An In Vitro Batch-culture Model to Estimate the Effects of Interventional Regimens on Human Fecal Microbiota
Summary
This protocol describes an in vitro batch-culture fermentation system of human fecal microbiota, using inulin (a well-known prebiotic and one of the most widely studied microbiota modulators) to demonstrate the use of this system in estimating effects of specific interventions on fecal microbiota composition and metabolic activities.
Abstract
The emerging role of the gut microbiome in several human diseases demands a breakthrough of new tools, techniques and technologies. Such improvements are needed to decipher the utilization of microbiome modulators for human health benefits. However, the large-scale screening and optimization of modulators to validate microbiome modulation and predict related health benefits may be practically difficult due to the need for large number of animals and/or human subjects. To this end, in vitro or ex vivo models can facilitate preliminary screening of microbiome modulators. Herein, it is optimized and demonstrated an ex vivo fecal microbiota culture system that can be used for examining the effects of various interventions of gut microbiome modulators including probiotics, prebiotics and other food ingredients, aside from nutraceuticals and drugs, on the diversity and composition of the human gut microbiota. Inulin, one of the most widely studied prebiotic compounds and microbiome modulators, is used as an example here to examine its effect on the healthy fecal microbiota composition and its metabolic activities, such as fecal pH and the fecal levels of organic acids including lactate and short-chain fatty acids (SCFAs). The protocol may be useful for studies aimed at estimating the effects of different interventions of modulators on fecal microbiota profiles and at predicting their health impacts.
Introduction
The human microbiota is a complex community consisting of bacteria, archaea, viruses and eukaryotic microbes1, that inhabit the human body internally and externally. Recent evidences have established the fundamental role of the gut microbiota and the gut microbiome (the entire collection of microbes and their genes found in the human gastrointestinal tract) in various human diseases including obesity, diabetes, cardiovascular diseases, and cancer1,2,3. Additionally, the microorganisms living in our gut produce a wide spectrum of metabolites which significantly affect our health and can also contribute to the pathophysiology of several diseases as well as a variety of metabolic functions4,5. Abnormal changes (perturbations) in the composition and function of this gut microbial population are generally termed as "gut dysbiosis". Dysbiosis is usually associated with an unhealthy state of the host and hence can be differentiated from the normal (homeostatic) microbial community associated with a healthy control state of the host. Specific patterns of gut microbiome dysbiosis are often found in various different diseases1,2,3,6,7.
The fermentation of undigested food, particularly the fermentable carbohydrates/fibers, by the gut microbiota not only yields energy but also produces divergent metabolites including short-chain fatty acids (SCFAs), lactate, formate, carbon dioxide, methane, hydrogen, and ethanol6. In addition, the gut microbiota also produces a number of other bioactive substances such as folate, biotin, trimethylamine-N-oxide, serotonin, tryptophan, gamma-aminobutyric acid, dopamine, norepinephrine, acetylcholine, histamine, deoxycholic acid, and 4-ethylphenyl sulfate. This occurs primarily through the utilization of intrinsic metabolic fluxes within the host-microbe niche, which contributes in several body processes, metabolic functions and epigenetic changes1,8,9,10. However, the effects of various interventions on such microbial products remain unkown or unclear due to the lack of easy, efficient and reproducible protocols. The human gut microbiota composition is an extremely complex and diverse ecosystem, and hence, many questions about its role in human health and disease pathology still remain unanswered. The effects of many common gut microbiome modulators (e.g., probiotics, prebiotics, antibiotics, fecal transplantation and infections) on the composition and metabolic functions of the intestinal microbiota remain largely elusive. In addition, the examination and validation of these effects in vivo is difficult, particularly because most of the nutrients and metabolites produced by the gut microbiota are absorbed or disposed of simultaneously and rapidly in the gut; therefore, measuring the production, amount and processing of these metabolites (e.g., SCFAs) in vivo still remains a practical challenge. Indeed, physiological models such as animals and human subjects are critical for determining the role of gut microbiome and its modulation on host health, but these may not be suitable for large-scale screening of different types of microbiome modulators due to ethical, monetary or time constraints. To this end, in vitro and/or ex vivo models, such as culturing of gut microbiota in vitro and then intervening with different microbiota modulators, can offer time- and money-saving opportunities and hence can allow for preliminary or large-scale screening of various components (such as probiotics, prebiotics, and other interventional compounds) to examine/predict their effects on the fecal microbiota diversity, composition and metabolic profiles. Studies using such in vitro and ex vivo systems of the gut microbiome may facilitate further understanding of the host-microbiome interactions that contribute to host health and disease, and could also lead to finding novel therapies that target the microbiome to ameliorate host health and prevent and treat various diseases1.
Although the in vitro gut microbiota culture systems cannot truly replicate the actual intestinal conditions, several laboratories have endeavored to develop such models, some of which have been found practicable to some extent and have been successfully used for different purposes. One of the recent gut models is the Simulator of the Human Intestinal Microbial Ecosystem, which mimics the entire human gastrointestinal tract, including the stomach, small intestine, and different regions of the colon. However, such technically complex models may not be accessible to other research facilities worldwide. Therefore, there is still a critical need for the development of new alternative models that are relatively simple, affordable and practical for laboratories studying the microbiome modulators and their effects on gut microbiota and host health. Hence, the use of an in vitro (or ex vivo) fecal microbiota culture system would be useful for studying the effects of such interventions11,12. Specifically, the effect of different prebiotics on the microbiota fermentation capacity in terms of periodic changes in the gut microbiota diversity and composition, the fecal pH, and the levels of microbial metabolites including SCFAs and lactate can be studied13. Herein, using inulin (one of the most widely studied prebiotic components) as an example of the microbiome modulator, a step-by-step protocol of this simple ex vivo microbiota batch-culture system is described to demonstrate its use to estimate the changes in the fecal microbiota and microbial metabolites following intervention with the microbiome modulators.
Protocol
CAUTION: Consult the appropriate Material Safety Data Sheets and follow the instructions and guidelines for appropriate Biosafety Level 2 (BSL-2) training. Follow all the culturing steps as per the standard biosafety rules and use a BSL-2 cabinet using aseptic conditions. Furthermore, fecal samples from different models and human subjects may have potential risk of spreading microbial borne diseases. Immediately seek medical aid in the occurrence of any injury and infection. In addition, the use of human and animal stool samples should be approved through institutional ethical committees and must compliant with protocols to use samples and subject information.
1. Preparation of Culture Media
- Preparation of the culture media, prepare nine types of stock solutions
- Solution A (1,000 mL): Dissolve 5.4 g of sodium chloride (NaCl), 2.7 g of potassium dihydrogen phosphate (KH2PO4), 0.16 g of calcium chloride dihydrate (CaCl2·2H2O), 0.12 g of magnesium chloride hexahydrate (MgCl2·6H2O), 0.06 g of manganese chloride tetrahydrate (MnCl2·4H2O), 0.06 g of cobaltous chloride hexahydrate (CoCl2·6H2O), and 5.4 g of ammonium sulfate (NH4)2SO4, in deionized water to make total volume to 1,000 mL.
- Solution B (1,000 mL): Dissolve 2.7 g Potassium Hydrogen Phosphate (K2HPO4) in deionized water to make total volume to 1000 mL.
- Trace mineral solution (1,000 mL): Dissolve 500 mg of disodium ethylenediamine-tetraacetate dihydrate (Na2EDTA), 200 mg of ferrous sulfate heptahydrate (FeSO4·7H2O), 10 mg of zinc sulfate heptahydrate (ZnSO4·7H2O), 3 mg of manganese(II) chloride tetrahydrate (MnCl2·4H2O), 30 mg of phosphoric acid (H3PO4), 20 mg of CoCl2·6H2O, 1 mg of cupric chloride dihydrate (CuCl2·2H2O), 2 mg of nickel(II) chloride hexahydrate (NiCl2·6H2O) and 3 mg of sodium molybdate dihydrate (Na2MoO4·2H2O) in deionized water to make total volume to 1,000 mL.
NOTE: This solution is light sensitive, therefore ensure to be stored in dark/black or aluminum wrapped tubes/bottles. - Water soluble vitamin solution (1,000 mL): Dissolve 100 mg of thiamine hydrochloride (Thiamin-HCl), 100 mg of D-pantothenic acid, 100 mg of Niacin, 100 mg of pyridoxine, 5 mg of P-aminobenzoic acid and 0.25 mg of Vitamin B12 in deionized water to make total volume to 1,000 mL.
- Folate: biotin solution (1,000 mL): Dissolve 10 mg of folic acid, 2 mg of D-biotin and 100 mg of ammonium bicarbonate (NH4HCO3) in deionized water to make total volume to 1,000 mL.
- Riboflavin solution (1,000 mL): Dissolve 10 mg of Riboflavin in 5 mM HEPES (1.19 g/L) solution to make total volume to 1,000 mL.
- Hemin solution (10 mL): Dissolve 5,000 mg of Hemin in 10 mM sodium hydroxide (NaOH) (0.4 g/L) solution to make total volume to 10 mL.
- Short-chain fatty acid mix (10 mL): Combine 2.5 mL of N-valerate, 2.5 mL of isovalerate, 2.5 mL of isobutyrate and 2.5 mL: DL-α-methylbutyrate.
NOTE: This solution is recommended to use in fume hood to avoid smell and fumes. - Resazurin (1,000 mL): Dissolve 1 g of resazurin in deionized water and make total volume to 1,000 mL.
- Medium used for in vitro anaerobic fermentation
- To prepare this media, mix 330 mL of Solution A, 330 mL of Solution B, 10 mL of Trace mineral solution, 20 mL of Water soluble vitamin solution, 5 mL of Folate:biotin solution, 5 mL of Riboflavin solution; 2.5 mL of Hemin solution, 0.4 mL of Short chain fatty acid mix, 1 mL of Resazurin, 0.5 g of yeast extract, 4 g of sodium carbonate (Na2CO3), 0.5 g of Cysteine HCl-H2O, and 0.5 g of Trypticase, and add 296.1 mL of distilled water.
- Check the pH and ensure it is around 7.0, if not, adjust the pH with 1 N HCl or 1 N NaOH. Sterilize by vacuum filtering the media using a bottle filter under the aseptic workstation.
- Alternatively, mix all the components (except vitamin and hemin solutions) and autoclave at 121 °C for 20 min and let it cool down to room temperature. Simultaneously, filter-sterilize vitamin and hemin solutions using 0.22 µm membrane filters and add these to the autoclaved and cooled media before dispensing.
2. Preparation of Anaerobic Chamber and Required Material
- Keep all the materials, solutions and tools needed for the fermentation experiment inside the anaerobic chamber at least 48 h before the start of the experiment, to ensure that any residual oxygen associated with tools and soluble oxygen in buffers/solutions is removed and all the materials are acclimatized to the set anaerobic conditions.
NOTE: Materials needed inside the anaerobic chamber (48 h earlier of experiment to start): (i) fermentation media; (ii) anaerobic solution (prepared according to section 4.1), (iii) vortexer, (iv) weighing balance, (v) muslin cheese cloths, (vi) scissors, (vii) funnel, (viii) 1.5, 2.0, 15, 50 mL tubes, (ix) pipettor (2, 20, 200 and 1,000 µL) and compatible pipet tips, (x) gun pipet and pipets (5 and 10 mL), (xi) paper tissues, (xii) markers, (xiii) tube stands for different tubes, (xiv) waste box, (xv) O2 indicator and (xvi) 70% ethanol spray bottle (disinfectant).
3. Preparation of Tubes and Fibers
- Weight 300 mg of inulin and transfer to a 50 mL tube followed by the aseptically addition of 26 mL of fermentation media already prepared and stored in the anaerobic chamber. Prepare one blank tube for each fecal sample type and experimental tube(s) (according to the number of compounds being tested), in triplicate.
- Leave these tubes inside the anaerobic chamber for around 24 h to allow hydration of samples before starting the fermentation experiment. Ensure the tube temperature is 37 °C at the time of inoculation, therefore bring the tubes in the incubator enclosed within the anaerobic chamber.
4. Preparation of Inoculum
- Anaerobic dilution solution (at least 48 h before fermentation experiment): Dissolve 5 g of NaCl, 2 g of glucose and 0.3 g of Cysteine-HCl in deionized water and make total volume to 1,000 mL. Autoclave and store it inside the anaerobic chamber at least 48 h before use.
- Fecal inoculum preparation (at the day of fermentation experiment): Weigh 5 g of fresh fecal sample in a 50 mL conical tube, add anaerobic dilution solution for a final volume of 50 mL (1:10 w/v) and vortex for 15 min or until completely homogenized. Filter the homogenized mixture through 4 layers of sterile cheesecloth (autoclaved) and use it immediately for inoculation in the tubes containing media.
NOTE: The fecal samples from a group of subjects can be pooled if the experimental objective is to compare the effect of a given compound/ingredient on overall healthy versus diseased fecal microbiota in general.
5. Fermentation and Sampling
- Prepare tubes according to the section 4.2 and inoculate blank/control and experimental tubes with 4 mL of diluted and filtered fecal inoculum. Incubate the inoculated tubes at 37 °C inside the anaerobic chamber. Shake the tubes once every hour by inverting gently to re-suspend the fibers and inoculum.
- Collect samples as frequently as needed, for example, hourly to 0, 3, 6, 9 and 24 h during fermentation by taking a 2 mL aliquot sample out into a 2 mL tube from respective fermenting tubes.
- Measure the pH of the aliquots using a laboratory pH meter (by directly inserting the pH electrode in the sample); centrifuge the remaining sample at 14,000 x g for 10 min at 4 °C. Immediately freeze the supernatant and pellet in liquid nitrogen, and after snap freezing, store the supernatant for SCFAs analysis and pellet for microbiome analysis at -80 °C.
6. Short Chain Fatty Acids (SCFAs) and Lactate Quantification
NOTE: The SCFAs and lactate in the supernatant of microbiome culture can be measured exactly according to the methods detailed elsewhere13,14,15,16.
- Briefly, thaw the snap frozen supernatants obtained in section 5 from control and treatment samples on ice and carry out all further processing steps on ice. Filter the supernatant through a 0.45 µm membrane filter and use the cell-free samples to measure the concentrations of SCFAs and lactate using HPLC system with DAD detector at 210 nm, equipped with an HPX-87H column. Use inject volume of 10 μL for each sample and use H2SO4 (0.005 N) to elute the column at a flow rate of 0.6 mL/min at 35 °C.
7. Fecal Microbiome Analysis
NOTE: Perform microbiome analysis following the methods and pipeline detailed elsewhere7,13,14,17.
- Briefly, extract genomic DNA from approximately 200 mg of fecal slurry pellets by using a fecal DNA extraction kit13.
- Amplify the hypervariable region of the bacterial 16S rRNA gene by using the barcoded primers as per the method described elsewhere13, using the primer sequences as described in the Earth Microbiome Project protocol18.
- Purify the resulting amplicons with magnetic purification beads and quantify by Picogreen or an equivalent method. Pool the purified PCR products in equal molar concentration and sequence on a sequencer13.
- Process the resulting sequences for de-multiplexing, quality filtering and clustering, taxonomic assignment and rarefaction, and downstream analyses by using the Quantitative Insights into Microbial Ecology (QIIME) software as per the methods described by Caporaso et al.19.
Representative Results
The protocol is used to demonstrate the effect of a specific prebiotic (i.e., inulin on the microbiota composition and metabolic activities in terms of changes in the fecal pH and the concentration of lactate and SCFAs in the feces of healthy human subjects over different time-points following treatment with inulin). The fecal pH, the fecal levels of lactate and SCFAs (Figure 1), and the microbiota composition (Figure 2 and Figure 3) are measured at 0 (baseline), 9 and 24 h of incubation with or without inulin. The results demonstrate how fecal microbiota composition and its metabolic activities are modulated during in vitro fermentation with or without inulin treatment.
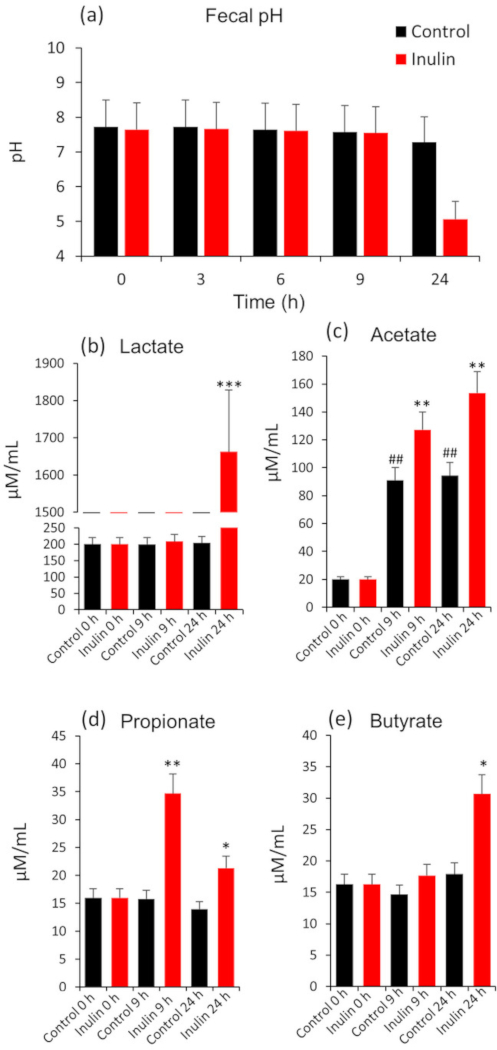
Figure 1: Changes in fecal pH (a), lactate (b) and short-chain fatty acids viz. acetate (c), propionate (d) and butyrate (e) in human feces at 0 (baseline), 9 and 24 h of anaerobic fermentation with or without inulin. Values presented here are Mean ± SEM of triplicate samples. *P < 0.05, **P < 0.01, ***P < 0.001, vs. baseline. Please click here to view a larger version of this figure.
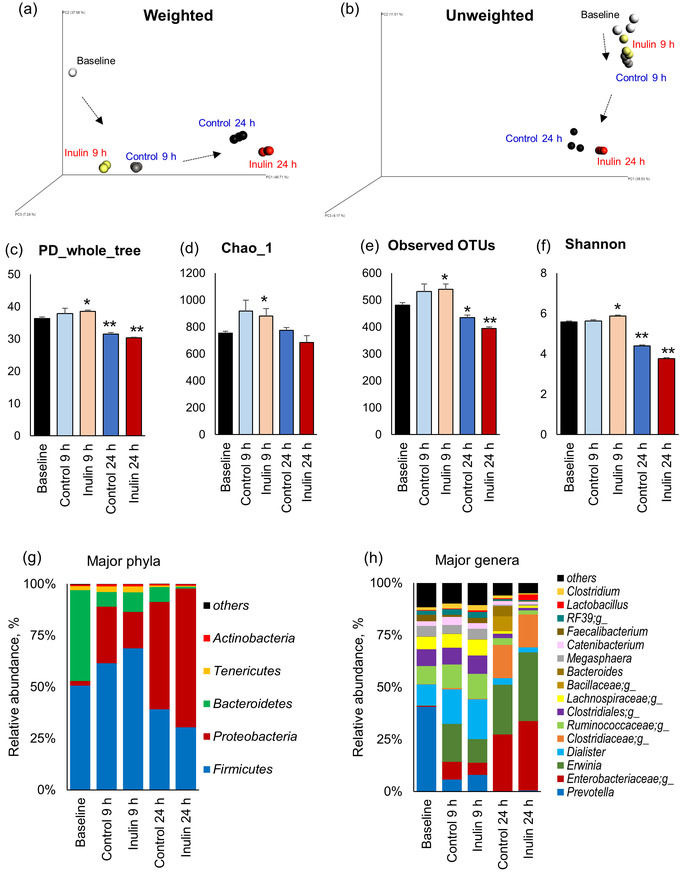
Figure 2: Changes in the microbiota diversity and composition in human feces at 0 (baseline), 9 and 24 h of anaerobic fermentation with or without inulin. (a) Weighted and (b) unweighted Unifrac measures of beta-diversity visualized using Principle Coordinate Analysis (PCoA). (c–f) Indices of alpha-diversity viz. phylogenetic diversity (PD whole tree (c); species richness (Chao1; d); observed number of operational taxonomic units (OTUs, e); and species evenness (Shannon index, f)). Relative abundance of major phyla (g) and genera (h). Values presented here are Mean ± SEM of triplicate samples. *P < 0.05, **P < 0.01, vs. baseline. Please click here to view a larger version of this figure.
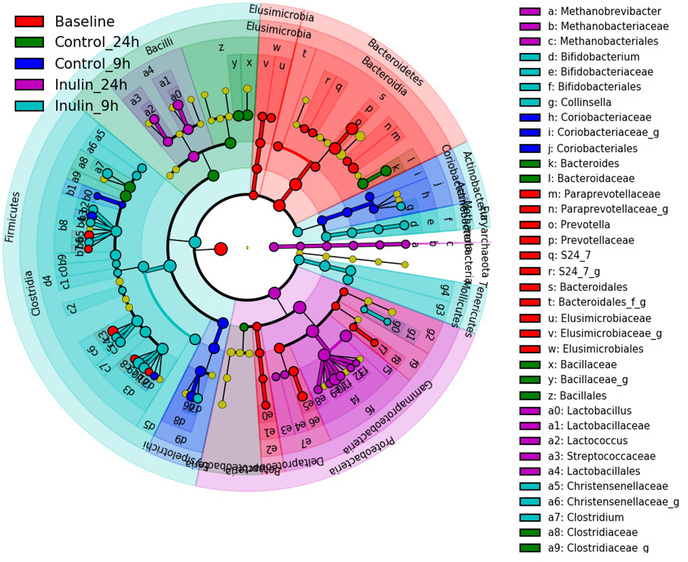
Figure 3: Linear discriminant analysis (LDA) effect size (LEfSe) analysis of gut microbiota changes following 9 h and 24 h incubation with (INU) or without (CTL) inulin. Taxonomic cladogram derived from LEfSe analysis of 16S sequences (relative abundance ≥ 0.5%) representing the differentially abundant taxa between different group of samples. The brightness of each dot is proportional to its effect size (i.e., the taxon abundance). Only taxa passing LDA threshold value of >2.4 are shown here. Please click here to view a larger version of this figure.
Discussion
The in vitro fecal slurry fermentation model presented here is a simple single-batch model to approximate the effects of different substrates and microbial strains (e.g., prebiotics and probiotics) on the composition of human fecal microbiota as well as its metabolic activities in terms of fecal pH and SCFAs levels. The results presented herein demonstrate that the inoculation of inulin decreases the fecal pH and significantly increases the levels of SCFAs and lactate in inulin-treated fecal specimen as compared to non-treated fecal microbiota culture (Figure 1). In addition, the gut microbiota signature also appears to be different between inulin-treated and untreated samples (Figure 2 and Figure 3). These data exemplify how this system can reflect the effects of inulin on fecal microbiome diversity and composition as well as its metabolic activities. In addition, depending on specific experimental objectives and hypotheses, a variety of other factors can also be measured using this system. Furthermore, in addition to 16S rRNA gene sequencing, other analyses such as whole microbial metagenome sequencing (using Whole genome sequencer) or qPCR and culture methods targeted at specific single or multiple genera, species and strains (e.g., bifidobacteria, lactobacilli, Akkermansia, Enterobacteriacaea, clostridia, etc.) can also be executed. In addition, different chromatographic procedures for the analysis of SCFAs, such as LC-MS, GC, GC-MS, GC-FID, and HPLC can also be exploited based on experimental requirement and availability. Nevertheless, it should be noted that sample preparation for these procedures may vary according to the instruments and conditions required for operation20.
Although the use of fresh fecal specimen would yield best reproducible results; however, snap frozen fecal sample (as used in the experiment presented herein) can also be used efficaciously as most of the bacteria can be revived from it once resuscitated to body temperature and no more decomposition happens after the freezing21. Using frozen sample can be particularly advantageous when it is impossible to obtain fresh fecal samples from specific donor(s) on planned day of the experiment, or from the same donor when an experiment needs to be replicated. It should be noted, however, that the fecal samples should be snap frozen using liquid nitrogen and immediately stored at -80 °C until further use. In addition, to avoid the exposure of the frozen fecal samples to air (oxygen), the samples should be transferred to the anaerobic chamber as soon as possible/immediately after taking out from the freezer and used right away (repeated thawing should be avoided). All subsequent experiments including sample thawing, inoculum preparation and processing should be done inside the anaerobic chamber.
The intestinal organic environment and pH during nutrient fermentation in large bowel are very important particularly in view of the fact that an abnormally reduced pH indicates increased acidity due to the substrate utilization. Hence, more rapid pH reduction can correspond to more rapid substrate utilization22. Although, in this model, the pH has not been controlled, however, inclusion of identical non-treated control is highly recommended to make direct comparisons. The SCFAs produced in the colon as a result of the fermentation of indigestible polysaccharides by the gut microbiota can further influence diverse mechanisms related to the maintenance of the host health. These metabolites contribute in gluconeogenesis and lipid biosynthesis, act as an energy source for colonocytes, and can also have health benefits including immune modulation, controlled/improved gut barrier functions, and neuromodulation23,24,25,26. In addition, SCFAs are also known to influence several biological pathways including hormones, endocannabinoid system, cell proliferation and death, bone health, mineral absorption, gut motility, intestinal pH, and invert effects on the gut microbiome and microbial metabolic function. Therefore, knowledge of the profile and concentrations of intestinal/ fecal SCFAs can be an important component while evaluating the efficacy of specific microbiota modulators6. Of course, besides SCFAs, the gut microbiome also produces many other important metabolites (e.g., ammonium, vitamins, histamine). Hence, the supernatant specimens collected during such in vitro fermentation experiments can also be evaluated for global metabolomics analyses to discover novel gut microbiome-derived metabolites that can be influenced by specific microbiome modulators.
The system described here has many advantages, such as the ease, simplicity, cost effectiveness, and the general adoptability of the experimental set-up. However, there are few limitations as well. For example, the system does not pertain to the interaction of prebiotics (or other interventions used) in the upper digestive system (e.g., saliva, stomach, small intestine) before being exposed to the microbiota of the large intestine. However, such steps can be adopted and incorporated according to specific experimental requirements. Also, an acidic and/or enzymatic hydrolysis process may need to be performed before fermentation if using specific substrates including whole food or digestible food because only the indigestible part of such foods reaches to the colon to be fermented by the lower gut microbiota. In addition, the composition of gut microbiota may shift due to specific culture conditions during fermentation. For example, it was observed that up to 9 h of incubation, microbiota changes are much closer to normal than at 24 h. However, after 24 h of incubation, although pH and SCFAs levels increased substantially, gut microbiota composition showed that Proteobacteria counts increased while the microbial diversity reduced. However, it remains unknown how precisely and closely the increased accumulation of microbial metabolites accompanied with the lowered fecal pH is associated with specific microbiota signatures.
In summary, a simple protocol to simulate the ex vivo microbiota ecosystem is described herein. The system enables researchers to test different interventions for modulation of the microbiota diversity, composition and metabolic function that can influence diverse features of the host intestinal and overall health.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge funding support from the Center for Diabetes, Obesity and Metabolism and the Clinical and Translational Science Center, the Wake Forest School of Medicine, the Department of Defense funding (Grant number: W81XWH-18-1-0118), the Kermit Glenn Phillips II Chair in Cardiovascular Medicine; the National Institutes of Health funded Claude D. Pepper Older Americans Center (funded by P30AG12232); R01AG18915; R01DK114224 and the Clinical and Translational Science Center (Clinical Research Unit, funded by UL1TR001420), is also thankfully acknowledged. We also thank the volunteers for providing fecal samples, and our other lab members for their technical helps during this experiment.
Materials
| Ammonium Bicarbonate (NH4HCO3) | Sigma-Aldrich | 217255 | |
| Ammonium Sulfate (NH4)2SO4 | TGI | C2388 | Toxic |
| Calcium Chloride Dihydrate (CaCl2•2H2O) | Sigma-Aldrich | C3306 | Irritating |
| Cobaltous Chloride Hexahydrate (CoCl2•6H2O) | Sigma-Aldrich | 255599 | |
| Cupric Chloride Dihydrate (CuCl2•2H2O) | Acros organics | 2063450000 | Toxic, Irritating |
| Cysteine-HCl | Sigma-Aldrich | C121800 | |
| D-biotin | Sigma-Aldrich | B4501 | |
| D-Pantothenic acid | Alfa Aesar | A16609 | |
| Disodium Ethylenediaminetetraacetate Dihydrate (Na2EDTA) | Biorad | 1610729 | |
| DL-α-methylbutyrate | Sigma-Aldrich | W271918 | |
| Ferrous Sulfate Heptahydrate (FeSO4•7H2O) | Sigma-Aldrich | F8263 | Toxic |
| Folic acid | Alfa Aesar | J62937 | |
| Glucose | Sigma-Aldrich | G8270 | |
| Hemin | Sigma-Aldrich | H9039 | |
| Hepes | Alfa Aesar | A14777 | |
| Isobutyrate | Alfa Aesar | L04038 | |
| Isovalerate | Alfa Aesar | A18642 | |
| Magnesium Chloride Hexahydrate (MgCl2•6H2O) | Sigma-Aldrich | M8266 | |
| Manganese Chloride Tetrahydrate (MnCl2•4H2O) | Sigma-Aldrich | 221279 | |
| Niacin (Nicotinic acid) | Sigma-Aldrich | N4126 | |
| Nickel(Ii) Chloride Hexahydrate (NiCl2•6H2O) | Alfa Aesar | A14366 | Toxic |
| N-valerate | Sigma-Aldrich | 240370 | |
| P-aminobenzoic acid | MP China | 102569 | Toxic, Irritating |
| Phosphoric Acid (H3PO4) | Sigma-Aldrich | P5811 | |
| Potassium Dihydrogen Phosphate (KH2PO4) | Sigma-Aldrich | P5504 | |
| Potassium Hydrogen Phosphate (K2HPO4) | Sigma-Aldrich | 1551128 | |
| Pyridoxine | Alfa Aesar | A12041 | |
| Resazurin | Sigma-Aldrich | R7017 | |
| Riboflavin | Alfa Aesar | A11764 | |
| Sodium carbonate (Na2CO3) | Sigma-Aldrich | 1613757 | |
| Sodium chloride (NaCl) | Fisher BioReagents | 7647-14-5 | |
| Sodium hydroxide (NaOH) | Fisher Chemicals | S320 | |
| Sodium Molybdate Dihydrate (Na2MoO4•2H2O) | Acros organics | 206375000 | |
| Thiamine Hydrochloride (Thiamin-HCl) | Acros organics | 148991000 | |
| Trypticase | BD Biosciences | 211921 | |
| Vitamin B12 | Sigma-Aldrich | V2876 | |
| Yeast extract | Sigma-Aldrich | 70161 | |
| Zinc Sulfate Heptahydrate (ZnSO4•7H2O) | Sigma-Aldrich | Z0251 | |
| 0.22 µm membrane filter | |||
| AMPure magnetic purification beads | Agencourt | ||
| Anaerobic chamber with incubatore | Forma anaerobic system, Thermo Scientific, USA | ||
| Bottle filter | Corning | ||
| Cheesecloth | |||
| Illumina MiSeq sequencer | Miseq reagent kit v3 | ||
| pH meter | |||
| Qiagen PowerFecal kit | Qiagen | ||
| Quantitative Insights into Microbial Ecology (QIIME) software | |||
| Qubit-3 fluorimeter | InVitrogen | ||
| Vortex | Thermoscientific | ||
| Waters-2695 Alliance HPLC system | Waters Corporation |
References
- Shreiner, A. B., Kao, J. Y., Young, V. B. The gut microbiome in health and in disease. Current Opinion in Gastroenterology. 31 (1), 69-75 (2015).
- Xu, Z., Knight, R. Dietary effects on human gut microbiome diversity. British Journal of Nutrition. 113, 1-5 (2015).
- Jiang, C., Li, G., Huang, P., Liu, Z., Zhao, B. The gut microbiota and Alzheimer’s disease. Journal of Alzheimers Disease. 58 (1), 1-15 (2017).
- Clemente, J. C., Ursell, L. K., Parfrey, L. W., Knight, R. The impact of the gut microbiota on human health: an integrative view. The Journal Cell. 148 (6), 1258-1270 (2012).
- Yadav, H., Jain, S., Marotta, F. Probiotics mediated modulation of gut flora might be biotherapeutical approach obesity and type 2 diabetes. Metabolomics : Open Access. 1 (3), 1-3 (2011).
- Ahmadi, S., et al. Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obesity and Control Theries: Open Access. 4 (3), (2017).
- Nagpal, R., et al. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. Journal of Diabetes Research. , 1-9 (2018).
- Paul, B., et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Journal of Clinical Epigenetics. 7 (1), 112 (2015).
- O’mahony, S., Clarke, G., Borre, Y., Dinan, T., Cryan, J. Serotonin tryptophan metabolism and the brain-gut-microbiome axis. Journal of Behavioural Brain Research. 277, 32-48 (2015).
- Sharon, G., et al. Specialized metabolites from the microbiome in health and disease. Journal of Cell Metabolism. 20 (5), 719-730 (2014).
- Faber, T. A., Bauer, L. L., Price, N. P., Hopkins, A. C., Fahey, G. C. In vitro digestion and fermentation characteristics of temulose molasses, a coproduct of fiberboard production, and select temulose fractions using canine fecal inoculum. Journal of Agricultural Food Chemistry. 59 (5), 1847-1853 (2011).
- Bourquin, L. D., Titgemeyer, E. C., Fahey, G. C. Vegetable fiber fermentation by human fecal bacteria: cell wall polysaccharide disappearance and short-chain fatty acid production during in vitro fermentation and water-holding capacity of unfermented residues. Journal of Nutrition. 123 (5), 860-869 (1993).
- Nagpal, R., et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Scientific Reports. 8 (1), 12649 (2018).
- Nagpal, R., et al. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate and human feces. Frontiers in Microbiology. 9, 2897 (2018).
- Thangamani, S., Guinan, J., Wang, S., Yadav, H. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids promote gastrointestinal colonization of Candida albicans. bioRxiv. , 428474 (2018).
- Ahmadi, S., et al. Prebiotics from acorn and sago prevent high-fat diet-induced insulin resistance via microbiome-gut-brain axis modulation. The Journal of Nutritional Biochemistry. , (2019).
- Nagpal, R., et al. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Frontiers in Nutrition. 5, 28 (2018).
- Caporaso, J. G., et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME Journal. 6 (8), 1621-1624 (2012).
- Caporaso, J. G., et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 7 (5), 335-336 (2010).
- Garcia-Villalba, R., et al. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. Journal of Seperation Science. 35 (15), 1906-1913 (2012).
- Lee, C. H., et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 315 (2), 142-149 (2016).
- Chen, M. -. H., et al. In vitro fermentation of xylooligosaccharides produced from Miscanthus× giganteus by human fecal microbiota. Journal of Agricultural and Food Chemistry. 64 (1), 262-267 (2015).
- Cook, S., Sellin, J. Short chain fatty acids in health and disease. Alimentary Pharmacology & Therapeutics. 12 (6), 499-507 (1998).
- Rastelli, M., Knauf, C., Cani, P. D. Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity. 26 (5), 792-800 (2018).
- Zou, J., et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host & Microbe. 23 (1), 41-53 (2018).
- Dinan, T. G., Cryan, J. F. Gut–brain axis in 2016: Brain–gut–microbiota axis—mood, metabolism and behaviour. Nature Reviews Gastroenterology & Hepatology. 14 (2), 69 (2017).

