Simple Homemade Tools to Handle Fruit Flies—Drosophila melanogaster
Summary
Described here is the use of several homemade tools to transfer, chill, and kill adult Drosophila, as well as to clean glass culture vials and collect eggs. These tools are easy to make and are rather efficient in handling Drosophila.
Abstract
The fruit fly, Drosophila melanogaster, is widely used both in biological research and biology education. Handling adult flies is common but difficult in practice, as adult flies fly. Demonstrated here is how to make some simple and cost-effective tools to address difficult issues in the handling of Drosophila. Holes in foam stoppers are made and pipette tips or funnels are inserted into the holes. Flies then move only in one direction into the pipette tip/funnel assemblage, allowing efficient control of the transfer of adult Drosophila into or out of a vial. Existing protocols have been modified for cool-anesthetizing flies by chilling in crushed ice and transferring them onto a cold, hard icepack surface. The icepack is covered with a piece of medical gauze that keeps immobilized flies from the condensed water when examined under a stereomicroscope. The flies are finally euthanized for counting and sorting or discarded by microwaving. A bottle-shaped cage has also been developed for collecting eggs, as well as a labor-saving device and accompanying protocol for cleaning glass culture vials.
Introduction
The fruit fly, Drosophila melanogaster, is a model organism widely used in biological research and biology education to study a wide range of topics1,2. The basic problems of handling Drosophila are the transfer of adults from vial to vial and immobilization of the flies so they are easier to handle, as all adults (except for some mutants3,4) can fly.
Conventionally, a researcher transfers flies from one vial to another by holding two vials mouth-to-mouth, tapping the flies down or allowing flies to fly up into another vial, then separating and replugging both vials4. Obviously, this requires that the opening of two vials with the same diameter, and it is hard to control the quantity of flies transferred. Meanwhile, this requires quick hands to get the job done, and escaping stray flies can result in problems for the laboratory or classroom. Adding extra virgin flies or male flies to an already prepared cross is another routine task in Drosophila experiments. Conventionally, flies must be immobilized in the cross vial before the addition of extra flies.
Adult Drosophila are routinely anaesthetized by ether, CO2, or chilling5. Compared to ether and CO2 exposure, chilling is the most cost-efficient agent for immobilizing adult Drosophila and the least harmful to both the flies and researchers (especially young students)6,7. However, water that condenses continuously on the cold surface or chamber wets the flies. It is difficult to determine the phenotypes of wet flies, and they can easily become damaged during manipulation8,9. This has kept the chilling method from becoming more widely accepted.
Tools for fly transferal and a method for fly cooling have been previously described10. Herein, a modified chilling anesthesia technique is reported that is safe, reliable, and feasible for Drosophila experiments. Also described in this paper are 1) methods for killing adults for counting, sorting, or discarding, 2) labor-saving devices and protocols for cleaning glass culture vials, and 3) a simple cage for collecting eggs. The easily designed and cost-effective tools described here can be used to address the difficult issues of fly handling, and these methods have been tested and are proven to be robust, reliable, and easy-to-handle for experienced and novice researchers.
Protocol
1. Preparing Tools and Accessories
- Tip/funnel stoppers
- Obtain two sponge plugs (the diameter of the plugs must be slightly larger than the internal diameter of the vials used to transfer flies). Make a hole in the centers of the sponge plugs with a heated electric soldering iron.
- Obtain two 1 mL pipette tips, cut one in half transversely with a sharp knife, and discard the pointed end. Then, cut 1.5 cm of the pointed end off the second pipette tip. Glue the remains of the two pipette tips together with an all-purpose adhesive to make an elongated pipette tip (Figure 1A).
- Insert a funnel and the elongated pipette tip into the sponge plugs to make a tip and funnel stopper (hereafter referred to as T- and F-stoppers) and cap the pipette tip with a 100 μL microcentrifuge tube (Figure 1A).
NOTE: The length of the funnel stem must be greater than the height of the plug. If it is shorter than or equal to the height of the plug, then flies will escape from the stem opening. The end of the funnel stem should be situated at least 2 cm above the surface of the culture medium or the bottom of an empty vial. Small funnels (e.g., disk diameter <60 mm) with small internal stem opening diameters (<5 mm) are preferable. Either a glass or a plastic funnel can be used to make an F-stopper. However, plastic funnels are preferable for biology classes, as they break less readily than glass funnels.
- Microdissecting needles
- Obtain mechanical pencils that feel comfortable in the hand and insect pins that match the diameters (e.g., 0.5 mm, 0.7 mm) of their lead refills.
- Cut the wide ends of the insect pins with a pair of pliers and file the cut flat. Replace the lead with the pins (Figure 1B). Press the click button and feed out 0.5–1 cm of a pin to conduct a dissection. Clean the pin and push it completely back into the pencil shaft after a dissection activity to make it safe for any person to handle.
NOTE: Microdissecting needles are useful not only in dissections of organs such as larval salivary glands but also in counting and sorting dead adult flies.
- Hard icepacks
- Obtain several refreezable hard icepacks (large-sized icepacks are preferable). Figure 1C shows an icepacks that worked well, which measures 26.5 cm x 14.5 cm x 2.5 cm and has top and bottom sides that are completely flat.
- Cut medical gauze (nonsterile) into pieces that are slightly smaller than the cold surfaces of the icepacks they cover. For example, a piece of medical gauze slightly smaller than 26.5 cm x 14.5 cm is preferable to cover an icepack shown in Figure 1C.
NOTE: The necessary accessories for these chilling tools include: an ice box (we used a 25 cm x 15 cm x 15 cm foam box for one person and 37 cm x 28 cm x 20 cm box for more than one person), which is used to store crushed ice; a pair of fine-point tweezers, which are used to grab chilled flies by their wings and transfer them to a vial; a pair of protective work gloves, which are used to take chilled icepacks out of a -20 °C freezer; and plastic film, which is used to cover the stage of a stereomicroscope.
- Drosophila egg collection cage
NOTE: Ready-made Drosophila egg collection cages are available from many biotechnology companies11. Described here is a small acrylic bottle-shaped egg collection cage for 60 mm Petri dishes (Figure 1D left; the cage design is shown in the middle). It can be adapted for other Petri dish sizes (e.g., 100 mm, 35 mm). This allows the transfer of flies into or out of the cage with ease. A simple cage can be prepared as follows.- Use a snap cutter to cut a soft plastic drink bottle (500 mL, internal diameter ca. 65 mm) into an approximate 2:1 (pointed end:blunt end) ratio and discard the blunt end.
- Wrap a strip of card paper around an apple juice plate (internal diameter 60 mm) with adhesive tape [the apple juice plate is used to collect eggs (Figure 1E, right)].
- Cordless tube brush driver
- Obtain a cordless drill driver (max speed = 500 rpm).
- Obtain a tube brush that has bristles along its sides as well as its front. Ideally, the diameter of the brush should be slightly larger than the diameter of the culture vials that need to be cleaned. Cut the end of its handle so it can be inserted into the drill chuck (Figure 1D).
NOTE: The necessary accessories for these cleaning tools include stainless steel sponges and long cuff rubber gloves.
2. Transferring Adult Flies from Vial A to Vial B
NOTE: Transferring adult flies from one vial to another is the most common practice conducted in Drosophila experiments [e.g., transferring flies from old culture (A) to fresh culture (B) or from a cross vial (A) to empty vial (B)] for anesthetizing. The protocol described here can be used for any adult fly transferring activities. Unless otherwise stated, this protocol is used to transfer flies from vial A to vial B throughout this paper.
- Check the stem of the funnel of an F-stopper and the pipette tip of a T-stopper carefully, then clear any flies that remain in the stoppers with a rubber air blower. This step is of paramount importance, especially when one set of T- and F-stoppers is used for the continuous transferring of different Drosophila lines.
- Tap down the flies in vial A and replace its plug with a T-stopper, then plug vial B with an F-stopper.
- Invert vial A over vial B, insert the pipette tip end of the T-stopper into the funnel opening of the F-stopper, knock the edge of inverted vial A to allow flies to slip out of the pipette tip and through the stem of the funnel, and drop into vial B. If any old food in vial A becomes less compact, it may drop when vial A is inverted and knocked. In such a situation, invert vial B over vial A and allow the flies to crawl up into vial B.
- Separate the T-stopper from the F-stopper. Cap the pipette tip end of the T-stopper with a 200 μL microcentrifuge tube if the remaining flies in vial A need to be transferred to other vials momentarily; otherwise, remove the T-stopper and replug vial A. Remove the F-stopper and replug vial B.
3. Immobilizing Flies by Chilling
- Keep hard refreezable icepacks in a -20 °C freezer for at least 24 h before use.
- Place a chilled, hard icepack at room temperature (RT) for 20 min. Slightly moisten a piece of non-aseptic medical gauze with some running water and allow it to closely cling to the surface of the icepack. The medical gauze can be reused in the next fly chilling. At the same time, chill an empty vial in crushed ice.
- Transfer adult flies that need to be immobilized into the chilled empty vial (CEV). When the two transfer vials are separated, cover the CEV with a Petri dish or a plug and knock the CEV against the crushed ice to tap all the flies in the CEV down to the bottom. Repeat this process several times until all flies are immobilized. The flies will be immobilized within 30 s. Next, place the CEV in the ice for 1 min. It is not advisable to transfer too many flies at one time for anesthetizing.
- Pour the chilled flies out onto the medical gauze that covers the ice pack. Spread out the overlapping flies with a paintbrush and make sure that each fly can be chilled by the cold surface of the icepack. If a chilled hard icepack swells slightly, place it on a towel and work on its flat side.
- Remove the stage clips from the stereomicroscope, cover the stage with a piece of plastic film, and put the icepack onto the stage. Turn on the top light (a cold light source is desirable), focus the stereomicroscope and move the icepack until the chilled flies can be seen clearly.
4. Killing Adult Flies for Counting, Sorting, or Discarding
- Transfer adult flies into an empty vial and cover it with a Petri dish.
- Invert the vial, heat it for 1 min + 20 s in a microwave oven, and allow the dead flies drop into the Petri dish.
- Put on protective work gloves and take the vial out of the microwave. Pour the dead flies onto a white paper card, count or examine the flies with a microdissecting needle under a stereomicroscope, and dispose of the fly bodies in a garbage can after observation.
- To kill unwanted flies, heat the flies for 2–3 min in a microwave oven, then tap the carcasses into a garbage can.
NOTE: It is not advisable to kill some wing mutant strains (e.g., wing length mutants) for examination, as it is difficult to judge from the carcasses if the wings extend beyond the tip of the abdomen, which is seen in wild-type flies.
5. Transferring Flies In/Out of Bottle-shaped Egg Collection Cage
NOTE: As mentioned above, T- and F-stoppers are used to transfer flies into and out of the egg collection cage. Flies do not need to be anesthetized throughout this process. Other details, such as preparing the apple juice medium, egg collection, and dechorionization, can be found in the literature12.
- Insert the egg collection cage into the apple juice plate or mount the apple juice plate to the cage made of a soft drink bottle. Seal the joint around the two components with a strip of paraffin film.
- Place as many flies as possible into the cage and replug the cage with a foam stopper after transferring the flies.
- To change the food for flies in the cage, transfer the flies in the cage to an empty vial.
- Replace the apple juice plate and reseal it, then transfer the flies from the vial back to the cage.
- When egg collection ends, transfer the flies into an empty vial and transfer them into culture vials.
6. Cleaning Glass Culture Vials
NOTE: Generally, an old culture vial contains live flies. In the protocol described here, these flies DO NOT need to be killed before cleaning unless they are transgenic flies.
- Remove any permanent marker ink from the glass culture vials with wet, stainless steel sponges.
- Soak the culture vials in running water.
- Fill a laboratory sink with water, add liquid dishwashing soap into the water, and mix.
- Immerse the culture vials into the water, then remove the plug, allowing the water to run into the vial. The dish detergent in the water will make any remaining adult flies sink to the bottom and drown in the water.
- Soak the old culture vials in water for at least 30 min.
- Loosen the chuck of the drill, insert the test tube brush and retighten the chuck. Check the direction of the rotation selector and ensure that the drill rotates clockwise. Adjust the speed trigger and ensure that the maximum speed is less than 500 rpm.
- Clean the culture vials.
- Clean the culture vials roughly.
- Put a long cuff rubber glove on the non-dominant hand and hold the vial in the water.
- Hold the cordless tube brush driver with the bare dominant hand, squeeze the brush into the culture vial, and squeeze the trigger.
NOTE: Do not dip the battery into the water. The rotating brush will break up old food, pupa, etc., and remove more than 95% of the waste. - Dump the waste into a separate garbage can. Repeat this process until most of the waste in each vial has been cleaned.
- Clean the culture vials thoroughly.
- Clean the tube brush, drain and clean the sink, and refill it with clean water.
- Remove the remaining waste from each culture vial as described in section 6.4.1.
- Clean the culture vials roughly.
Representative Results
The T- and F-stoppers were developed as a set of simple tools that can be adapted and used in any fly transferring activities. Transferring flies from an old culture into several fresh cultures involves removing the plugs of the fresh vials, replacing them with F-stoppers, then tapping down the flies in the old vial, quickly removing its plug, and replacing it with a T-stopper. If the old food is compact, then it is important to flip the old vial and insert the tip of T-stopper into the opening of an F-stopper, then tap the flies down into the fresh vial. Then, replacing the T- and F-stoppers and replugging the vials is performed. If the old food becomes less compact, it is advised to flip the fresh vial over, mount the F-stopper to the T-stopper, and allow the flies to crawl up into the fresh vial.
To add extra flies to an already prepared cross, it is important to tap the flies in the cross vial down and replace its plug with an F-stopper. Then, the experimenter must examine the chilled flies under a stereomicroscope, pick up a desired fly by its wing using a pair of pointed tweezers, and allow it to slip into the cross vial through the stem of the funnel. If a fly is trapped in the stem of a funnel, it is advised to gently blow on it with an air blower and let it slip into the vial. Replacing the F-stopper and replugging the vial when enough flies for a cross have been collected is then required. T- and F-stoppers were introduced in 201013,14; thus far, more than 1,200 students have benefitted from these fly transferring devices. T- and F-stoppers have also been introduced to instructors and researchers through a laboratory guide15, which has been adopted for use in teaching and research labs.
Existing chill anesthetizing methods have been modified for use in this study. Crushed ice or ice-water mixtures are used to chill the adult flies then transfer the immobilized flies onto the cold surface of an icepack covered with a piece of nonsterile medical gauze. The gauze fibers soak up the condensed water and keep the flies dry when they are examined. At the same time, the tiny holes between the warp/weft threads allow flies to touch the cold surface of the icepack and keep them immobile (Figure 2). At a room temperature of 25 °C, the temperature of the surface of a chilled, hard icepack increases dramatically from -19 °C to -2 °C within 20 min and reaches a plateau that is safe for both old and newly hatched flies (Figure 3). An icepack works quite well within the plateau, and the chilled flies regain consciousness at room temperature within 30 s. Because a hard icepack is thin, it can then be placed under a stereomicroscope to examine the flies. The hard icepack described here costs less than $2; furthermore, 60 hard icepacks for a class of 100-150 students each semester have been used, and they are reusable for many years. This modified version of the chilling anesthesia technique was introduced to a specific genetics class three years ago, and its robustness has been tested by more than 300 students and those in other universities.
It has been found that microwave dielectric heating is a faster, more convenient agent to kill adult flies (if they are no longer needed after observation) compared to agents such as overetherizing or deep freezing (Table 1). Microwave dielectric heating requires a far shorter time to kill flies than overetherizing or deep freezing. All flies die within 80 s, so counting and sorting a large batch of flies within a short timeframe is feasible16. Assuming that the experimenter needs to kill flies 20x to count and sort batches for an experiment, it will take 3 h + 20 min and 5 h to kill the flies by overetherizing and deep freezing, respectively; however, only 27 min are needed using a microwave.
Similar to overetherized flies, microwaved flies extend the wings at right angles from the bodies. Generally, fly carcasses killed by microwaving were significantly lighter than those killed by ether or chilling, but the heat does not distort the body shape, and the carcasses do not become crisp or turgid. Characteristics (e.g., body color, eye color, and wing shape) of microwaved flies are similar to those killed by ether or freezing (Figure 4), and there are no significant differences in wing sizes (area, length, width) of flies killed by the three agents (Table 1). Therefore, carcasses of flies killed by microwaving can be used for counting, sorting, and measuring of some traits, such as wing size. Microwave heating is also a good method to kill unwanted flies and dispose of them in a timely manner. Additionally, fly morgues (bottles containing flammable ethanol, methanol, or soap solutions), which are used to store dead or discarded flies, are no longer needed in fly labs or biology classes3.
A small, bottle-shaped egg collection cage was designed for this protocol. Using T- and F-stoppers, a large number of flies can be transferred into or out of the cage, and the apple juice medium plates can be changed with greater ease. Finally, the flies do not need anesthetizing before and after egg collection.
A cordless tube brush driver and protocol for use of this equipment to clean culture vials was also developed for the protocol. This battery-powered tube brush can easily break down old food and pupa attached to a glass culture vial, a vial can be cleaned within 30 s, and the efficiency of cleaning is increased greatly; therefore, cleaning large amounts of glass culture vials is no longer a tedious task.

Figure 1: Tools used in handling Drosophila. (A) Shown are fly-transferring tools and the necessary accessories. They are (from left to right) an air blower (used to blow out adult flies remaining in the funnel stem); T- and F-stoppers (inserted into vials); and an empty vial covered with a Petri dish (36 mm, the bottom half of a 40 mm Petri dish). The foam stoppers are larger than the openings of the vials so they can be used with vials of variable opening sizes. The size described here can be changed if necessary. (B) Shown are materials needed for the micro-dissecting needles. (C) Shown is the hard icepack used to chill flies. (D) Shown is the bottle-shaped egg collection cage (left), its design plan (middle), and a simple egg collection cage made of a soft drink bottle (right) (E) Shown are the materials needed for the cordless tube brush driver. The white-colored round brush that can be mounted to the drill driver is used to clean Petri dishes. Please click here to view a larger version of this figure.

Figure 2: Chilled flies on the cold surface of an icepack. The condensing water is absorbed by the medical gauze, and the chilled flies are kept dry. Please click here to view a larger version of this figure.
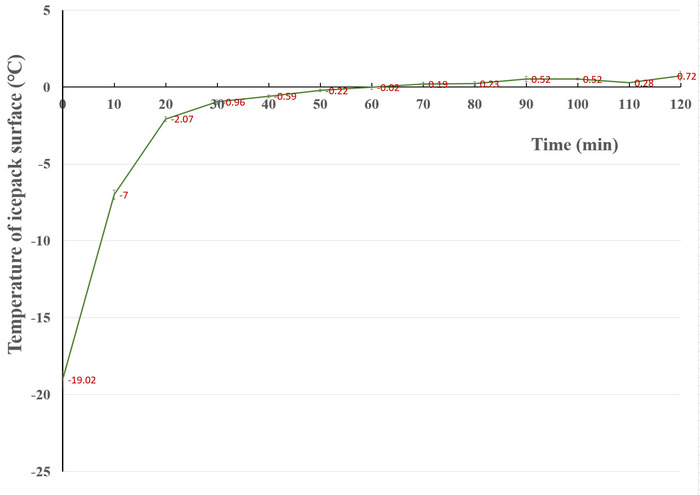
Figure 3: Variation of the temperature of the icepack surface with time. The data were collected from five hard icepacks, and the temperatures were measured in two locations in the center of an icepack with an infrared thermometer at a RT of 25 °C and relative humidity of 29%. The freezer temperature was -24.5 °C. Please click here to view a larger version of this figure.

Figure 4: Comparison of fly carcasses killed by microwaving to those killed by ethyl ether and deep freezing. When the fly carcasses killed by microwaving were examined under a stereomicroscope, no scorches or distortions were found on the bodies, and no noticeable differences were found in body color, eye color, and wing shape. Please click here to view a larger version of this figure.
| Killing agentb | Time used to kill flies | Weight (mg/30 flies) | Wing (mm or mm2)d | ||||||
| Female | Male | Female | Male | ||||||
| Areans | Lengthns | Widthns | Areans | Lengthns | Widthns | ||||
| Heat | 1 min 20 s | 36.60±0.00 a Ac | 22.65±0.95 a A | 1.51±0.16 | 2.30±0.12 | 0.92±0.05 | 1.20±0.09 | 2.06±0.08 | 0.83±0.03 |
| Chill | 15 min | 41.20±0.10 b B | 25.70±1.00 ab A | 1.57±0.15 | 2.37±0.12 | 0.94±0.05 | 1.23±0.12 | 2.07±0.10 | 0.84±0.05 |
| Ether | 10 min | 43.35±0.85 b B | 26.9±0.70 b A | 1.57±0.16 | 2.36±0.11 | 0.94±0.05 | 1.18±0.10 | 2.05±0.10 | 0.83±0.04 |
| a The adult Drosophila are wild type Drosophila melanogaster. They are captured in Beijing, China and kept in my lab for more than 5 years, and maintained at 25 °C in cornmeal medium. | |||||||||
| b The equipments used are heat: 1,300 W microwave oven; chill: fridge (-30 °C); ether: 2 mL ether, and the inner size of the etherizer is 170 mL. | |||||||||
| c Within each column, means followed by the same letter are not significantly different by the Duncan’s Multiple Range Test, lower/upper case letters indicate p = 0.05/0.01 | |||||||||
| d Flies are selected randomly from the same culture vial. Twenty right wings of the same gender were collected from the flies killed by the same agent and two replications were maintained. Digital photographs of each wings were taken and wing size was measured using ImagePro Plus software | |||||||||
| ns: Non-significant at p = 0.05 | |||||||||
Table 1: The effects of the three killing agents on the carcass weights and wing sizes of adult Drosophila.
Discussion
Some homemade tools for handling basic activities involved in Drosophila rearing and experimentation are described in this paper. These tools are simple but rather effective. Virtually, any lab can make these tools with ease, and a research or a teaching laboratory does not need to find a ready-made alternative that is perhaps not available locally.
Fly transferring is the most common practice and a difficult task in Drosophila experiments. Unfortunately, until now, there have been no described transferring tools3,4,12,17 Here, T- and F-stoppers are described. These simple tools make transferring the flies much easier and more controllable, and fewer flies escape during transfer, as evidenced by the fact that few stray flies have been found in the genetics classes in recent years. As the sponge plug is elastic, it does not require that the opening of the vials have the same inside diameter. Additionally, only one fly is allowed to pass through the opening of the pipette tip at a time; therefore, T-stoppers prevent flies from surging into a vial, and the experimenter can easily stop the process and control the number of flies transferred. T-stoppers can also prevent old food from dropping into a fresh vial. T- and F-stoppers are easy to make and use, and even an inexperienced handler can complete fly transfers quickly and easily.
F-stoppers are used to guide flies into a new vial. The adult flies tend to associate under the stopper and do not escape from the stem of the funnel. This makes some work easier and more controllable (e.g., transferring flies from one vial to another or adding extra virgin flies or male flies to a prepared cross). It has been found that when a vial is placed in the laboratory for a fairly long time (e.g., 1 h), only very few flies will escape from the stem of the funnel.
In this paper, a feasible chilling method for immobilizing flies is described. This method is a great alternative to ether and CO2 and can be used in both research and teaching labs. This method is particularly friendly to a teaching lab, as an instructor does not need to be as concerned about potential health risks to students or make great efforts to construct an expensive staging area in a crowded teaching lab. This method is cost-effective, as the icepacks are inexpensive and reusable. A researcher or student can chill and inspect flies anywhere, as this “cold pad” does not connect to any pipe. This method is not only safe for people but also to flies, as the system works at temperatures higher than -2 °C. Flies are lightly knocked out and remain immobile as long as they remain on the cold surface and are not killed. The flies regain consciousness quickly once they return to room temperature. Those who apply this method does not require a training period, and there are no concerns with excessive or inadequate anesthesia concentrations. However, experimenters should pay close attention to the size of the icepack, as small-sized icepacks (e.g., 400-500 mL, ca. 19 cm x 11 cm x 2.5 cm) are not desirable for fly chilling since they swell when they are frozen and it becomes awkward to work on the surfaces.
A bottle-shaped egg collection cage was also developed for the protocol. Taking advantage of the T- and F-stoppers, large amounts of flies to the cage can be added or transferred without requiring immobilization of the flies beforehand. It has been found that microwave heating is an efficient way to kill flies for inspection or discarding. A mechanical pencil-based microdissection needle and drill-based a cleaning tool were also utilized. All of these tools are simple and work well.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None
Materials
| a pair of pliers | |||
| cordless drill driver | max speed: 500 rpm | ||
| electric soldering iron | |||
| file | |||
| funnel | diameter of disk<60mm | ||
| ice box | |||
| insect pins | |||
| infrared thermometer | HCIYET HT-830 | ||
| long cuff rubber gloves | |||
| mechanical pencils | |||
| medical gauze | |||
| microcentrifuge tube | 100 ul | ||
| microwave oven | |||
| Parafilm | |||
| peri dish | internal diameter 60 mm | ||
| pipette tips | 1 ml | ||
| plastic film | |||
| plastic peri dish | Φ36 mm used to cover the empty vial | ||
| point tweezers | |||
| protective work gloves | |||
| re-freezable hard icepacks | 26.5×14.5×2.5 cm or larger | ||
| rubber air blower | |||
| snap cutter | |||
| soft drink bottle | 500 ml, internal diameter c.a. 65 mm | ||
| sponge stopper | |||
| stainless steel sponges | |||
| tube brush | |||
| vial | Φ34 mm × 90 mm |
References
- Jennings, B. H. Drosophila – a versatile model in biology & medicine. Materials Today. 14 (5), 190-195 (2011).
- JoVE Science Education Database. . Biology I: yeast, Drosophila and C. elegans. An Introduction to Drosophila melanogaster. , (2018).
- Ashburner, M., Roote, J., Sullivan, W., Ashburner, M., Hawley, R. S. Laboratory Culture of Drosophila. Drosophila Protocols. , (2000).
- Greenspan, R. J. Fly pushing: The theory and practice of Drosophila genetics. Cold Spring Harbor Laboratory Press. , (2004).
- Ashburner, M., Thompson, J., Ashburner, M., Wright, T. R. F. The laboratory culture of Drosophila. The genetics and biology of Drosophila. 2a, 1-109 (1978).
- Ratterman, D. M., O'Donnell, M. A. Eliminating ether by using ice for Drosophila labs. Tested Studies For Laboratory Teaching. , 259-265 (2003).
- . Culturing techniques for Drosophila Available from: https://www.ptbeach.com/cms/lib/NJ01000839/Centricity/Domain/113/ap%20biology%20Labs/Culturing%20techniques%20for%20Drosophila.pdf (2019)
- Markow, T. A., O'Grady, P. M. . Drosophila: A Guide to Species Identification and Use. , (2006).
- Artiss, T., Hughes, B. Taking the Headaches Out of Anesthetizing Drosophila: A Cheap & Easy Method of Constructing Carbon Dioxide Staging. The American Biology Teacher. 69 (8), e77-e80 (2007).
- Qu, W. -. H., Zhu, T. -. B., Yang, D. -. X. A Modified Cooling Method and its Application in Drosophila Experiments. Journal Of Biological Education. 49 (3), 302-308 (2015).
- . Egg-laying cages for drosophila Available from: https://www.kisker-biotech.com/frontoffice/product?produitId=0H-19-17 (2018)
- Roberts, D. B. . Drosophila: a practical approach. , (1998).
- Tang, M., Peng, Q. -. F., Yang, D. Two devices for Drosophila experiments (in Chinese). Bulletin of Biology. 45 (11), 49-50 (2010).
- Zhou, T. -. y., Gan, J., Yang, D. Preparation of sponge plug and sponge plug based fly transferring device for Drosophila experiments (in Chinese). Bulletin of Biology. 46 (6), 49-50 (2011).
- Yang, D. . Genetics laboratory investigation. , (2016).
- Yang, D. Carnivory in the larvae of Drosophila melanogaster and other Drosophila species. Scientific Reports. 8, (2018).
- Stocker, H., Gallant, P., Dahmann, C. Getting Started: An Overview on Raising and Handling Drosophila. Drosophila: Methods and Protocols. , (2008).

