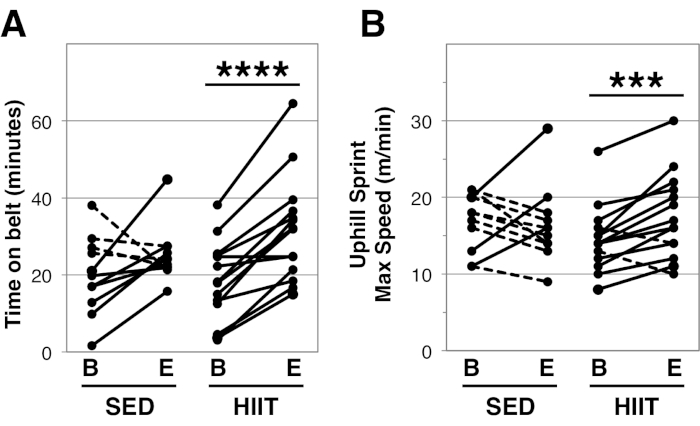
Figure 4. Impacts of HIIT on treadmill performance in aged mice. Twenty-four month old female mice were given HIIT (n=14) or remained sedentary (SED, n=11) for 2 months. Treadmill endurance (A) was assessed before and after the exercise intervention as a best of two trials given on consecutive days on a flat treadmill. Uphill sprint capacity (B) was assessed as the best of two trials administered with no less than 30 min of rest on an inclined (25°) treadmill. Lines indicate individual mice. B is baseline and E is endpoint, *** indicate a p-value < 0.001 and **** indicates p < 0.0001 as determined by a paired student's T-test comparing within group change between baseline and endpoint. Please click here to view a larger version of this figure.
Table 1: Program parameters for treadmill endurance acclimatization and assessment.
| Program parameter | Acclimation | Assessment |
| Interval | 1 | 0.5 |
| Duration | 5 | 90 |
| Enable Profile Mode | Unchecked | Unchecked |
| Accel Step | 1.0 m/min | 1 m/min |
| Accel Int | 60 seconds | 120 seconds |
| Max Speed | 10 m/min | 35 m/min |
| S_speed | 5 m/min | 5 m/min |
| Shock Rep Rate | 1 hz | 1 hz |
| Shock Intensity | 1 then 2 | 3 |
Table 2: Program parameters for uphill sprint acclimatization and assessment.
| Program parameter | Acclimation | Assessment |
| Interval | 0.5 | 0.5 |
| Duration | 10 | 90 |
| Enable Profile Mode | Checked | Checked |
| Accel Step | N/A | N/A |
| Accel Int | N/A | N/A |
| Max Speed | 15 m/min | 35 m/min |
| S_speed | 5 m/min | 5 m/min |
| Shock Rep Rate | 1 hz | 1 hz |
| Shock Intensity | 2 | 3 |
| PROFILE MODE (Refer to Figures 1 and 2 to set up program parameters) | ||
| Test intervals | 8, 10, 10, 10 m/min | 6, 8, 10, 11, 12 … 51, 52 m/min |
Table 3: Intensity designations for HIIT exercise mice.
| Intensity Group | Baseline Uphill Sprint Score | Initial Exercise Speeds | ||
| Base | Sprint | Dash | ||
| 0 | < 8 m/min | 5 m/min | 7 m/min | 10 m/min |
| 1 | 8-16 m/min | 5 m/min | 10 m/min | 15 m/min |
| 2 | 17-25 m/min | 8 m/min | 13 m/min | 18 m/min |
| 3 | >25 m/min | 11 m/min | 16 m/min | 21 m/min |
Discussion
Benefits from short sessions are a key aspect of high intensity interval training that captures scientific and public interest. However, animal studies rarely investigate HIIT regimens that are 10 min or less. Here, we describe a protocol for a 10 min short session HIIT uphill treadmill exercise regimen that enhanced treadmill performance in aged female mice and which we previously have shown to increase physical performance in aged male mice17. A strength of our protocol is that in addition to the protocol being only 10 min in length, the design is modular such that the number of intervals can be added or removed to make protocols that are even one min in length. Additionally, the length of the intervals can be modified providing multiple strategies to titrate exercise and evaluate the impacts.
Another strength of this protocol is that we include a system to tailor the exercise regimen to the physical capabilities of individual mice, which to our knowledge only one other study33 aside from our report in aged male mice17 has used. Although individually tailoring an exercise intervention introduces variability into the experimental design, an important advantage of this method is that it better models the administration of prescribed exercise in human clinical settings. Furthermore, animals do not train at intensities that are too easy or difficult, which could be a significant factor in experiments where the animal population exhibits diversity in exercise capacity (i.e. during aging). One other advantage of this methodology is that it does not require the use of metabolic treadmills to determine VO2max as the exercise intensity is tailored to the performance of the animal, a concept that is further examined by Picoli et al.34.
This protocol includes two assessments for treadmill performance, in the form of a slowly accelerating flat treadmill test and an inclined treadmill incrementing interval test, respectively. There are multiple protocols that have been published for determining continuous treadmill performance in mice using treadmills16,34,35, including a JoVE article36. Multiple parameters differ across these studies and across the literature, including: inclination (usually 0° or 5° for endurance testing), shock intensity (ranging from 0.25-1.12 mA), and rules for defining exhaustion. It was also noticed that 5 consecutive shocks were commonly used to define exhaustion across multiple studies. Although this strategy may induce greater exhaustion than the rules we have used in our study and this protocol, this system also assumes all mice have similar pain thresholds, which may not be true depending on experimental conditions. Our definition of exhaustion of 10 visits or 20 total shocks provides a framework to assess if there are different pain thresholds. Ultimately, there are advantages and disadvantages to both strategies and the decision for defining the endpoint should be best aligned with the goals of the study. Additionally, some treadmill assessment protocols for mice have been designed without the use of shocks as a stimulus37. Although there are advantages to this approach37, some drawbacks to consider include the use of a determination of exhaustion that is subjective and the potential difficulty of assessing multiple mice simultaneously.
Additionally, others have used work and power as parameters to describe treadmill performance35,38,39. The speed of the belt and rate of acceleration are also diversely represented in the literature. As most protocols feature increasing speed, the result will yield a blend of aerobic and anaerobic capacity in the mouse, with slower protocols providing more focus on the aerobic component. We therefore designed our uphill treadmill interval test to provide greater focus on the anaerobic component17. To achieve this, our protocol features short test intervals with active recovery between tests, allowing for mice to achieve higher speeds and therefore greater anaerobic utilization relative to our flat continuous treadmill endurance assessment. For our protocol we used an active recovery period of 20 s between intervals, the half time for reoxygenation of muscle tissue in human tissue40. However, we note a limitation of this method is that the specific impacts on anaerobic metabolism have yet to be elucidated, and including an examination of anaerobic parameters such as glycolytic metabolism, phosphocreatine utilization, and lactate kinetics would strengthen this method. In addition, new studies that investigate the impacts of changing stage and active rest durations, inclination, and different speed increments would also strengthen this method.
The methodology described in this protocol, including the two treadmill assessments, are designed for older animals in particular, and as such includes greater time for acclimation of mice to the protocols. Proper acclimation is a critical step to experimental design to ensure endpoints are due to exhaustion and not insufficient learning by the mouse. Improper acclimation is likely apparent in longitudinal studies – yet, importantly, may not be noticed in crosssectional studies. Although, shorter acclimation protocols might be possible for younger cohorts, in our experience older mice require greater acclimation and a published protocol for exercise training in aged rats initiates acclimation 1 month prior with 10 total days given for treadmill acclimatization41. However, we agree with Castro et al. that providing excessive acclimation may impact the behavior of and impacts on the mice35 and his recommendation of 3 to 10 days total35, for which our acclimatization protocol is in line with these recommendations. Furthermore, older animals also exhibit greater diversity in exercise performance and for this reason we include two trials per assessment time point. Younger animals display greater homogeneity in performance and therefore a single trial may be sufficient. Furthermore, the diversity of aged animals may make direct cross-sectional comparisons of an intervention difficult to interpret, and more success may be achieved by comparing the change from baseline as was done in the analysis of the aged female mice. We also indicate as a caveat that we have not tested our short session HIIT protocol in younger mice, where it is possible a ceiling effect in performance may mask the benefits of our short session HIIT protocol. The protocol was also used only in the C57BL/6J mouse strain, and therefore the impact of this exercise in other mouse strains remains to be elucidated. Additionally, the C57BL6/J mice used in this experiment carried a SIRT1loxp-exon4-loxp mutation29, that was not induced. These mice were aged in house from birth; however, insufficient numbers were available at 24 months of age to power 4 groups. We therefore focused the experiment on just HIIT and sedentary groups. We note that both the present cohort of aged female mice and our previously published cohort of aged male mice17 both improved in physical performance using this short session HIIT protocol.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We wish to thank the animal care personnel at the University at Buffalo Animal Laboratory Animal Facility. This research was supported by a Veteran Affairs Rehabilitation Research and Development Grant RX001066 and the Indian Trail Foundation.
Materials
| Exer-3/6 Open Treadmill w/ Shock, Detection, auto-calibration and PC Interface/Software | Columbus Instruments | 1055-SDRM | The Columbus Instruments 3/6 treadmill allow up to 6 mice or 3 rats simultaneously. The device comes with controllers to allow manual control of treadmill belt speed and shock intensity, or connections to a computer and software to run and control these elements. |
| Bleach | Varies | Varies | 0.25-0.5% Bleach solution (V/V) is used to clean the treadmill belt between sessions |
| Ethanol | Varies | Varies | 70% ethanol solution (V/V) can alternatively be used to clean treadmill belt between runs and sesions. |
| Make-up Brush (large) | Varies | Varies | A make-up brush provides a soft surface and ample length to motivate mice to continue exercise. |
References
- Cameron, I. D., et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Medicine. 11 (65), (2013).
- Manas, A., et al. Reallocating Accelerometer-Assessed Sedentary Time to Light or Moderate- to Vigorous-Intensity Physical Activity Reduces Frailty Levels in Older Adults: An Isotemporal Substitution Approach in the TSHA Study. Journal of the American Medical Directors Association. 19 (185), (2018).
- Rogers, N. T., et al. Physical activity and trajectories of frailty among older adults: Evidence from the English Longitudinal Study of Ageing. PLoS One. 12, e0170878 (2013).
- Yamada, M., Arai, H., Sonoda, T., Aoyama, T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. Journal of the American Medical Directors Association. 13, 507-511 (2012).
- de Rezende, L. F., Rey-Lopez, J. P., Matsudo, V. K., do Carmo Luiz, O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. 14 (333), (2014).
- Wullems, J. A., Verschueren, S. M., Degens, H., Morse, C. I., Onambele, G. L. A review of the assessment and prevalence of sedentarism in older adults, its physiology/health impact and non-exercise mobility counter-measures. Biogerontology. 17, 547-565 (2016).
- Tjonna, A. E., et al. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS One. 8, e65382 (2013).
- Burgomaster, K. A., et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Journal of Physiology. 586, 151-160 (2008).
- Cavar, M., et al. Effects of 6 Weeks of Different High-Intensity Interval and Moderate Continuous Training on Aerobic and Anaerobic Performance. Journal of Strength and Conditioning Research. , (2018).
- Gillen, J. B., et al. Twelve Weeks of Sprint Interval Training Improves Indices of Cardiometabolic Health Similar to Traditional Endurance Training despite a Five-Fold Lower Exercise Volume and Time Commitment. PLoS One. 11, e0154075 (2016).
- Metcalfe, R. S., Babraj, J. A., Fawkner, S. G., Vollaard, N. B. Towards the minimal amount of exercise for improving metabolic health: beneficial effects of reduced-exertion high-intensity interval training. European Journal of Applied Physiology. 112, 2767-2775 (2012).
- Belmonte, L. A. O., et al. Effects of Different Parameters of Continuous Training and High-Intensity Interval Training in the Chronic Phase of a Mouse Model of Complex Regional Pain Syndrome Type I. The Journal of Pain. , (2018).
- Chavanelle, V., et al. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Scientific Reports. 7 (204), (2017).
- de Oliveira Sa, G., et al. High-intensity interval training has beneficial effects on cardiac remodeling through local renin-angiotensin system modulation in mice fed high-fat or high-fructose diets. Life Sciences. 189, 8-17 (2017).
- Marcinko, K., et al. High intensity interval training improves liver and adipose tissue insulin sensitivity. Molecular Metabolism. 4, 903-915 (2015).
- Niel, R., et al. A new model of short acceleration-based training improves exercise performance in old mice. Scandinavian Journal of Medicine and Science in Sports. 27, 1576-1587 (2017).
- Seldeen, K. L., et al. High Intensity Interval Training (HIIT) improves physical performance and frailty in aged mice. The Journals of Gerontology Series A Biological Sciences. 73 (4), 429-437 (2017).
- Tuazon, M. A., McConnell, T. R., Wilson, G. J., Anthony, T. G., Henderson, G. C. Intensity-dependent and sex-specific alterations in hepatic triglyceride metabolism in mice following acute exercise. Journal of Applied Physiology. 118, 61-70 (2015).
- Wang, N., Liu, Y., Ma, Y., Wen, D. High-intensity interval versus moderate-intensity continuous training: Superior metabolic benefits in diet-induced obesity mice. Life Sciences. 191, 122-131 (2017).
- Wilson, R. A., Deasy, W., Stathis, C. G., Hayes, A., Cooke, M. B. Intermittent Fasting with or without Exercise Prevents Weight Gain and Improves Lipids in Diet-Induced Obese Mice. Nutrients. 10, (2018).
- Hafstad, A. D., et al. High intensity interval training alters substrate utilization and reduces oxygen consumption in the heart. Journal of Applied Physiology. 111, 1235-1241 (2011).
- Brown, M. B., et al. High-intensity interval training, but not continuous training, reverses right ventricular hypertrophy and dysfunction in a rat model of pulmonary hypertension. American Journal of Physiology Regulatory. Integrative and Comparative Physiology. 312, R197-R210 (2017).
- Hoshino, D., Yoshida, Y., Kitaoka, Y., Hatta, H., Bonen, A. High-intensity interval training increases intrinsic rates of mitochondrial fatty acid oxidation in rat red and white skeletal muscle. Applied Physiology, Nutrition, and Metabolism. 38, 326-333 (2013).
- Rahimi, M., et al. The effect of high intensity interval training on cardioprotection against ischemia-reperfusion injury in wistar rats. EXCLI Journal. 14, 237-246 (2015).
- Songstad, N. T., et al. Effects of High Intensity Interval Training on Pregnant Rats, and the Placenta, Heart and Liver of Their Fetuses. PLoS One. 10, e0143095 (2015).
- Motta, V. F., Aguila, M. B., Mandarim-De-Lacerda, C. A. High-intensity interval training (swimming) significantly improves the adverse metabolism and comorbidities in diet-induced obese mice. The Journal of Sports Medicine and Physical Fitness. 56 (5), 655-663 (2015).
- Pimenta, M., et al. High-intensity interval training beneficial effects on body mass, blood pressure, and oxidative stress in diet-induced obesity in ovariectomized mice. Life Sciences. , 75-82 (2015).
- Vieira, J. M., et al. Caffeine prevents changes in muscle caused by high-intensity interval training. Biomedicine and Pharmacotherapy. 89, 116-123 (2017).
- Price, N. L., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabolism. 15, 675-690 (2012).
- Bains, R. S., et al. Assessing mouse behaviour throughout the light/dark cycle using automated in-cage analysis tools. Journal of Neuroscience Methods. 300, 37-47 (2018).
- Hanell, A., Marklund, N. Structured evaluation of rodent behavioral tests used in drug discovery research. Frontiers in Behavioral Neuroscience. 8 (252), (2014).
- Hopkins, M. E., Bucci, D. J. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behavioral Neuroscience. 124, 868-872 (2010).
- Hollinski, R., et al. Young and healthy C57BL/6 J mice performing sprint interval training reveal gender- and site-specific changes to the cortical bone. Scientific Reports. 8, 1529 (2018).
- Picoli, C. C., et al. Peak Velocity as an Alternative Method for Training Prescription in Mice. Frontiers in Physiology. 9 (42), (2018).
- Castro, B., Kuang, S. Evaluation of Muscle Performance in Mice by Treadmill Exhaustion Test and Whole-limb Grip Strength Assay. Bio-protocol. 7, (2017).
- Dougherty, J. P., Springer, D. A., Gershengorn, M. C. The Treadmill Fatigue Test: A Simple, High-throughput Assay of Fatigue-like Behavior for the Mouse. Journal of Visualized Experiments. , 111 (2016).
- Conner, J. D., Wolden-Hanson, T., Quinn, L. S. Assessment of murine exercise endurance without the use of a shock grid: an alternative to forced exercise. Journal of Visualized Experiments. 90, e51846 (2014).
- Aguiar, A. S., Speck, A. E., Amaral, I. M., Canas, P. M., Cunha, R. A. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Scientific Reports. 8, 10742 (2018).
- Barbato, J. C., et al. Spectrum of aerobic endurance running performance in eleven inbred strains of rats. Journal of Applied Physiology. 85, 530-536 (1998).
- Nagasawa, T. Slower recovery rate of muscle oxygenation after sprint exercise in long-distance runners compared with that in sprinters and healthy controls. Journal of Strength and Conditioning Research. 27, 3360-3366 (2013).
- Arnold, J. C., Salvatore, M. F. Getting to compliance in forced exercise in rodents: a critical standard to evaluate exercise impact in aging-related disorders and disease. Journal of Visualized Experiments. (90), (2014).

