Assessing Mineral Availability in Fish Feeds using Complementary Methods Demonstrated with the Example of Zinc in Atlantic Salmon
Summary
This article explains in detail a systematic approach to assess micro-mineral availability in Atlantic salmon. The methodology includes tools and models with increasing biological complexity: (1) chemical speciation analysis, (2) in vitro solubility, (3) uptake studies in cell lines, and (4) in vivo fish studies.
Abstract
Assessing the availability of dietary micro-minerals is a major challenge in mineral nutrition of fish species. The present article aims to describe a systematic approach combining different methodologies to assess the availability of zinc (Zn) in Atlantic salmon (Salmo salar). Considering that several Zn chemical species can be present in an Atlantic salmon feed, it was hypothesised that Zn availability is influenced by the Zn chemical species present in the feed. Thus, in this study, the first protocol is about how to extract the different Zn chemical species from the feed and to analyze them by a size exclusion chromatography-inductively coupled plasma mass spectroscopy (SEC-ICP-MS) method. Subsequently, an in vitro method was developed to evaluate the solubility of dietary Zn in Atlantic salmon feeds. The third protocol describes the method to study the impact of changing Zn chemical species composition on the uptake of Zn in a fish intestinal epithelial model using a rainbow trout gut cell line (RTgutGC). Together, the findings from the in vitro methods were compared with an in vivo study examining the apparent availability of inorganic and organic sources of Zn supplemented to Atlantic salmon feeds. The results showed that several Zn chemical species can be found in feeds and the efficiency of an organic Zn source depends very much on the amino acid ligand used to chelate Zn. The findings of the in vitro methods had less correlation with that outcome of the in vivo study. Nevertheless, in vitro protocols described in this article provided crucial information regarding Zn availability and its assessment in fish feeds.
Introduction
Fish meal and fish oil were traditionally used in Atlantic salmon feed. However, these ingredients are being increasingly replaced by plant-based ingredients1. The aforementioned shift in feed composition has resulted in low dietary availability and an increased need for improving mineral availability in Atlantic salmon feeds, especially zinc (Zn)2. The reduced availability might be a result of a change in the Zn level, Zn chemical species or/and antinutritional factors present in the feed matrix. In this scenario, a new array of additives generically considered as 'organic sources' have emerged with potential of being a better available source of dietary minerals to fish. Therefore, it is important to understand fundamental chemistry and physiology governing the availability of minerals and their sources to fish. Zinc is an essential trace element for all living organisms3. The role of Zn as a signaling molecule has been described at both the paracellular and intracellular level in fish4. In Atlantic salmon, Zn deficiency has been associated with skeletal abnormalities and reduced activity of various Zn metalloenzymes5,6.
This study describes a systematic approach to understand Zn availability by categorizing it into four different compartments of varied chemical and biological complexity. The methods involved are described in four sections, as can be seen in Figure 1: (1) evaluation of Zn chemical species in the soluble fraction of an Atlantic salmon feed using a size exclusion chromatography-inductively coupled plasma mass spectroscopy (SEC-ICP-MS) method7; (2) in vitro solubility of supplemented Zn in Atlantic salmon feed; (3) evaluation Zn chemical species uptake by in vitro intestinal model (RTgutGC)8; and (4) apparent availability of Zn in Atlantic salmon (Salmo salar)9. Similar protocols can be developed for other minerals (e.g., manganese, selenium, copper) of nutritional interest to aquaculture fish species.
Protocol
The feeding trial in section 4 was performed according to Norwegian (FOR-2015-06 - 18-761) and European legislation (Directive 2010/63/EU).
1. Evaluation of Zn chemical species in the soluble fraction of an Atlantic salmon feed using a SEC-ICP-MS method
- Extraction buffer (100 mM Tris-HCl, pH 8.5)
- Prepare the extraction buffer by dissolving an appropriate amount of tris(hydroxymethyl)aminomethane to reach the desired ionic strength (100 mM) in ultrapure H2O.
- Adjust the pH of the solution to pH 8.5 with HCl solution, monitoring the pH change with a pH meter.
- Preparation of feed samples
NOTE: The feed sample used was formulated based on commercial feed for Atlantic salmon, containing protein sources mainly from plant-based ingredients (i.e., approximately 5% fish protein, 10% fish oil, 68% plant-based protein and 12% plant-based oil). Zinc sulphate was supplemented to the feed.- Grind the feed sample by hand using a pestle and a mortar.
- Sieve the feed sample to ensure that the extraction is performed in a feed fraction with similar particle size (from 850 µm to 1.12 mm).
- Proceed to perform the Zn extraction.
- Zinc extraction from a feed sample
- Weigh approximately 0.5 g of feed in triplicate into 15 mL conical tubes.
- Add the extraction buffer (5 mL of 100 mM Tris-HCl, pH 8.5) to the samples.
- Extract the samples in a rotator (20 rpm) at 4 °C for 24 h.
- Separate the soluble and non-soluble fractions by centrifugation for 10 min at 3000 x g.
- Use a 0.45 µm disposable syringe filter to filter the soluble fraction.
- Transfer the filtered samples to clean tubes.
- Perform the Zn speciation analysis in the soluble fractions using SEC-ICP-MS, as described in step 1.6.
NOTE: A summary of the procedure for Zn extraction from a feed sample is described in Figure 2.
- Mobile phase solution (50 mM Tris-HCl + 3% MeOH, pH 7.5)
- Prepare the mobile phase solution dissolving 6.057 g of tris(hydroxymethyl)aminomethane in 1 L of 3% MeOH solution (v/v).
- Adjust the pH of the solution to pH to 7.5 with HCl solution, monitoring the pH change with a pH meter.
- Filter the mobile phase solution through a 0.45 μm membrane filter.
- Molecular weight calibration of the SEC column separation range
- Calibrate the separation range by performing a molecular weight calibration.
NOTE: In this study thyroglobulin (660 kDa), Zn/Cu superoxide dismutase (32 kDa), myoglobin (17 kDa) and vitamin B12 (1.36 kDa) were used. - Prepare each of the standards with a known concentration in ultrapure H2O.
- Prepare a high-performance liquid chromatography (HPLC) vial by adding 250 µL of standard to a vial.
- Load the vials with standards to the sequence run of the samples.
- Run the molecular weight calibration at the beginning and at the end of the analytical sequence, monitoring 127I (thyroglobulin), 66Zn (Zn/Cu superoxide dismutase), 57Fe (myoglobin) and 59Co (vitamin B12).
NOTE: The molecular weight calibration is simultaneously performed with the Zn speciation analysis.
- Calibrate the separation range by performing a molecular weight calibration.
- Zinc speciation analysis using SEC-ICP-MS
NOTE: The Zn speciation analysis by the SEC-ICP-MS method was developed based on principles described elsewhere10,11 and further optimization was performed for the analysis of an Atlantic salmon feed7.- Perform the Zn speciation analysis on the soluble fractions by using a size exclusion chromatography (SEC) column and an HPLC coupled with inductively coupled plasma mass spectroscopy (ICP-MS).
- Prepare an HPLC vial by adding 250 µL of soluble fraction to a vial.
- Before analysis, spike all the samples with 0.5 µL of vitamin B12. This step allows to correct for retention times shifts, monitoring 59Co.
- Dilute the soluble fraction with extraction buffer (100 mM Tris-HCl, pH 8.5) and adjust to a final volume of 1 mL.
- Prepare the sequence run of the samples in random order.
- Tune the ICP-MS according to manufacturer’s instructions.
- Follow the instrument settings for the HPLC and ICP-MS performing the Zn speciation analysis (see Table 1).
2. In vitro solubility of supplemented Zn in Atlantic salmon feed
NOTE: The feed sample used was formulated based on commercial feed for Atlantic salmon, containing protein sources mainly from plant-based ingredients (i.e., approximately 5% fish meal, 10% fish oil, 68% plant-based ingredients and 12% vegetable oil).
- Grind the Atlantic salmon feed samples for 10 s at 3000 rpm using a knife mill and store at 4 °C until further analysis.
- Weigh ~0.2 g of the feed samples ground in step 2.1 and add Zn radiotracer (65Zn) of known specific activity in a 5 mL volume sample tube (with a cap).
CAUTION: This procedure must be performed inside a radionuclide suite. The person performing this step should be trained and certified to handle radio isotopes. The safety and precautionary measures advised by the radiation safety administration of the institute must be strictly followed. - Then prepare the freshwater intestinal luminal buffer solution as described below.
- For salt solution A, weigh 11.65 g of NaNO3, 0.55 g of KNO3 and 0.4 g of MgSO4. Dissolve the salts in ultrapure H2O and adjust to a final volume of 60 mL.
- For salt solution B, weigh 0.31 g of Ca(NO3)2·4H2O. Dissolve the salts in ultrapure H2O and adjust to a final volume of 10 mL.
- Use 500 mM HEPES stock solution as salt solution C.
- For salt solution D, weigh 1.2 g of MgCl2, dissolve the salt in ultrapure H2O and adjust to a final volume of 20 mL.
- For salt solution E, weigh 0.9 g of MgSO4, dissolve the salt in ultrapure H2O and adjust to a final volume of 20 mL.
- Prepare pyruvate solution by dissolving 0.55 g of CH3COCOONa in 10 mL of ultrapure H2O.
- Dissolve 0.9 g of galactose (C6H12O6) in ultrapure H2O.
- Dissolve well using a magnetic stirrer and sterilize salt solutions A, B, D and E by autoclaving, and solution C, pyruvate and galactose by filtration through a 200 µm syringe filter.
- After preparation of the different stock solutions, to prepare 100 mL of working solution of the buffer, mix the above prepared solutions in the following proportion: 6.8 mL of salt solution A, 4.14 mL of B, 5 mL of C, 2.5 mL of D, 1.5 mL of E, and 1.14 mL each of pyruvate and galactose. Make up the volume to 100 mL using deionized water.
NOTE: The above buffer will now represent the ionic composition of the intestinal lumen found in freshwater salmonids.
- Prepare six other aliquots of the buffer described in step 2.6 and add one of the following amino acids (cysteine, methionine, glycine, histidine, lysine and arginine) to reach a final molar concentration of 5 mM.
- Add the freshwater intestinal luminal buffer (reaction volume = 3 mL; pH 7.4) to the feed sample.
- Repeat step 2.5 with the buffers described in 2.4 (in the presence of different amino acids at 5 mM concentration).
- Close the tubes and allow them to spin in a rotary spinner for 30 min at 25 rpm.
- Separate the soluble and non-soluble fractions by centrifuging for 10 min at 1157 x g.
- Use a gamma teller to measure the counts per minute (cpm) of 65Zn in the soluble and non-soluble fractions.
- Calculate the proportion of the radio isotopes of Zn (65Zn) present in the soluble and non-soluble fractions.
3. Evaluation of Zn chemical species uptake using an in vitro intestinal model (RTgutGC)
- RTgutGC cells culture
NOTE: All working materials used in this step should be sterile.- Revive the frozen RTgutGC cells gently in a water bath set at 20 °C.
- Gently pipette out the solution containing the cells and suspend them in 10 mL of L15 medium containing 10% fetal bovine serum (FBS).
NOTE: 10% FBS is only used for reviving the frozen cells. For subsequent passage, FBS is used at 5%. The composition of FBS can vary between batches, so it is advisable to buy and stock as much as required from a single batch to avoid inter-batch variations in the serum composition. - Add the cell suspension to 75 cm2 cell culture flasks and incubate in an incubator set at 19 °C under normal atmosphere.
- Check the cells and when confluent (80% confluence, assess visually by examining the density of the cell surface under a microscope), split the cells to new flasks (subsequent passage) or harvest to use in experiments.
NOTE: An example of the RTgutGC cells 1 h and 1 week after seeding to the cell culture flasks is shown in Figure 3.
- Cell harvest and preparing for exposure experiments
- Wash cells twice with 1 mL ethylenediaminetetraacetic acid (EDTA) solution. After each wash, siphon out the EDTA solution using a sterile suction tube.
- Treat the cells with trypsin (0.7 mL of trypsin, in 0.25% in phosphate-buffered saline [PBS]).
- Gently rotate the flask at acute angles to spread the trypsin all along the surface of the flask.
- Continue rotation for 2 min, while the cells detach.
- After gently rotating for 2 min, add 10 mL of L15/FBS medium to neutralize trypsin.
- Decant the resulting cell suspension into a conical bottom centrifuge tube using a sterile pipette and centrifuge for 3 min at 130 x g.
- Determine the density of the harvested cells by manual counting using hemocytometer.
- Add required volume of L15/FBS medium to attain a cell density of 5 x 104 cells/mL.
- Seed the cells onto 24-well plates by pipetting 1 mL of cell suspension per well to attain the final cell density of 5 x 104 cells/well.
NOTE: Preferably use multi-dispensing pipettes to minimize variation and reduce time. - Place the seeded plates in an incubator under normal atmosphere at 19 °C for 48 h prior to experiments.
NOTE: Trypsin to be stored at -20 °C; EDTA solution and L15/FBS media at 4 °C. Adjust temperature of all working solutions and media to 19 °C just before use.
- Preparation of exposure media
NOTE: This step has to be done under the fume hood under aseptic and sterile conditions.- Prepare L15/ex by mixing 6.8 mL of salt solution A (step 2.3.1), 1.14 mL of B (step 2.3.2), 5 mL of C (step 2.3.3), and 1.14 mL each of pyruvate (step 2.3.6) and galactose (step 2.3.7). Make up the volume to 100 mL using sterile cell culture-grade distilled water.
- Prepare FW by mixing 6.8 mL of salt solution A (2.3.1), 4.14 mL of B (2.3.2), 5 mL of C (2.3.3), 2.5 mL of D (2.3.4), 1.5 mL of E (2.3.5), and 1.14 mL each of pyruvate (2.3.6) and galactose (2.3.7). Make up the volume to 100 mL using sterile, cell culture-grade distilled water.
- Quantify the concentrations of ions in the exposure media using ICP-MS as described elsewhere12.
NOTE: Ionic concentrations analyzed in the media preparations are presented in Table 2.
- Zinc (65Zn) influx assays
- Seed the RTgutGC cells onto 24-well plates (5 x 104 cells/well) in complete L15/FBS medium.
- Incubate for 48 h in an incubator with normal atmosphere at 19 °C.
- Adjust all experimental media preparations to pH 7.4 using 0.5 M NaOH in the presence or absence of L-methionine (L-Met) or DL-methionine (DL-Met) at 2 mM concentration.
NOTE: The pH adjustment of the buffers described in 3.4.3 needs to be done freshly before treatment of the cells in step 3.4.5. - After the completion of the incubation period, remove the medium from the wells, and rinse thoroughly with PBS.
- Add the pH-adjusted FW experimental media, and allow to acclimatize for 20 min.
- Expose the RTgutGC cells to nominal concentrations of 3.07, 6.14, 12.27 and 24.55 µM 65Zn(II) (as ZnCl2; ~4 kBq/mL) in the media described in step 3.4.3.
- Immediately after, keep the cells in the incubator at 19 °C for 15 min.
- After the 15 min incubation is over, aspirate the culture supernatant and remove from the well.
- Rinse the cells with ice cold FW medium (with 200 µM Zn, pH 7.4) and then quench by adding the 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) buffer (pH 7.4) for 5 min, to get rid of any adsorbed 65Zn(II).
- Expose the cells to the above media preparations in the presence or absence of 10 mM 2-Aminobicyclo [2.2.1] heptane-2-carboxylic acid (BCH), an amino acid transport inhibitor.
- After the exposure period of 15 min, repeat steps 3.4.8 and 3.4.9.
- The RtgutGC cells will be adhered to the bottom of the wells as a monolayer. Digest the cells by using 0.2% hot sodium dodecyl sulfate (SDS) detergent (100 µL/well).
NOTE: The SDS solution needs to be placed for 1 h in a water bath set to 90 ˚C prior to use. - Aspirate and recover the cell digestate into a 1.5 mL tube.
- Measure radioactivity of the cell digests using a gamma counter.
NOTE: The counts per minute (cpm) needs to be corrected for radioactive decay, background activity and are subjected to specific activity calculations following the formulae described by Glover and Hogstrand13. - To quantify the protein concentration of the cells, homogenize the cells with 500 µL of 0.5 M NaOH.
- Use a Bradford assay kit to measure the protein concentration in the cell sample, with bovine serum albumin (BSA) as the standard.
NOTE: Once the protein concentration is quantified, the rate of Zn uptake by RTgutGC cells can be expressed as pmoles Zn min-1 mg-1 protein.
4. Apparent availability of dietary Zn in Atlantic salmon (Salmo salar)
NOTE: The Atlantic salmon feeds were formulated based on commercial feeds, containing protein sources mainly from plant-based ingredients (i.e., approximately 5% fish protein, 10% fish oil, 68% plant-based protein and 12% plant oil). Two feeds were supplemented with an inorganic source (Zn sulphate) or an organic source (Zn chelate of glycine) to achieve a Zn concentration of 150 mg/kg of feed. In addition, Yttrium oxide (feed grade) was added to the feed at 0.01% as the inert marker to enable calculation of apparent availability coefficient.
- Acclimatize the Atlantic salmon (SalmoBreed strain, age 1+ years, mixed-sex groups) in their respective tanks until the fish are used to the experimental conditions.
- Assess the acclimation of the Atlantic salmon by monitoring their daily feed intake.
NOTE: This trial was performed in triplicate tanks, thus a total of six tanks were used. During the feeding trial the water temperature was 11.9 ± 0.3 °C and dissolved oxygen saturation was 101 ± 5%. - Feed the fish with experimental feeds for 11 days.
- Euthanize the fish by overdose using 6 mL of tricaine methanesulphonate stock solution per liter of water.
- Collect a pooled sample of feces from the fish from the same tank into a plate by striping from the ventral fin to anus.
- Remove the feces from the plate with a spatula into a 50 mL conical tube and immediately store the samples at -20 °C.
NOTE: The samples were kept at -20 °C until further analysis. - Freeze dry the feces samples for 72 h at -80 °C.
- Manually homogenize manually the feces sample into a fine powder using a pestle and mortar.
- Determine the concentration of Zn and Yttrium in the feed and feces samples using an ICP-MS (as described elsewhere9).
- Determine apparent availability coefficient (AAC, %) using the following formula:

Representative Results
Evaluation of Zn chemical species in the soluble fraction of an Atlantic salmon feed using a SEC-ICP-MS method
The SEC-ICP-MS method provides data about the Zn chemical species found in the soluble fraction of the Atlantic salmon feed. Figure 4 illustrates the chromatographic profile of Zn found in the soluble fraction. This chromatogram was obtained using the SEC-ICP-MS method. Five Zn containing peaks were found in the soluble fractions of the Atlantic salmon feed. Each peak has a different molecular weight; peak one (~ 600 kDa), peak two and peak three (from 32 to 17 kDa), peak four (from 17 to 1.36 kDa) and peak five (> 1.36 kDa). Peak four was the most abundant, followed by peak two, three, five and one, respectively. The Zn chemical species found in the soluble fraction can have different sources because the feed used contains both marine-based and plant-based ingredients, and supplemented form (i.e., Zn sulphate). The molecular weight range of the Zn chemical species suggested that these compounds might be metalloproteins.
In vitro solubility of supplemented Zn in Atlantic salmon feed
Solubility of supplemented 65Zn increased in the presence of amino acids. All the tested amino acids increased the solubility of supplemented 65Zn. Methionine, glycine, cysteine, histidine, and lysine improved 65Zn solubility; higher solubility was found with histidine and lysine (Figure 5).
Evaluation of Zn species uptake using an in vitro intestinal model (RTgutGC)
Apical zinc uptake in RTgutGC cells were significantly influenced by the presence of L-Met or DL-Met at 2 mM concentrations. Furthermore, the impact of methionine on Zn uptake in RTgutGC cells was negatively affected by the presence of BCH (an amino acid transport system blocker), when compared to cells untreated with BCH (Figure 6).
Apparent availability of dietary Zn in Atlantic salmon (Salmo salar)
In practical feeds for Atlantic salmon, apparent Zn availability was the same when supplementing with an inorganic source (Zn sulphate) or an organic source (Zn chelate of glycine). The estimated values for apparent availability of Zn (%, n = 3) in Atlantic salmon were 31% ± 12% when supplementing with an inorganic source (Zn sulphate) and 31% ± 3% when supplementing an organic source (Zn chelate of glycine).
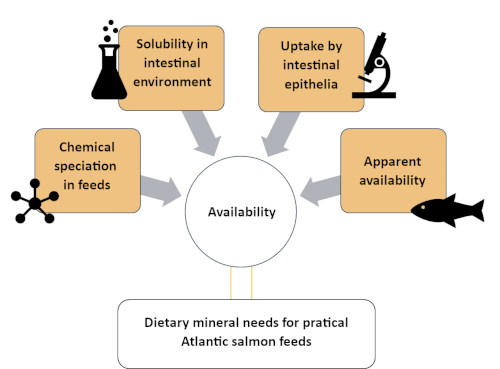
Figure 1: A summary of the systematic approach to assess mineral availability using complementary methods. This approach was used to study zinc availability in Atlantic salmon, including Zn speciation, Zn solubility in intestinal environment, Zn uptake by intestinal cells and Zn apparent availability. Please click here to view a larger version of this figure.
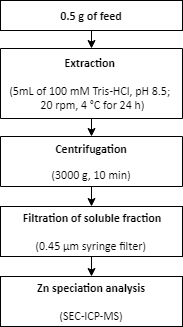
Figure 2: A summary of the procedure for Zn extraction from a feed sample. Zinc is extracted from a feed sample using mild extraction conditions. The extraction is followed by Zn speciation analysis. Please click here to view a larger version of this figure.
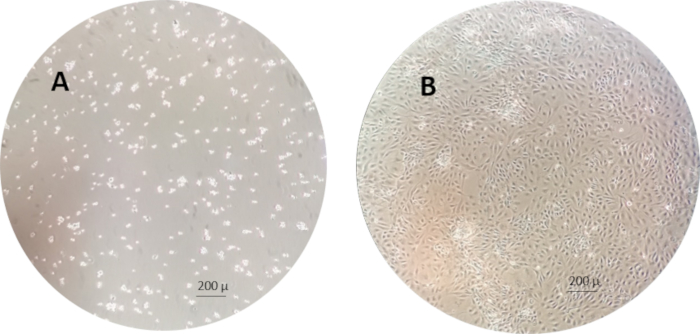
Figure 3: An example of the RTgutGC cells 1 h (left) and 1 week (right) after seeding in the cell culture flasks. Please click here to view a larger version of this figure.
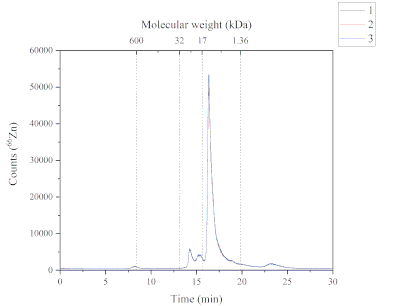
Figure 4: A chromatogram showing the Zn-containing peaks from the soluble fraction of Atlantic salmon feed and analyzed by SEC-ICP-MS. The three replicates are characterized by the blue, red and black lines. A molecular weight calibration was performed using thyroglobulin (660 kDa, monitoring 127I), Zn/Cu superoxide dismutase (32 kDa, monitoring 66Zn), myoglobin (17 kDa, monitoring 57Fe), vitamin B12 (1.36 kDa, monitoring 59Co); Peak 1 (P1): ~600 kDa, retention time (RT) 8.2 min; Peak 2+3 (P2+3): from 32 to 17 kDa, RT 14.2 + 15.3 min; Peak 4 (P4): from 17 to 1.36 kDa, RT 16.3 min; Peak 5 (P5): > 1.36 kDa, Rt 23.2 min. Please click here to view a larger version of this figure.
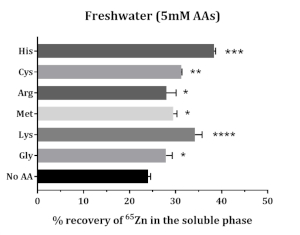
Figure 5: The impact of amino acids on the in vitro solubility of supplemented Zn in Atlantic salmon feed. Data are presented as mean ± SD (n = 3). Data were analyzed through one-way ANOVA, followed by Dunnet’s multiple comparison test, comparing the mean of each AA group with that of control group (No AA). The asterisks denote the level of significance of ANOVA (P-values < 0.05 (*), < 0.01 (**), < 0.001 (***) and < 0.0001 (****)). Please click here to view a larger version of this figure.
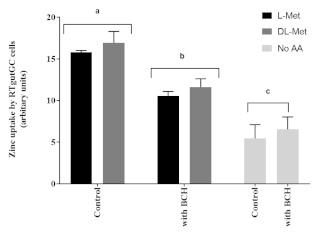
Figure 6: The influence of methionine and an amino acid transport inhibitor (2-Aminobicyclo [2.2.1] heptane-2-carboxylic acid, BCH, 10 mM). Data are presented as mean ± SD (n = 3). Data were analyzed through two-way ANOVA, followed by Tukey’s multiple comparison test with p < 0.05 level of significance. Post-hoc differences among groups are represented as superscript letter above the bars; bars with different superscripts are statistically different (p < 0.05). Please click here to view a larger version of this figure.
| HPLC settings | |
| Column | SEC column (30 cm x 7.8 mm, 5 µm particle size) + guard column (7 µm particle size) |
| Calibration range | 1.0 × 104 – 5.0 × 105 Da |
| Mobile phase | 50 mM Tris-HCl + 3% MeOH (pH 7.5) |
| Flow rate | 0.7 mL min−1 |
| Injection volume | 50 μL |
| ICP–MS settings | |
| Forward power | 1550 W |
| Plasma gas flow | 15.0 L min−1 |
| Carrier gas flow | 0.86 L min−1 |
| Makeup gas flow | 0.34 L min−1 |
| Dwell time | 0.1 s per isotope |
| Isotopes monitored | 127I, 66Zn, 59Co, 57Fe |
Table 1. An overview of instrument settings for the HPLC and ICP-MS.
| Chemical composition (mM) | L15/ex | Experimental medium (L15/FW) |
| Sodium nitrate | 155 | 155 |
| Potassium nitrate | 6.2 | 6.2 |
| Magnesium sulfate | 3.8 | 19.5 |
| Calcium nitrate | 1.5 | 5.4 |
| HEPES | 5 | 5 |
| Magnesium chloride | – | 15 |
| Sodium pyruvate | 5.7 | 5.7 |
| Galactose | 5.7 | 5.7 |
| pH | 7.1 | 7.4 |
| Ionic strength | 178 | 258 |
| Ionic composition (mM) | ||
| Calcium, Ca2+ * | 1.6 ± 0.1 | 5.3 ± 0.2 |
| Magnesium, Mg2+ * | 3.9 ± 0.3 | 32.5 ± 0.7 |
| Potassium, K+ * | 8.2 ± 1.2 | 8.6 ± 1.1 |
| Sodium, Na+ * | 160 ± 3 | 157 ± 2 |
| Nitrate, NO3– ** | 164 | 172.4 |
| Sulfate, SO4– ** | 3.8 | 18.7 |
| Chloride, Cl– ** | 1.5 | 31.5 |
Table 2. The chemical and ionic composition of the experimental media tested.
Discussion
The intestinal absorption of Zn seems to be influenced by the chemical form of the Zn species13. In this regard, the use of the protocols described in this article allowed the sequentially study of the chemical and biological aspects underlying the 'availability' of Zn in Atlantic salmon.
This study reported the use of a Zn speciation analysis method. The SEC-ICP-MS method provided qualitative data concerning the molecular weight of Zn chemical species present in the soluble fraction of an Atlantic salmon feed. This was achieved by comparison of the retention times of the molecular weight calibration standards (i.e., thyroglobulin (660 kDa), Zn/Cu superoxide dismutase (32 kDa), myoglobin (17 kDa) and vitamin B12 (1.36 kDa)) with the retention times of Zn containing peaks. A challenge found in the Zn speciation analysis was the identification of the unknown Zn chemical species due to lack of analytical standards. In SEC, the separation of the molecules is based on their sizes relative to the pores in the stationary phase. In principle, larger molecules will travel faster, eluting first, and smaller molecules will travel slower, eluting later14. Consequently, each Zn containing peak might contain several compounds with similar molecular weight15. This also contributes to the challenge of identifying unknown Zn chemical species. Moreover, several mild extraction conditions were tested for extraction of Zn. The extracted Zn was low (~10%). Mild extraction conditions were applied to keep the Zn chemical species intact but this may have compromised the extraction efficiency7.
In the in vitro solubility assay, the solubility of supplemented Zn (as radio isotope 65ZnCl2) indicated that the amino acids, especially histidine and lysine, increased the solubility of Zn (Figure 5). Using feed samples directly for in vitro solubility assays under simulated gastrointestinal conditions is based on the knowledge that change in Zn speciation is pH dependent16. However, acidic conditions at the beginning of the GI tract, might result in some change in the speciation which might be irreversible (e.g., ZnO -> ZnCl2, in the presence of HCl under acidic conditions in the stomach). Nevertheless, the Zn source used here is ZnSO4 and the solubility of which was improved by amino acids in the medium. The next question to be answered was, can the increased solubility be translated to availability? The RTgutGC intestinal cell line was used to study this question. In the context of mineral nutrition in animals, the term 'availability' is hard to define and can be regulated differentially in the cells (in vitro) compared to an animal (in vivo). Hence, the term 'uptake' was used when it came to the in vitro evaluation using intestinal cell line. The cell line provided useful information on the Zn uptake mechanisms at the intestinal epithelium which is part of the complex regulatory process which govern mineral availability in animals. The RTgutGC cells elicited a better capacity for apical uptake of Zn in the presence of an amino acid (i.e., methionine; Figure 6). However, the apparent availability in vivo did not significantly differ between inorganic and organic Zn sources in Atlantic salmon. In the in vivo availability study, the Zn source comparison was made at dietary Zn levels well exceeding the known Zn requirements of Atlantic salmon17, total Zn concentration of 150 mg/kg feed. The differences in availability are better visualized when the dietary levels tested fall in the linear dynamic range before the animal reaches saturation. In the present in vivo study, it is possible that the Atlantic salmon were well saturated to observed difference in Zn absorption between sources used.
In summary, the first method provided qualitative information regarding different Zn chemical species found in the soluble fraction of an Atlantic salmon feed; the second method, in vitro solubility of supplemented Zn was improved in the presence of amino acid ligands; the third method confirmed that improved solubility by amino acids can improve uptake at intestinal epithelium; conversely, the fourth method failed to find differences in availability of Zn from inorganic or organic source to Atlantic salmon. To conclude, although not in alignment with the in vivo findings, the in vitro protocols did provide interesting insights into understanding the different components of the Zn availability.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was performed under the project APREMIA (Apparent availability and requirement of minerals in Atlantic salmon, grant no. 244490) funded by the Norwegian Research Council.
Materials
| 0.45 µm syringe filter | Sartorius | ||
| 0.45 μm membrane filter | Pall | ||
| 10 % fetal bovine serum | Eurobio | ||
| 1282 Compugamma Laboratory Gamma Counter | LKB Wallac | ||
| 24 well plates (Falcon, TPP microplates) | Thermo Fisher Scientific | 10048760 | |
| 2-aminobicyclo(2.2.1)heptane-2-carboxylic acid | Sigma Aldrich | A7902 | |
| 75 cm2 cell culture flasks (Falcon, TPP tissue culture flasks) | TPP Techno Plastic Products AG | 90075 | |
| L-Arginine | Sigma Aldrich | A5006 | |
| Bradford assay kit | Bio-Rad | 5000001 | |
| Centrifuge | Eppendorf Centrifuge 5702 | ||
| L-Cysteine | Sigma Aldrich | 30089 | |
| DL-methionine | Alfa Aesar | 59-51-8 | |
| D-methionine | Sigma Aldrich | M9375 | |
| Experimental fish feeds | Skretting | ||
| Glycine | Sigma Aldrich | 410225 | |
| Guard column, TSKgel SWxl Type (7 μm particle size) | Tosoh | ||
| L-Histidine | Sigma Aldrich | 53319 | |
| HPLC coupled with a 7500ce ICP-MS | Agilent Technologies | ||
| Hydrochloric acid | Emsure ACS, ISO, 37% w/w, Merck | 1.00317 | |
| Knife mill | GM 300, Retsch Gmbh | ||
| L-15 medium | Invitrogen/Gibco | 21083027 | |
| L-methionine | Sigma Aldrich | M9625 | |
| L-Lysine | Sigma Aldrich | 23128 | |
| Methanol | LiChrosolv, HPLC grade, Merck | 1.06035 | |
| Milli-Q water (18.2 MΩ cm) | EMD Millipore Corporation | ||
| Myoglobin | Sigma Aldrich | M1882 | |
| NexION 350D ICP-MS | Perkin Elmer | ||
| Pasteur pipette | VWR | ||
| pH meter | inoLab | ||
| Phosphate-buffered saline (PBS) | Sigma Aldrich | 806552 | |
| RTgutGC cells | Obtained in kind from Professor Dr. Kristin Schirmer, Dept. of Environmental Toxicology, Eawag, Swiss Federal Institute of Aquatic Science and Technology, Switzerland | ||
| SEC column, TSKgel G3000SWxl | Tosoh | ||
| Sieve stainless steel (850 μm – 1.12 mm) | Retsch | ||
| Sodium dodecyl sulphate (SDS) | Sigma Aldrich | 436143 | |
| Superoxide dismutase | Sigma Aldrich | S7571 | |
| Thyroglobulin | Sigma Aldrich | T1001 | |
| Tricaine methanesulphonate | PharmaQ | ||
| Tris(hydroxymethyl)aminomethane | Sigma Aldrich | 252859 | |
| Trypsin in 0.25% in phosphate-buffer saline | Biowest | L0910 | |
| Versene EDTA solution | Invitrogen/Gibco | 15040-033 | |
| Vitamin B12 | Sigma Aldrich | V2876 | |
| Zinc chelate of glycine | Phytobiotics | ||
| Zinc sulphate | Vilomix |
References
- Ytrestoyl, T., Aas, T. S., Asgard, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture. 448, 365-374 (2015).
- Prabhu, P. A. J., et al. Evaluating dietary supply of microminerals as a premix in a complete plant ingredient-based diet to juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture Nutrition. 24 (1), 539-547 (2018).
- Maret, W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Advances in nutrition. 4 (1), 82-91 (2013).
- Hogstrand, C., Wood, C. M., Farrell, A. P., Brauner, C. J. . Fish Physiology. 31, 135-200 (2011).
- Baeverfjord, G., et al. Mineral nutrition and bone health in salmonids. Reviews in Aquaculture. , (2018).
- Maage, A., Julshamn, K. Assessment of zinc status in juvenile Atlantic salmon (Salmo salar) by measurement of whole body and tissue levels of zinc. Aquaculture. 117 (1), 179-191 (1993).
- Silva, M. S., Sele, V., Sloth, J. J., Araujo, P., Amlund, H. Speciation of zinc in fish feed by size exclusion chromatography coupled to inductively coupled plasma mass spectrometry – Using fractional factorial design for method optimization and mild extraction conditions. Journal of Chromatography B. , (2018).
- Prabhu, A. J., et al. Zinc uptake in fish intestinal epithelial model RTgutGC: Impact of media ion composition and methionine chelation. Journal of Trace Elements in Medicine and Biology. 50, 377-383 (2018).
- Silva, M. S., et al. Apparent availability of zinc, selenium and manganese as inorganic metal salts or organic forms in plant-based diets for Atlantic salmon (Salmo salar). Aquaculture. 503, 562-570 (2019).
- Persson, D. P., Hansen, T. H., Laursen, K. H., Schjoerring, J. K., Husted, S. Simultaneous iron, zinc, sulfur and phosphorus speciation analysis of barley grain tissues using SEC-ICP-MS and IP-ICP-MS. Metallomics. 1 (5), 418-426 (2009).
- Lothian, A., Roberts, B. R. Standards for Quantitative Metalloproteomic Analysis Using Size Exclusion ICP-MS. Journal of Visualized Experiments. (110), (2016).
- Minghetti, M., Schirmer, K. Effect of media composition on bioavailability and toxicity of silver and silver nanoparticles in fish intestinal cells (RTgutGC). Nanotoxicology. 10 (10), 1526-1534 (2016).
- Glover, C. N., Hogstrand, C. Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. Journal of Experimental Biology. 205 (1), 151-158 (2002).
- Ekman, R., Ekman, R. . Mass spectrometry: Instrumentation, interpretation, and applications. Wiley Series on Mass Spectrometry. , 105-115 (2009).
- Hong, P., Koza, S., Bouvier, E. S. P. A Review Size-Exclusion Chromatography for the Analysis of Protein Biotherapeutics and Their Aggregates. Journal of Liquid Chromatography & Related Technologies. 35 (20), 2923-2950 (2012).
- Krezel, A., Maret, W. The biological inorganic chemistry of zinc ions. Archives of Biochemistry and Biophysics. 611, 3-19 (2016).
- National Research Council. . Nutrient Requirements of Fish and Shrimp. , (2011).

