Cultivation of the Marine Pelagic Tunicate Dolioletta gegenbauri (Uljanin 1884) for Experimental Studies
Summary
Doliolids, including the species Dolioletta gegenbauri, are small gelatinous marine zooplankton of ecological significance found on productive subcontinental shelf systems worldwide. The difficulty of culturing these delicate organisms limits their investigation. In this study, we describe cultivation approaches for collecting, rearing, and maintaining the doliolid Dolioletta gegenbauri.
Abstract
Gelatinous zooplanktons play a crucial role in ocean ecosystems. However, it is generally difficult to investigate their physiology, growth, fecundity, and trophic interactions primarily due to methodological challenges, including the ability to culture them. This is particularly true for the doliolid, Dolioletta gegenbauri. D. gegenbauri commonly occurs in productive subtropical continental shelf systems worldwide, often at bloom concentrations capable of consuming a large fraction of daily primary production. In this study, we describe cultivation approaches for collecting, rearing, and maintaining D. gegenbauri for the purpose of conducting laboratory-based studies. D. gegenbauri and other doliolid species can be captured live using obliquely towed conical 202 µm mesh plankton nets from a drifting ship. Cultures are most reliably established when water temperatures are below 21 °C and are started from immature gonozooids, maturing phorozooids, and large nurses. Cultures can be maintained in rounded culture vessels on a slowly rotating plankton wheel and sustained on a diet of cultured algae in natural seawater for many generations. In addition to the ability to establish laboratory cultures of D. gegenbauri, we demonstrate that the collection condition, algae concentration, temperature, and exposure to naturally conditioned seawater are all critical to the culture establishment, growth, survival, and reproduction of D. gegenbauri.
Introduction
Zooplankton account for the largest animal biomass in the ocean, are key components in marine food webs, and play important roles in ocean biogeochemical cycles1,2. Zooplankton, although comprised of a huge diversity of organisms, can be grossly distinguished into two categories: gelatinous and non-gelatinous with few intermediate taxa3,4. Compared to the non-gelatinous zooplankton, gelatinous zooplankton are especially difficult to study because of their complex life histories5, and their delicate tissues are easily damaged during capture and handling. Gelatinous zooplankton species are, therefore, notoriously difficult to culture in the laboratory and generally less studied compared to non-gelatinous species6.
Among gelatinous zooplankton groups, one abundant and of ecological importance in the world’s ocean are the Thaliaceans. Thaliaceans are a class of pelagic tunicates that include the orders Salpida, Pyrosomida, and Doliolida7. Doliolida, collectively referred to as doliolids, are small barrel-shaped free-swimming pelagic organisms that can reach high abundances in productive neritic regions of subtropical oceans. Doliolids are among the most abundant of all the zooplankton groups4,8. As suspension feeders, doliolids collect food particles from the water column by creating filter currents and capturing them on mucus nets9. Taxonomically, doliolids are classified in the phylum Urochordata10. Ancestral to the chordates, and in addition to their ecological significance as key components of marine pelagic systems, Thaliaceans are of significance to understanding the origins of colonial life history10,11 and the evolution of the chordates5,7,10,12,13,14.
The life history of doliolids is complex and contributes to the difficulty in culturing and sustaining them through their life cycle. A review of the doliolid life cycle and anatomy can be found in Godeaux et al.15. The doliolid life cycle, which involves an obligatory alternation between sexual and asexual life-history stages, is presented in Figure 1. Eggs and sperm are produced by the hermaphroditic gonozooids, the only solitary stage of the life cycle. Gonozooids release sperm to the water column and eggs are internally fertilized and released to develop into larvae. Larvae hatch and metamorphose into oozooids that can reach 1-2 mm. Presuming conducive environmental conditions and nutrition, oozooids become early nurses within 1-2 days at 20 °C and initiate the colonial stages of the life cycle. Oozooids asexually produce buds on their ventral stolon. These buds leave the stolon and migrate to the dorsal cadophore where they line up in three paired rows. The central double rows become phorozooids and the outer two double rows become trophozooids. The latter provide food to both the nurse and the phorozooids16,17. The trophozooids supply the nurse with nutrition as she loses all internal organs. As the abundance of trophozooids increases, the size of the nurse can reach 15 mm in the laboratory. As the phorozooids grow, they increasingly ingest planktonic prey and reach ~ 1.5 mm in size prior to being released as individuals17. A single nurse may release > 100 phorozooids during its lifespan18. After the phorozooids are released from the cadophore, they continue to grow and are the second colonial stage of the life cycle. Once they reach ~ 5 mm in size, each phorozooid develops a cluster of gonozooids on their ventral peduncle. These gonozooids can ingest particles when they reach ~1 mm in length. After the gonozooids have reached ~ 2 to 3 mm in size they are released from the phorozooid and become the only solitary stage of the life cycle. Once they reach ~ 6 mm in size, gonozooids become sexually mature17. Gonozooids can reach 9 mm or greater in length. Gonozooids are hermaphroditic, sperm is released intermittently while the fertilization of the eggs occurs internally16,17. When the gonozooid is ≥ 6 mm in size, it releases up to 6 fertilized eggs. Successful culturing requires supporting the specific needs of each of these unique life history stages.
Due to the ecological and evolutionary significance of Thaliaceans, including doliolids, there is a need for the cultivation methodologies to advance the understanding of this organism’s unique biology, physiology, ecology, and evolutionary history19. Doliolids have considerable promise as experimental model organisms in developmental biology and functional genomics because they are transparent and likely have streamlined genomes20,21. The lack of reliable cultivation methods, however, impedes their usefulness as laboratory models. Although a handful of laboratories have published results based on cultured doliolids, to our knowledge cultivation approaches and detailed protocols have not been previously published. Based on years of experience, and trial and error cultivation attempts, the purpose of this study was to review experiences and to share protocols for the collection and cultivation of doliolids, specifically the species Dolioletta gegenbauri.
Protocol
1. Preparing culturing facilities for rearing D. gegenbauri
NOTE: All materials and equipment required are listed in the Table of Materials.
- Prepare 1 M Sodium Hydroxide (NaOH), 0.06 M Potassium Permanganate (KMnO4) solution. To prepare this solution, dissolve 400 g of NaOH into 10 L deionized water. Add 100 g of KMnO4 to the NaOH solution and mix well.
- Prepare a 0.1 M Sodium bisulfite (NaHSO3) solution by dissolving 100 g of NaHSO3 into 10 L deionized water and mix well.
CAUTION: These reagents are irritants that may cause respiratory problems if inhaled. Place in a well-ventilated area such as a fume hood. Avoid any skin contact. Wear protective gloves, protective clothing, eye protection, and face protection when handling. - Before establishing and rearing doliolid cultures in the laboratory, clean and sterilize the culture jars.
- Rinse 1.9 L and 3.8 L culture jars at least 3 times with deionized water. Allow the screw caps to dry, as the caps are not included in the following cleaning steps.
- Clean and sterilize 1.9- and 3.8 L glass culture jars by immersing them in the NaOH/KMnO4 solution. Allow the jars to soak overnight.
- Remove the jars from the NaOH/KMnO4 solution and immerse the jars into the sodium bisulfite (NaHSO3) solution. Allow the jars to soak overnight.
- Remove the jars from the NaHSO3 solution and rinse thoroughly with deionized water. Allow the jars to dry.
- Place the plankton wheel (Figure 2) in a temperature-controlled space (environmental chamber). Equilibrate the temperature to 20 °C. For a more detailed description of the custom plankton wheel please refer to the Supplementary Figure 1.
2. Phytoplankton culture
- Obtain algal cultures from the National Center for Marine Algae and Microbiota (NCMA) or other sources to be used as food for D. gegenbauri. Mixtures of two flagellate species including Isochrysis galbana (CCMP 1323), Rhodomonas sp (CCMP 740), and a small diatom, Thalassiosira weissflogii (CCMP 1051) were obtained from the NCMA and have been used in previous laboratory studies to rear doliolids successfully17.
- Prepare L1 and L1-Si growth media22 as recommended by the NCMA.
- Follow the instructions provided by the supplier to initiate the new algal cultures.
- To maintain stock cultures, using rigorous axenic culture techniques, transfer 0.5 mL of old senescing culture to 25 mL of fresh growth media in sterile 55 mL glass culture tubes every two weeks.
NOTE: It is not possible to store living algal cultures without transferring them regularly. If cultures will not be used for long periods, and it is not possible to maintain cultures for the duration of the non-use period, it is recommended re-acquiring these common algal cultures from their original sources (e.g., NCMA). - Prepare larger volumes of phytoplankton for feeding doliolids in clean 500 mL plastic tissue culture flasks containing 200 mL of growth media.
- Inoculate phytoplankton from axenic stocks (4 mL) into 200 mL of growth media (1:50 dilution).
- Incubate at 20 °C with a 12:12 h light:dark cycle under cool white light illumination of 65-85 µE/m2. Lay culture flasks flat to maximize illumination. Gently swirl culture daily.
- Determine the concentration of cells using a particle counter or microscope to monitor the growth of the cultures.
NOTE: After 7-10 days from inoculation, the flagellate cultures will contain ~105-106 cells/mL and the diatom culture will contain ~104-105 cells/mL. These concentrations are enough to maintain the doliolid cultures. - Initiate new feeding stocks at a minimum of every two weeks to provide enough algal biomass for supporting all culture activities.
3. Collection of wild doliolids and seawater for culture
NOTE: An overview of collection and cultivation approaches is outlined in Figure 3. A description of the specialized collection plankton net and cod-end is provided in Figure 4.
- Locate doliolids by detecting them using either plankton nets or in situ imaging systems23.
NOTE: Because doliolids are rarely present in surface waters and are not detectable by remote sensing technology, guided by prerequisite knowledge of conditions favorable to doliolids (see Discussion), the presence of doliolids must be determined prior to sampling. - Collect particle-rich seawater prior to collecting live doliolids in preparation for initiating a D. gegenbauri culture.
- Deploy Niskin bottles mounted on a CTD rosette or equivalent equipment to collect water from the site where doliolids are located and from the depth containing the highest estimates of chlorophyll a concentration estimated by in situ fluorometry.
NOTE: Chlorophyll a concentration is used as an indicator of particle concentrations. On the South Atlantic Bight (SAB) mid-continental shelf, the subsurface chlorophyll a maximum is usually close to the bottom, but in other locations, it may not be.
- Deploy Niskin bottles mounted on a CTD rosette or equivalent equipment to collect water from the site where doliolids are located and from the depth containing the highest estimates of chlorophyll a concentration estimated by in situ fluorometry.
- Once doliolids are located, recover undamaged doliolid zooids using the specialized plankton net and cod-end. Before deploying the net, fill the cod-end with seawater.
- From a drifting ship, lower and raise the net through the water column maintaining an oblique towing angle of ~15 – 25° and vertical deployment and retrieval speed not greater than 15 m/min.
- Once the net is onboard, gently transfer and divide the contents of the cod-end into 3, 5-gallon (~20 L) plastic buckets each containing ~ 10 L of surface seawater collected from the site.
NOTE: New plastic buckets should be conditioned by the addition of seawater days before living doliolid collection. The aim is to reduce the leaching of chemicals from plastic. If seawater is not available, use purified (e.g., Milli Q) or tap water free of toxic contaminants to condition the buckets. - Isolate doliolid zooids from other plankton.
- In small batches (~ 2 L) transfer mixed planktons from the net tow contents (now in 20 L plastic buckets) to a 2 L glass beaker.
- Using a wide-bore glass pipette (8 mm ID x 38 cm length), carefully siphon and transfer actively swimming doliolid zooids from the beaker into clean glass culture jars containing particle-rich seawater collected using Niskin bottles from where doliolids were located.
- Gently release the doliolid zooids beneath the surface of the seawater.
NOTE: Collect gonozooids, phorozooids containing attached developing gonozooids, and nurse stages containing attached trophozooids (Figure 1).
- After the addition of doliolids, add Rhodomonas sp. culture to a final concentration of ~ 5 x 103 – 104 cells/mL (~50 mL of a culture containing ~ 5 x 104 – 1 x 105 cells/mL in a 3.8 L jar). This is to determine if the doliolids are actively feeding. When doliolids ingest Rhodomonas sp., their digestive tract will appear red in color. Remove zooids that do not appear to be feeding.
- To prevent doliolids from being trapped at the air-water interface, avoid the headspace in the culture jars by completely filling the jars with unfiltered particle-rich seawater and placing a piece of plastic wrap over the jar opening (89 mm wide).
- Avoid creating air bubbles that can also damage the animals. Carefully screw the cap onto the jar and gently invert the jar to determine if bubbles are present. If bubbles are present, remove them.
- After jars are filled, wipe the excess water from the outside of the jar.
- Mount each jar onto the plankton wheel (Figure 2) by placing the jar on the vertical metal bars covered with rubber tubing, and between a stainless-steel hose clamp.
- Ensure that the back of the jar is cushioned against the rubber tubing. Tighten the hose clamp around the jar by adjusting the screw.
- Check that the jar is not moving once it is securely fastened in place. Allow the jars to rotate at 0.3 rpm to keep the doliolids in suspension.
CAUTION: It is important not to overtighten the jar to prevent the jar from cracking.
- On the ship, maintain the culture vessels on the plankton wheel at 20 °C in dim light until they can be transferred to the laboratory culture facility.
- Upon returning to the laboratory, transfer the jars containing doliolids into the prepared culture facility. Mount jars on the plankton wheel (see step 3.8) and allow the jars to continue to rotate at 0.3 rpm.
NOTE: All rearing of doliolids in this study was conducted at 20 °C.
4. Maintaining D. gegenbauri cultures
- From the ship to the lab, allow the animals to acclimate in the original jars to the laboratory conditions for 3 days.
- During the acclimation period, use a wide bore glass pipette to exchange 10% of the water with unfiltered particle-rich seawater from the collection site every day for 3 days.
- Keep several copepods in the jar but remove all other zooplankton, large fecal pellets, and large aggregated particles that may clog the doliolid’s filtering apparatus (mucus net). If the culture consists of early nurses, keep one large gonozooid (≥ 6 mm) in the jar.
NOTE: It is not important which copepod species are included in the culture, but in this experiment, the most abundant species present from where the doliolids were captured was used.
- Following the acclimation period, transfer doliolid zooids and copepods from the original jar to a clean cultivation jar containing 80% glass fiber filter (GF/F) filtered seawater and 20% of the seawater from the original jar. Prepare filtered seawater by filtering seawater through a GF/F with a nominal pore size of 0.7 µm filter paper.
- Maintain the new culture by exchanging 10% of the water with GF/F filtered seawater every 3 days and by removing aggregates and fecal pellets. Weekly, transfer animals to a new jar as described in step 4.2.
- Feed doliolids by maintaining phytoplankton concentrations in the culture jars between 40 – 95 µg C/L.
NOTE: These concentrations mimic environmental conditions that are known to support bloom conditions for D. gegenbauri17. The mixture of algal species varies depending on the life stage and the number of zooids in each jar. During early life stages, add 1:1 mixture (by carbon content) of the cryptomonad algae (Isochrysis galbana and Rhodomonas sp.) only. Larger prey species can easily clog the feeding apparatus of small nurses and developing trophozooids. Add the diatom Thalassiosira weissflogii to the algal mixture, also at equal carbon content, when feeding larger nurses, phorozooids, and gonozooids.- Monitor algal concentrations pre- and post-feeding to guide the decision of how frequently and how much algae to add to the cultures. Use a particle counter to determine algal concentrations, because algal concentrations in the culture jars are relatively dilute.
- Remove enough zooids to maintain algal concentrations of 40 – 95 µgC/L so that the remaining doliolids will have enough food to grow.
NOTE: The most difficult life stage to maintain successfully under laboratory conditions is the developing larvae and oozooid (early nurse). During this phase of the culture, keep one large gonozooid (≥ 6 mm) in addition to several copepods in the jar with developing larvae and oozooids (~ 20 per 3.8 L jar). - Transfer at least 4 nurses into to a new culturing jar once a minimum of 8 trophozooids are visible on the nurse’s cadophore (Figure 1B).
NOTE: Trophozooids will double in number every 1 – 2 days at 20 °C. Trophozooids are large enough to be visible to the naked eye.- Remove two of the nurses once nurses develop 20 trophozooids.
- Remove one nurse when the nurses develop > 30 trophozooids on their cadophores. Allow the remaining nurse to develop phorozooids on its cadophore.
- Remove the nurse once the nurse releases up to 30 phorozooids.
- Reduce the number of animals in the jar once the phorozooids reach 3 mm in size.
- Remove all but four phorozooids when the phorozooids become larger (> 5 mm) and have developed gonozooid clusters.
- Reduce the culture to two phorozooids when the number of gonozooids clusters increase in size and begin to feed.
- Remove the phorozooids once the phorozooids release up to 30 gonozooids.
- Reduce the number of gonozooids from 30 zooids to 2 per jar. Allow fertilized eggs to be released into the jar.
- Remove one gonozooid leaving a single gonozooid in the jar once the oozooids develop.
NOTE: Discarded nurses, phorozooids, and gonozooids can be used to seed additional cultures and to conduct further experiments.
- Remove one gonozooid leaving a single gonozooid in the jar once the oozooids develop.
Representative Results
Following the described procedures for collecting and culturing the doliolid, D. gegenbauri outlined in Figure 3, it is possible to maintain a culture of D. gegenbauri throughout its complex life history (Figure 1) and sustain it for many generations. Although cultivation of D. gegenbauri is described here, these procedures should also be relevant for the cultivation of other doliolid species.
Capturing healthy and undamaged doliolid zooids requires the application of specialized nets and towing procedures (Figure 4). As delicate animals with no hard structures, care should be taken to minimize procedures that may result in any physical damage. These factors can include turbulence, pressure, and interactions with surfaces including the net, air and air bubbles. Despite their delicate nature, however, undamaged doliolid zooids can be collected using a conical plankton net with an opening diameter to length ratio of 1:5 and equipped with a relatively large weighted non-filtering cod-end. Routinely we have used a 202 µm mesh 2.5 m (length) plankton net with a 0.5 m opening mounted in a swivel harness and equipped with a 4 L weighted non-filtering cod-end (Figure 4). Although the effect of plankton mesh size on the capture of cultivatable D. gegenbauri zooids has not been systematically investigated, theoretically, the use of a net with a larger mesh size may result in further improvement as larger mesh size would reduce the pressure field generated during towing. Alternatively, greater mesh size will result in greater water flow through the net, potentially damaging doliolid zooids. Towing speeds and net angle should be optimized to minimize tow time and damage during collection. In our experience, we have found that sufficiently gentle towing conditions can be achieved by towing the net obliquely at an angle of 15-25° from a drifting ship with vertical deployment and retrieval speeds not greater than 15 m/min. To orient the net to the direction of water flow, the plankton net is mounted in a swivel harness. It is usually the case that the distribution of doliolids in the water column is not random and generally greatest in the region with the highest particulate loads24. Therefore, the water column from below the subsurface chlorophyll maximum to the surface should be sampled. In the shallow SAB mid-continental shelf (20 – 45 m), the water column from ~ 1 m above the bottom to the surface is sampled.
Once healthy zooids have been collected, it is critical to maintaining them in a manner that minimizes exposure to surfaces. To minimize encounters with surfaces doliolids are kept in rounded jars filled with seawater and gently tumbled on a slowly rotating plankton wheel (Figure 2).
Although it is theoretically possible to start a culture with zooids of any life stage, exploration of successes and failures at establishing new cultures of D. gegenbauri from 6 attempts between 2015 – 2018 in the South Atlantic Bight suggest that success is most often achieved when zooids are collected from waters that are < 21°C, and when life stages other than large mature gonozooids are utilized to start a new culture (Table 1 and Table 2). In practice, it is helpful, or at least not detrimental, to include multiple life stages of doliolid zooids when initiating a new culture.
Success in sustaining a culture of D. gegenbauri, as has been described for other pelagic tunicate species20, depends on providing sufficient, but not excessive, food and food diversity required to support each life stage. As diet requirements vary throughout the life cycle, the amount of algae provided at each feeding time must be varied to maintain food concentrations at the desired target levels (40 – 95 µg C/L) (Table 3). Concentrations above or below these levels can result in increased mortality rates (G.A. Paffenhöfer pers. comm.). Although the natural diet of D. gegenbauri remains poorly understood6, cultures can be maintained by supplying relatively simple mixtures of cultured algae and utilizing procedures that allow diverse microbial communities to establish in the culture. Increasing the potential diversity of the prey field is achieved by retaining a fraction of particle laden-water from older cultures and the inclusion of a small number of living copepods and large doliolids at each water change or transfer. Presumably, these organisms process algae and detrital material and serve to diversify the particle size and quality spectrum available for doliolid nutrition, but additional studies are required to confirm this hypothesis.
The availability of doliolid cultures provides the means to investigate, under controlled experimental conditions, many important aspects of doliolid biology, physiology, ecology, and molecular biology. For example, although doliolids are abundant in numerous regions of the coastal ocean and are major planktonic grazers25, data on rates of feeding and growth remain scarce26. Utilizing cultures of D. gegenbauri, a focus of culture-based research has been to quantify feeding and growth rates in response to critical environmental parameters including temperature and food concentrations26. Results from these studies have indicated that clearance rates are similar at concentrations from 20 to 60 μg C/L and decrease as the food concentrations increase (Figure 5A). Clearance rates increase proportionally over temperature ranges supportive of D. gegenbauri growth (Figure 5B). Growth rates (k) range from 0.1 – 0.7/day as a function of temperature and food availability (Figure 6). These studies, in addition to providing practical information for culturing, have allowed the determination of quantitative relationships between doliolid feeding and growth rates as a function of environmental parameters and provide critical insights into the biology and ecology of doliolids required for including this important zooplankton group into modeling frameworks27.
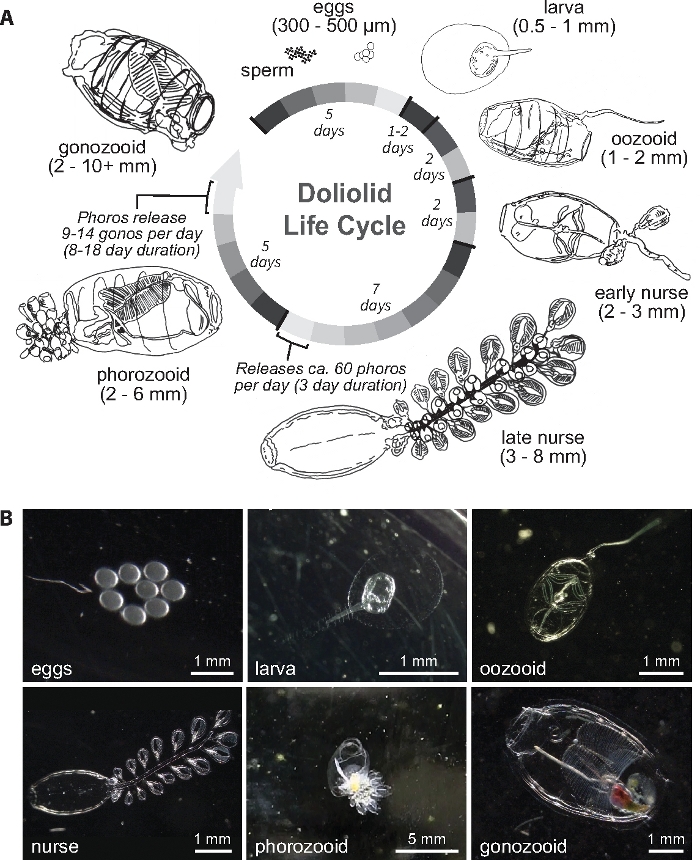
Figure 1: The Life cycle of D. gegenbauri at 20 °C.
The life cycle drawing (1A) has been modified after Walters et al. 20186 and re-drawn with permission. Please click here to view a larger version of this figure.
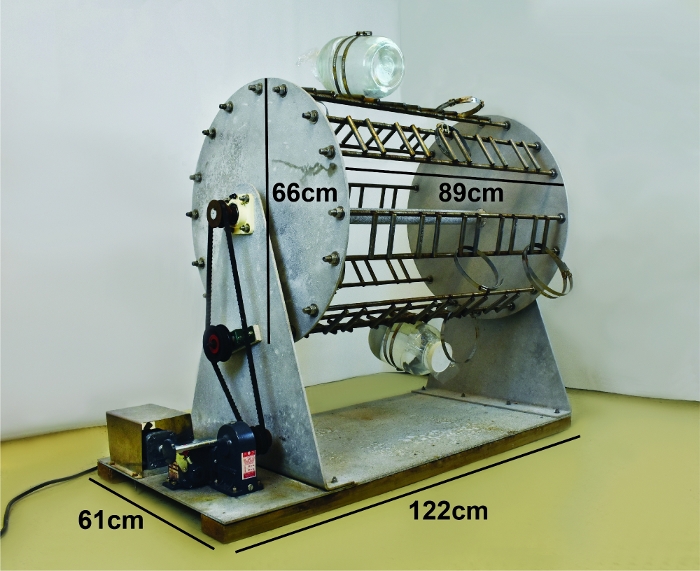
Figure 2: Plankton wheel used to culture D. gegenbauri. Please click here to view a larger version of this figure.
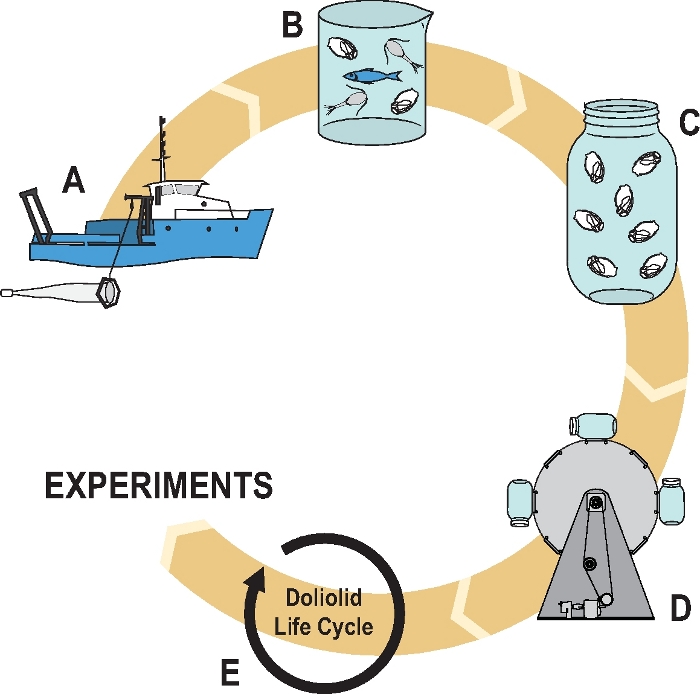
Figure 3: Schematic overview of D. gegenbauri collection and cultivation approach.
Collection at sea (A), transfer from concentrated buckets to small glass beaker in small batches (B), isolation of doliolid zooids into cultivation jars containing particle-rich seawater (C), maintenance on the plankton wheel throughout the life cycle (D,E). Please click here to view a larger version of this figure.
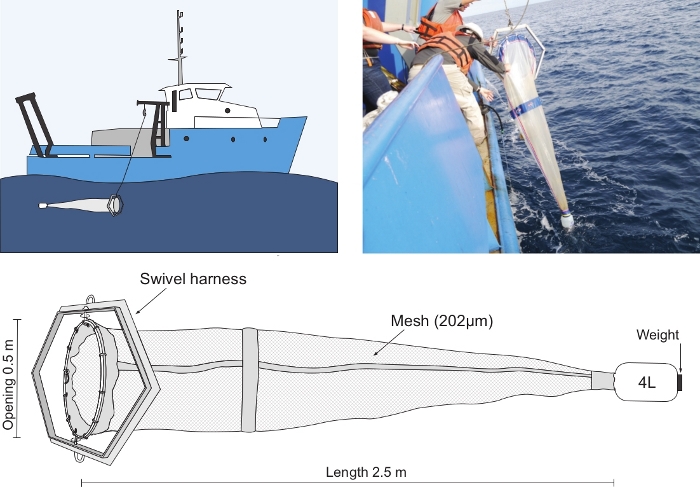
Figure 4: Plankton net and deployment.
Deployment (top left), retrieval (top right), and schematic of net and cod end (bottom). Please click here to view a larger version of this figure.
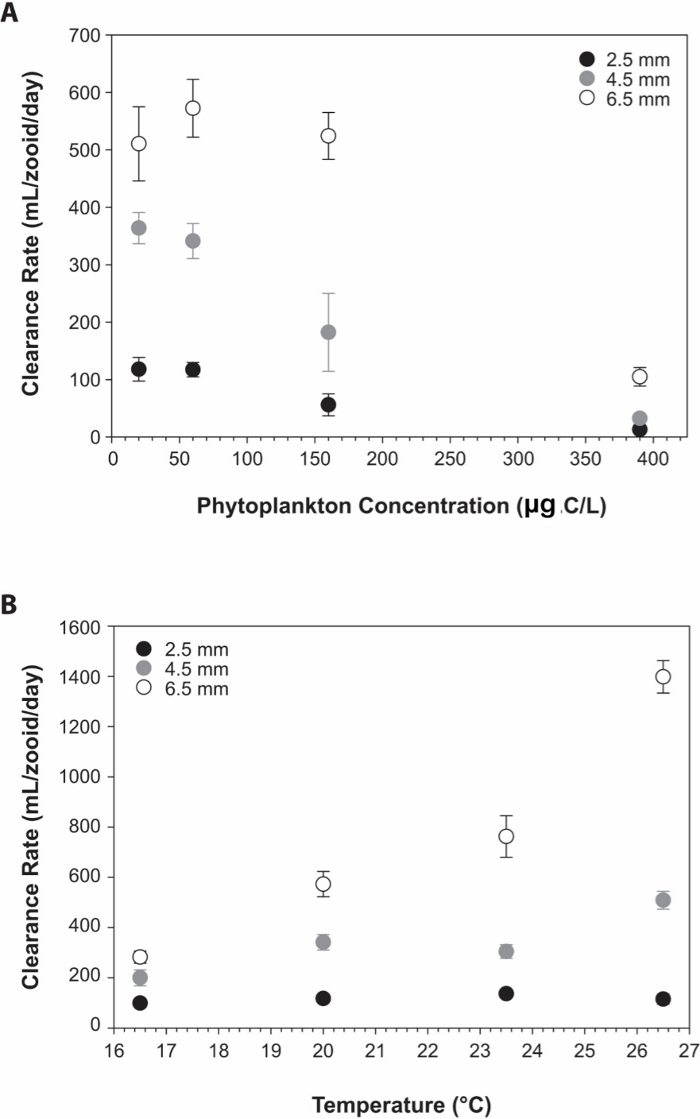
Figure 5: Algal clearance rates of D. gegenbauri gonozooids.
(A) relationship between (A) Mean (± S.E.) clearance rates (mL/zooid/day) versus phytoplankton concentration (µg C/L) for three sizes of D. gegenbauri gonozooids. Each point represents 4–11 observations. (B) Mean (± S.E.) clearance rates (mL/zooid/day) versus temperature (°C) for three sizes of D. gegenbauri gonozooids. Each point represents 4–12 observations. Gonozooids sizes are 2.5 mm ( ), 4.5 mm (
), 4.5 mm ( ), and 6.5 mm (
), and 6.5 mm ( ). Figures have been re-drawn with permission26. Please click here to view a larger version of this figure.
). Figures have been re-drawn with permission26. Please click here to view a larger version of this figure.
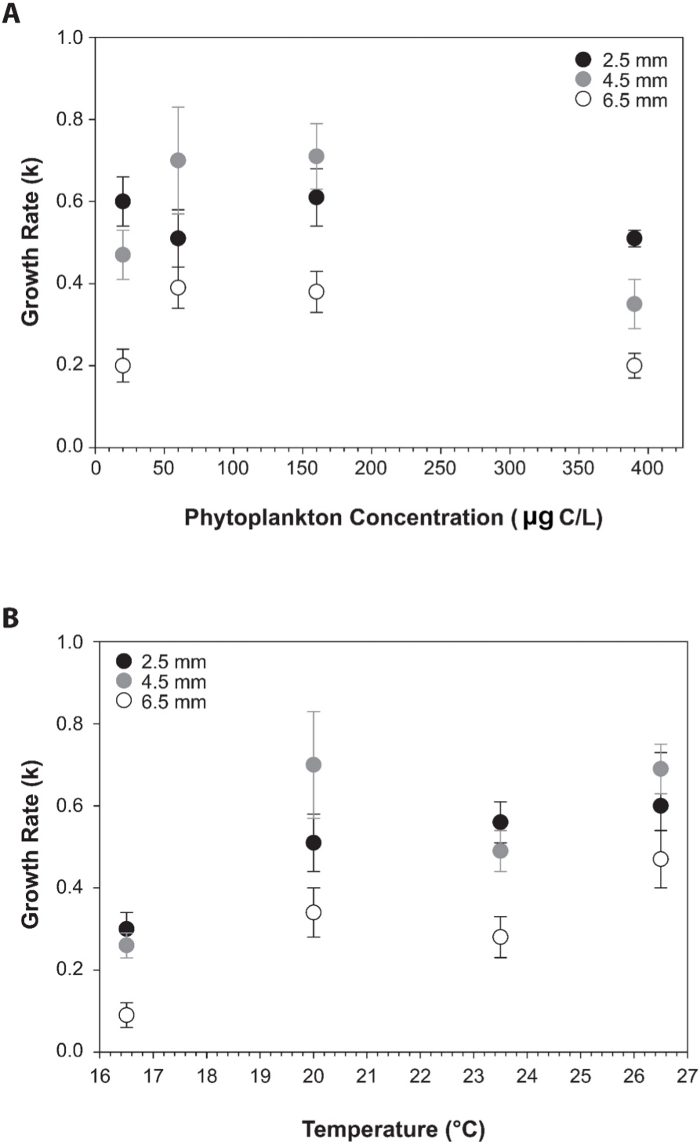
Figure 6: Growth rates of D. gegenbauri gonozooid.
Relationship between (A) Mean (± S.E.) growth rates (k) versus phytoplankton concentration (μg C/L) for three sizes of Dolioletta gegenbauri gonozooids. Each point represents 4–11 observations. (B) Mean (± S.E.) growth rates (k) versus temperature (°C) for three sizes of Dolioletta gegenbauri gonozooids. Each point represents 4–12 observations. Gonozooids sizes are 2.5 mm ( ), 4.5 mm (
), 4.5 mm ( ), and 6.5 mm (
), and 6.5 mm ( ). Figures have been re-drawn with permission from Gibson and Paffenhöfer26. Please click here to view a larger version of this figure.
). Figures have been re-drawn with permission from Gibson and Paffenhöfer26. Please click here to view a larger version of this figure.
| Table 1. Oceanographic Conditions and Doliolid Abundance | |||||||||||
| Surface | Bottom | Surface | Bottom | Surface | Bottom | Doliolid Abundance | |||||
| Date | Cruise ID | Latitude (N) | Longitude (W) | Depth (m) | Temperature (⁰C) | Temperature (⁰C) | Salinity (PSU) | Salinity (PSU) | Chla (µg/L) | Chla (µg/L) | zooids/m3 |
| 20/05/2015 | SAV-15-10 | 31.1889 | 80.1527 | 41.30 | 25.26 | 22.43 | 33.58 | 36.96 | NA | 0.20 | NA |
| 04/08/2015 | SAV-15-19 | 29.5687 | 80.3269 | 40.00 | 26.40 | 21.75 | 36.26 | 36.32 | 1.04 | 1.35 | 218 |
| 02/12/2015 | SAV-15-31 | 31.1674 | 80.1249 | 40.80 | 23.24 | 22.60 | 35.91 | 35.81 | 1.06 | 1.70 | 13 |
| 02/02/2017 | SAV-17-03 | 31.2139 | 80.1823 | 41.00 | 18.72 | 18.84 | 36.00 | 36.12 | 0.83 | 1.50 | 3 |
| 07/11/2017 | SAV-17-23 | 31.2144 | 80.1822 | 42.00 | 24.19 | 23.85 | 36.00 | 36.04 | 0.63 | 1.30 | 254 |
| 01/02/2018 | SAV-18-02 | 31.1835 | 80.1466 | 43.00 | 16.85 | 16.45 | 36.50 | 36.48 | 0.56 | 0.89 | NA |
| NA: data Not Available | |||||||||||
Table 1: Oceanographic conditions and doliolid abundance on the South Atlantic Bight mid-continental shelf at the time and location where D. gegenbauri zooids were collected and used to initiate new cultures.
| Table 2. Outcomes of D. gegenbauri Culturing Attempts | ||||
| Date | Cruise ID | Zooids Collected | Outcome | Comments |
| 20/05/2015 | SAV-15-10 | Sexually mature large (6-7 mm) gonozooids | Failed | All gonozooids had died after 4 days. Oozooid and early nurse life stages were produced but failed to thrive. |
| 04/08/2015 | SAV-15-19 | Sexually mature large (8-10 mm) gonozooids | Failed | Gonozooids died shortly after collection. Oozooids and early nurses were produced but failed to thrive. |
| 02/12/2015 | SAV-15-31 | Mixed collection including late nurse (4-5 mm) with attached trophozooids, sexually mature large (6 mm) gonozooids, and oozooids (2 mm) | Successful | Cultured for 4 full generations, additional gonozooids and nurses collected in January and March 2016 were added to the culture. Laboratory was evacuated for 4 days during Hurricane Matthew in October 2016 and the culture did not survive. |
| 02/02/2017 | SAV-17-03 | Mixed collection including gonozooids (1.5-5 mm) and large phorozooids (6 mm) with attached gonozooid clusters | Successful | Cultured for 4 full generations, additional gonozooids collected in April 2017 were added to the culture. Terminated culture in September 2017 in advance of Hurricane Irma. |
| 07/11/2017 | SAV-17-23 | Gonozooids (3-6 mm) | Failed | Large gonozooid died after 1 day. The immature gonozooid survived in culture for 14 days. Eggs were released by both gonozooids. Oozooids were produced but failed to develop into nurse stages. Culture failed after 1 month. |
| 01/02/2018 | SAV-18-02 | Large (6-7 mm) late nurse without trophozooids | Successful | In culture the nurse produced trophozooids. Culture was maintained for 3 generations and was terminated at the end of June 2018 when experiments were concluded. |
Table 2: Outcome of attempts to establish laboratory cultures of D. gegenbauri collected from the South Atlantic Bight mid-continental shelf.
| Dolioletta gegenbauri | zooid number per | zooid number per | |||
| life stage | 3.9 L jar | 1.9 L jar | Isochrysis galbana | Rhodomonas sp. | Thalassiosira weissflogii |
| oozooid | 20 | 10 | INCLUDE | INCLUDE | DO NOT INCLUDE |
| early nurse | 20 | 10 | INCLUDE | INCLUDE | DO NOT INCLUDE |
| late nurse with 8 trophozooids | 4 | 2 | INCLUDE | INCLUDE | INCLUDE |
| late nurse with 20 trophozooids | 2 | 1 | INCLUDE | INCLUDE | INCLUDE |
| late nurse with 30 trophozooids | 1 | 1 | INCLUDE | INCLUDE | INCLUDE |
| phorozooid (1 to 3 mm) | 30 | 15 | INCLUDE | INCLUDE | INCLUDE |
| phorozooid gonozooid cluster (> 5 mm) | 2 | 1 | INCLUDE | INCLUDE | INCLUDE |
| gonozooid (1 to 3 mm) | 30 | 15 | INCLUDE | INCLUDE | INCLUDE |
| gonozooid (> 5 mm) | 2 | 1 | INCLUDE | INCLUDE | INCLUDE |
| Target concentrations of algae should be maintained between 40 – 95 µg C/L with equal mixtures (by carbon content) of each algal species | |||||
Table 3: Target culture conditions for each D. gegenbauri life cycle phase.
Supplementary Figure 1: Detailed description of the custom plankton wheel. Please click here to download this figure.
Discussion
The capacity to culture doliolids has been established over the past several decades and has been used to support research in several areas. Experimental studies in our laboratories have supported the publication of at least 15 scientific studies focused on the feeding and growth18,26, reproduction18,28, diet6,29, physiology30, ecology31, and ecological modelling27 of doliolids.
Although the culture of these delicate animals is currently labor-intensive and time consuming, the cultivation of doliolids is feasible and, if undertaken by the broader community will foster the advancement of the understanding of this ecologically and evolutionarily important group of animals. The objective of this study was to describe current approaches for collecting, rearing, and maintaining D. gegenbauri in culture for the purpose of conducting laboratory-based studies.
The establishment of a doliolid culture requires the collection of healthy and undamaged animals and, once captured, gentle treatment, appropriate nutrition, and husbandry. Doliolids, specifically the species D. gegenbauri, occurs circumglobally on subtropical continental shelves but abundance can be highly variable. For example, in a recent study focused on the mid-shelf region of the SAB, although the abundance varied dramatically from <1/m3 to > 20,000/m3, doliolids were present throughout the year6. Because of the high variability of doliolids in space and time and the relative difficulty involved with sampling continental shelf margin environments, reliable knowledge of doliolid community dynamics where studies are being conducted is an important prerequisite to the successful establishment of the culture.
Once doliolid zooids have been located and captured, it can be difficult to determine whether the animals have been damaged. Animals may appear to be undamaged and exhibit active swimming and escape behaviors, but even the smallest injury can result in their failure to thrive. One characteristic that is especially pertinent to the health assessment of captured doliolid zooids is their ability to feed. Feeding activity can be assessed simply by providing pigmented algae to freshly captured animals. If an animal is feeding, the gut will become colored within a short period of time. In our experience, we have found that adding a small amount of the red pigmented algae, Rhodomonas sp., quickly provides information about feeding activity. If feeding is not observed, it is highly unlikely that a culture can be established.
Vigilance and good husbandry are critical for establishing and sustaining doliolids throughout their complex life cycle. Perhaps the most problematic stage is the development of a viable nurse from the larval stage and the production (sprouting) of the feeding trophoozoids. At this life stage, we speculate that the food requirements, with respect to quantity, quality, and particle size is most limited. To our knowledge, there have been no previous studies that have investigated the feeding activity of D. gegenbauri larvae and oozooids. For example, although the developing gonozooids and phorozooids are able to ingest particles over a wide range of sizes, the capacity of larvae, oozooids, and small nurses is likely more limited. In practice, we find that successful cultivation at these life stages can be achieved by omitting diatoms from the algal food mixture, by maintaining food concentrations at moderate levels, by conducting frequent feedings at lower concentrations, by maintaining a single larger gonozooid and a few copepods with the culture, and by manually removing large aggregates of detritus.
Although we have maintained cultures of D. gegenbauri for multiple generations originating from a single collection, when possible we routinely supplement existing cultures with freshly collected animals to increase genetic diversity and robustness of the culture. A potential danger of this practice is the introduction of parasites or diseases into the culture, but to our knowledge, we have never encountered this problem. Although there have been few reports of parasites of doliolids32, undoubtedly, they do exist. Interestingly, in a recent study comparing the diet of cultured D. gegenbauri gonozooids exposed to natural waters with field-caught D. gegenbauri gonozooids, presumptive Apicomplexa parasites were detected in the wild population that were absent in the cultured animals6.
An existing limitation of the described culture technology is the limitation of zooid production volume. Particularly because the described techniques involve cultivation in sealed jars at low densities on a rotating plankton wheel, it is unclear if this approach could be scaled-up or that the work flow would be amenable to automation. Larger scale cultivation systems, however, for another delicate small gelatinous marine zooplankton species, the larvacean Oikopleura dioica, have been described20,33,34, suggesting that it may be possible to design similar systems for doliolids in the future. However, the complex life history of D. gegenbauri compared to the simpler life history of O. dioica will remain a significant challenge to large scale cultivation.
In conclusion, following the protocols described here, D. gegenbauri can be reliably cultivated under controlled laboratory conditions throughout its complex life history. This capacity makes the species amenable to a variety of controlled experimental studies, and perhaps to develop doliolids as a new animal model in developmental biology and evolution. Limitations of production scale, however, will need to be overcome before this goal can be achieved.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are grateful to the many persons who have contributed accumulated knowledge to this project over the years including G.-A. Paffenhöfer and D. Deibel who originally developed these protocols. M. Köster, and L. Lamboley have also contributed significantly to the development of these procedures. N.B. López-Figueroa and Á.E. Rodríguez-Santiago generated the estimates of doliolid abundance provided in Table 1. This study was supported in part by the US National Science Foundation awards OCE 082599, 1031263 to MEF, collaborative projects OCE 1459293 and OCE 14595010 to MEF and DMG and, the National Oceanic and Atmospheric Administration award NA16SEC4810007 to DMG. We are grateful to the hardworking and professional crew of the R/V Savannah. Lee Ann DeLeo prepared the figures, Charles Y. Robertson proofread the manuscript and, James (Jimmy) Williams manufactured the plankton wheel
Materials
| Algal culture tubes (55 mL sterile disposable glass culture tubes) | Any | NA | For algal cultures |
| Autoclave | Any | NA | For sterilizing equipment and seawater for algal cultures |
| Beakers (2 L glass) | Any | NA | For sorting diluted plankton net tow contents |
| Buckets (5 gallon, ~20L) | Any | NA | For diluting contents of planton net tow – should be seawater conditioned before first use |
| Carboys (20 L) | Any | NA | For storing seawater |
| Doliolid glass culturing jar (1.9 L narrow mouth glass jar with cap) | Qorpak | GLC-01882 | Container for culture |
| Doliolid glass culturing jar (3.8 L narrow mouth glass jar with cap) | Qorpak | GLC-01858 | Container for culture |
| Environmental Chamber (Temperature controlled enviromental chamber) | Any | NA | To accommodate plankton wheel and culture maintenance |
| Filtration apparatus for 47 mm filters | Any | NA | For filtering seawater for cultures |
| Glass microfiber filters, 47 mm | Whatman | 1825-047 | For filtering seawater for cultures |
| Glass pipette (borosillicate glass pipette (glass tubing), OD 10mm, ID 8 mm, wall thickness 1mm) | Science Company | NC-10894 | Custom cut and edges polished |
| Hose clamps, stainless steel, #104 (178 mm) | Any | NA | For holding culturing jars to the plankton wheel |
| Isochrysis galbana strain CCMP1323 | National Center for Marine Algae and Microbiota (NCMA) | strain CCMP1323 | For feeding doliolid cultures |
| L1 Media Kit, 50 L | National Center for Marine Algae and Microbiota (NCMA) | MKL150L | For culturing algae |
| Lamp (Fluorescent table lamp with an adjustable arm) | Any | NA | For illuminating doliolids in the jars and beakers |
| Lighted temperature controlled incubator | Any | NA | For algal cultures |
| Micropipettes and sterile tips (0-20 µl, 20-200 µl, 200-1000 µl) | Any | NA | For algal cultures |
| Plankton Net (202 µm 0.5 m, 5:1 length) with cod end ring and 4 L aquarium cod-end | Sea-Gear Corporation | 90-50×5-200-4A/BB | For collecting living doliolids (see Figure 4) |
| Plankton Wheel | NA | NA | Custom built (see Figure 2) |
| Plastic wrap | Any | NA | To cover inside of lid of doliolid culture jars |
| Potassium Permanganate | Fisher Scientific | P279-500 | Reagent for cleaning jars and glassware |
| Rhodomonas sp. strain CCMP740 | National Center for Marine Algae and Microbiota (NCMA) | strain CCMP740 | For feeding doliolid cultures |
| Rubber Tubing | NA | NA | For holding culturing jars to the plankton wheel (can be made from tygon tubing) |
| Sodium Bisulfite | Fisher Scientific | S654-500 | Reagent for cleaning jars and glassware |
| Sodium Hydroxide | Fisher Scientific | BP359-212 | Reagent for cleaning jars and glassware |
| Sterile serological pipettes (1 mL, 5 mL, 10 mL, 25 mL) | Any | NA | For algal cultures |
| Thalassiosira weissflogii strain CCMP1051 | National Center for Marine Algae and Microbiota (NCMA) | strain CCMP1051 | For feeding doliolid cultures |
| Tissue culture flasks (250 mL) | Any | NA | For algal cultures |
References
- Banse, K. Zooplankton – Pivotal role in the control of ocean production. Ices Journal of Marine Science. 52 (3-4), 265-277 (1995).
- Wilson, S. E., Ruhl, H. A., Smith, K. L. Zooplankton fecal pellet flux in the abyssal northeast Pacific: A 15 year time-series study. Limnology and Oceanography. 58 (3), 881-892 (2013).
- Bode, A., Álvarez-Ossorio, M. T., Miranda, A., Ruiz-Villarreal, M. Shifts between gelatinous and crustacean plankton in a coastal upwelling region. Ices Journal of Marine Science. 70 (5), 934-942 (2013).
- Kiorboe, T. Zooplankton body composition. Limnology and Oceanography. 58 (5), 1843-1850 (2013).
- Holland, L. Z. Tunicates. Current Biology. 26 (4), R146-R152 (2016).
- Walters, T. L., et al. Diet and trophic interactions of a circumglobally significant gelatinous marine zooplankter, Dolioletta gegenbauri (Uljanin, 1884). Molecular Ecology. , (2018).
- Piette, J., Lemaire, P. Thaliaceans, the neglected pelagic relatives of ascidians: a developmental and evolutionay enigma. Quarterly Review of Biology. 90 (2), 117-145 (2015).
- Acuña, J. L. Pelagic tunicates: Why gelatinous?. American Naturalist. 158 (1), 100-107 (2001).
- Alldredge, A. L., Madin, L. P. Pelagic tunicates – unique herivores in the marine plankton. Bioscience. 32 (8), 655-663 (1982).
- Wada, H. Evolutionary history of free-swimming and sessile lifestyles in urochordates as deduced from 18S rDNA molecular phylogeny. Molecular Biology and Evolution. 15 (9), 1189-1194 (1998).
- Zeng, L. Y., Jacobs, M. W., Swalla, B. J. Coloniality has evolved once in stolidobranch ascidians. Integrative and Comparative Biology. 46 (3), 255-268 (2006).
- Delsuc, F., Brinkmann, H., Chourrout, D., Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 439 (7079), 965-968 (2006).
- Garstang, W. The morphology of the Tunicata, and its bearing on the phylogeny of the chordata. Quarterly Journal of Microscopical Science. 72 (285), 51-187 (1929).
- Govindarajan, A. F., Bucklin, A., Madin, L. P. A molecular phylogeny of the Thaliacea. Journal of Plankton Research. 33 (6), 843-853 (2011).
- Godeaux, D., Bone, Q., Braconnot, J. C., Bone, Q. . The Biology of Pelagic Tunicates. , 1-24 (1998).
- Deibel, D., Lowen, B. A review of the life cycles and life-history adaptations of pelagic tunicates to environmental conditions). Ices Journal of Marine Science. 69 (3), 358369 (2012).
- Paffenhöfer, G., Köster, M. From one to many: on the life cycle of Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). Journal of Plankton Research. 33 (7), 1139-1145 (2011).
- Paffenhöfer, G. A., Gibson, D. M. Determination of generation time and asexual fecundity of doliolids (Tunicata, Thaliacea). Journal of Plankton Research. 21 (6), 1183-1189 (1999).
- Conley, K. R., Lombard, F., Sutherland, K. R. Mammoth grazers on the ocean’s minuteness: a review of selective feeding using mucous meshes. Proceedings of the Royal Society B-Biological Sciences. 285 (1878), (2018).
- Bouquet, J. M., et al. Culture optimization for the emergent zooplanktonic model organism Oikopleura dioica. Journal of Plankton Research. 31 (4), 359370 (2009).
- Denoeud, F., et al. Plasticity of Animal Genome Architecture Unmasked by Rapid Evolution of a Pelagic Tunicate. Science. 330 (6009), 1381-1385 (2010).
- Harrison, P. J., Waters, R. E., Taylor, F. J. R. A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. Journal of Phycology. 16 (1), 2835 (1980).
- Ohman, M. D., et al. Zooglider: An autonomous vehicle for optical and acoustic sensing of zooplankton. Limnology and Oceanography-Methods. 17 (1), 69-86 (2019).
- Takahashi, K., et al. In situ observations of a doliolid bloom in a warm water filament using a video plankton recorder: Bloom development, fate, and effect on biogeochemical cycles and planktonic food webs. Limnology and Oceanography. 60 (5), 1763-1780 (2015).
- Deibel, D., Bone, Q. . The biology of pelagic tunicates. , 171-186 (1998).
- Gibson, D. M., Paffenhöfer, G. A. Feeding and growth rates of the doliolid, Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). Journal of Plankton Research. 22 (8), 1485-1500 (2000).
- Haskell, A., Hofmann, E., Paffenhöfer, G., Verity, P. Modeling the effects of doliolids on the plankton community structure of the southeastern US continental shelf. Journal of Plankton Research. 21 (9), 1725-1752 (1999).
- Gibson, D. M., Paffenhöffer, G. A. Asexual reproduction of the doliolid, Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). Journal of Plankton Research. 24 (7), 703-712 (2002).
- Frischer, M. E., et al. Reliability of qPCR for quantitative gut content estimation in the circumglobally abundant pelagic tunicate Dolioletta gegenbauri (Tunicata, Thaliacea). Food Webs. 1, 7 (2014).
- Köster, M., Paffenhöfer, G., Baker, C., Williams, J. Oxygen consumption of doliolids (Tunicata, Thaliacea). Journal of Plankton Research. 32 (2), 171-180 (2010).
- Paffenhöfer, G. A hypothesis on the fate of blooms of doliolids (Tunicata, Thaliacea). Journal of Plankton Research. 35 (4), 919-924 (2013).
- Takahashi, K., et al. Sapphirinid copepods as predators of doliolids: Their role in doliolid mortality and sinking flux. Limnology and Oceanography. 58 (6), 1972-1984 (2013).
- Martí-Solans, J., et al. Oikopleura dioica Culturing Made Easy: A Low-Cost Facility for an Emerging Animal Model in EvoDevo. Genesis. 53 (1), 183-193 (2015).
- Nishida, H. Development of the appendicularian Oikopleura dioica: Culture, genome, and cell lineages. Development Growth & Differentiation. 50, S239-S256 (2008).

