Assessment of Lymphocyte Migration in an Ex Vivo Transmigration System
Summary
In this protocol, lymphocytes are placed in the top chamber of a transmigration system, separated from the bottom chamber by a porous membrane. Chemokine is added to the bottom chamber, which induces active migration along a chemokine gradient. After 48 h, lymphocytes are counted in both chambers to quantitate transmigration.
Abstract
Herein, we present an efficient method that can be executed with basic laboratory skills and materials to assess lymphocyte chemokinetic movement in an ex vivo transmigration system. Group 2 innate lymphoid cells (ILC2) and CD4+ T helper cells were isolated from spleens and lungs of chicken egg ovalbumin (OVA)-challenged BALB/c mice. We confirmed the expression of CCR4 on both CD4+ T cells and ILC2, comparatively. CCL17 and CCL22 are the known ligands for CCR4; therefore, using this ex vivo transmigration method we examined CCL17– and CCL22-induced movement of CCR4+ lymphocytes. To establish chemokine gradients, CCL17 and CCL22 were placed in the bottom chamber of the transmigration system. Isolated lymphocytes were then added to top chambers and over a 48 h period the lymphocytes actively migrated through 3 µm pores towards the chemokine in the bottom chamber. This is an effective system for determining the chemokinetics of lymphocytes, but, understandably, does not mimic the complexities found in the in vivo organ microenvironments. This is one limitation of the method that can be overcome by the addition of in situ imaging of the organ and lymphocytes under study. In contrast, the advantage of this method is that is can be performed by an entry-level technician at a much more cost-effective rate than live imaging. As therapeutic compounds become available to enhance migration, as in the case of tumor infiltrating cytotoxic immune cells, or to inhibit migration, perhaps in the case of autoimmune diseases where immunopathology is of concern, this method can be used as a screening tool. In general, the method is effective if the chemokine of interest is consistently generating chemokinetics at a statistically higher level than the media control. In such cases, the degree of inhibition/enhancement by a given compound can be determined as well.
Introduction
This original transmigration method was presented by Stephen Boyden in 1962 in the Journal of Experimental Medicine1. Much of what we know about chemotaxis and chemokinetics would not be possible without the development of the Boyden chamber. Prior to the discovery of the first chemokine in 1977, ex vivo transmigration systems were used to learn about serum-factors that could arrest cellular movement in macrophages while amplifying cellular motility in neutrophils1,2. A massive wealth of knowledge has been developed regarding immune cell migration, and to date, 47 chemokines have now been discovered with 19 corresponding receptors3,4. In addition, multitudes of inhibitors/enhancers of these chemokine pathways have undergone development for therapeutic purposes5,6,7,8. Many of those compounds have been tested in similar transmigration chambers to understand direct interactions between the compounds and immune cell responsiveness to a given chemokine9.
Transmigration, or diapedesis, into inflamed tissue is an essential process to a healthy inflammatory response to clear infection10,11. A Boyden chamber, transmigration system, or transwell apparatus are generally composed of two chambers separated by a porous membrane1,12. The bottom chamber most often holds media containing the chemokine of interest, while leukocytes are placed in the top chamber. The size of the pore in the membrane can be selected based on the size of the cell of interest. For this project, we selected a 3 µm porous membrane, as lymphoid cells are 7-20 µm in size, depending on the stage of cellular development. This pore size ensures that these cells are not passively falling through the pores, but that they are actively migrating in response to the chemokine gradient.
The major advantage of this protocol is its cost effectiveness. In vivo transmigration is difficult because it requires extensive training in animal handling and surgery, and often involves high-powered microscopy that is not always available to a researcher. Cost effective screening of compounds thought to enhance or inhibit transmigration can be accomplished in advance of in vivo imaging. Because the transmigration system is tightly controlled, cells may be treated initially then added to the transwell apparatus, or, vice versa, the chemokine may be treated first with a chemokine inhibitor then cells added to the transwell apparatus. Lastly, endothelial cells and/or basement membrane proteins can be added to the bottom of the transwell insert 1-2 days prior to the transmigration experiment to understand the involvement of these barrier cells in chemokinetics. Again, these manipulations of the system provide a powerful means of determining important information about the effectiveness of a given compound in advance of more complicated in vivo studies.
Utilizing a transmigration chamber system is an effective way to assess lymphocyte mobility under various in vivo and in vitro conditions12,13,14. Herein, we describe an optimized method for assessing ex vivo lymphocyte responsiveness to chemokines in a transmigration chamber. In this example experiment, CD4+ T cells and group 2 innate lymphoid cells (ILC2) were isolated from male and female, BALB/c mice following OVA-allergen exposure. A hypothesis was generated that CCR4+ CD45+ Lineage- (LIN-) ILC2 from allergen-challenged mice would migrate more efficiently towards CCL17 and CCL22 than CCR4+ CD4+ T helper cells. CCL17 and CCL22 are chemokines commonly produced by dendritic cells and macrophages of the M2 (allergic) phenotype, among other cells, in allergy15,16. CCL17 and CCL22 can be thought of as biomarkers of allergic inflammation as they are readily detected in the lungs during airway exacerbations16,17,18. Importantly, CCR4 expression is elevated in comparison to untreated controls, as revealed in bioinformatic data generated from ILC2 isolated from house dust mite treated animals, and similarly ILC2 from naïve animals treated ex vivo with IL-33 (allergen-promoting innate cytokine) upregulates CCR419,20. Furthermore, according to data for ILC2 in the Immunological Genome Project database (www.immgen.org), CCR4 mRNA is highly expressed in these innate immune cells. To date, little is known regarding trafficking of ILC2 into tissues, but it is likely that the ILC2 and CD4+ T cells use similar chemokines and receptors for chemotaxis and chemokinetics as they express similar transcription factors and receptors. Thus, we compared CCL17 versus CCL22 responsiveness, of ILC2 and CD4+ T lymphocytes, from both male and female, OVA-challenged animals.
Protocol
All methods described here were reviewed and approved by the Institutional Animal Care and Use Committees at the University of Nebraska Medical Center (UNMC) and the University of Utah.
1. Setup and Preparation of Reagents
- Prepare complete RPMI (Roswell Park Memorial Institute) media.
- Add 10 mL of heat-inactivated fetal bovine serum (FBS) to 90 mL of RPMI.
- Add 1 mL of 100x Penicillin-Streptomycin-Glutamine to 100 mL of 10% FBS RPMI.
- Prepare ILC2 Expansion Media.
- Add IL-2 and IL-33 (20 ng/mL each cytokine) to 10 mL of complete RPMI.
- If stock cytokines are 10 µg/mL, pipette 20 µL of IL-2 and IL-33 into a 15 mL tube containing 10 mL of complete RPMI media.
- Prepare Lung Dissociation Medium.
- Add 50 mg of type 1 collagenase to 250 mL of unsupplemented RPMI.
- Add 2.5 mL of 100x penicillin-streptomycin-glutamine to the 250 mL of media in step 1.3.1.
- Gently mix the media to ensure the type 1 Collagenase is completely dissolved before use.
- Prepare Serum-free RPMI.
- Dilute 1 g of lyophilized bovine serum albumin (BSA) in 200 mL of RPMI.
- Add 2 mL of 100x penicillin-streptomycin-glutamine.
- Gently mix the media to ensure the BSA is completely dissolved in the media before use.
- Prepare Migration Medium with CCL17.
- Acquire 10 mL of serum-free RPMI and add CCL17 [50 ng/mL].
- If stock CCL17 is 10 µg/mL, add 50 µL of CCL17 stock to 10 mL of serum-free RPMI media.
- Acquire 10 mL of serum-free RPMI and add CCL17 [50 ng/mL].
- Prepare Migration Medium with CCL22.
- Acquire 10 mL of serum-free RPMI and add CCL22 [50 ng/mL].
- If stock CCL22 is 10 µg/mL, add 50 µL of CCL22 stock to 10 mL of serum-free RPMI media.
- Prepare CCR4 Antibody Staining Cocktail.
- For 10 tests total, add 5 µL of each of the following antibodies to a 5 mL tube: anti-mouse CCR4, CD19, CD11b, CD45, ST2, and ICOS.
NOTE: Example of how to make antibody cocktail for 10 samples: 0.5 µL of each antibody x 10 samples = 5 µL of each of the antibodies listed in 1.7.1. - For 10 tests total, add 2.5 µL of the following antibodies to the antibody cocktail from step 1.7.1: anti-mouse CD3, CD11c, and NK1.1.
NOTE: Example to complete the antibody cocktail for 10 samples: 0.25 µL x 10 samples = 2.5 µL of CD3, CD11c and NK1.1 antibodies. - Store the CCR4 Antibody Staining Cocktail at 4 °C until ready to add to the samples. Discard antibody cocktail after 1 week if not used.
- For 10 tests total, add 5 µL of each of the following antibodies to a 5 mL tube: anti-mouse CCR4, CD19, CD11b, CD45, ST2, and ICOS.
- Prepare 1x Stabilizing Fixative.
- Add 10 mL of deionized-distilled water to 5 mL of 3x stabilizing fixative concentrate
2. Preparation of Allergen-challenged BALB/c Mice
NOTE: Male and female BALB/c mice were purchased from Charles River (UNMC) or Jackson Laboratories (University of Utah) at 6 to 8 weeks of age.
- After acclimation (1 week), sensitize all animals to OVA.
- Combine 100 µg/mL OVA adsorbed to aluminum hydroxide (20 mg/mL) in a 5 mL polystyrene tube.
- Mix the tube and immediately draw 500 µL of the OVA-alum suspension into a 1 mL, 28 G syringe (insulin syringe).
- Place a mouse in a bell jar containing isoflurane (1–2 mL under a false floor, so that the animal is not standing directly in isoflurane). Allow the mouse to go under anesthesia for approximately 1–2 min, or until the rate of respiration drops.
- Quickly pick up the mouse by the fur on the back and shoulders and inject 100 µL of OVA-alum intraperitoneally per mouse21,22.
- Place the mouse back in its cage and watch to make sure they regain mobility; this should occur within 2–5 min.
- Seven days after sensitization, subject all animals to intranasal (i.n.) 1.5% OVA diluted in sterile saline in a nebulization chamber (Data Sciences International) for 20 min.
- Remove mice from their cages and place them in the animal nebulization chamber. Close the lid on the chamber.
- Attach the nebulization hose to the input spout on the nebulization chamber.
- Add 30 mL of 1.5% OVA diluted in sterile saline to the nebulization cup on the nebulizer.
- Turn on the nebulizer and allow the chamber to fill with mist for 20 min.
- Turn off the nebulizer and let the mist settle.
- Return animals to their cages.
- Repeat step 2.2 for a total of 5 times, on 5 consecutive days, to induce allergic inflammation.
3. Isolation of CD4+ T Cells from Spleens and Lungs of OVA-challenged Mice
- Humanely euthanize all OVA-treated male and female animals by CO2 asphyxiation according to approved IACUC protocols, using 2 to 3 animals per group, per experiment.
- Excise lungs and spleens from animals and place tissues in separate dissociation tubes based on the tissue type and sex of the animal23.
- Dissociate lung tissue in 500 µL of Lung Dissociation media (25 U/mL; collagenase, type 1) in the automated tissue dissociator using the ‘lung’ protocol.
- Repeat 3.2 and 3.3 a total of two times.
- Dissociate spleen tissue in 500 µL of complete RPMI media using the ‘spleen’ protocol on the automated tissue dissociator.
NOTE: The remaining steps should be performed in a Biological Safety cabinet using sterile technique. - Rinse the dissociation tubes containing lung and spleen homogenates with 5 mL of additional Lung Dissociation media or Complete RPMI, respectively.
- Filter cell suspensions through a 40 µm cell strainer and collect into 50 mL conical tubes.
- Incubate lung homogenates for 15–30 min in a 37 °C incubator with 5% CO2 to further dissociate lung tissue.
- Add 5 mL of Complete RPMI to the lung and spleen homogenates and pellet the cells at the bottom of the 50 mL tubes using centrifugation; 378 x g at room temperature (RT) for 5 min.
- Combine splenocytes and lung cells into a single 50 mL conical tube and determine total cell counts using the automated cell counter.
- Adjust male and female cell suspensions to 1 x 108 cells/mL in separation buffer and add to a 5 mL polystyrene tube.
NOTE: The Enrichment Protocol can be adjusted up to 14 mL polystyrene tubes when more cells are acquired from spleen and lungs. This protocol was designed for tissues from 2–3 mice per group; therefore a 5 mL tube should suffice. - Use approximately two thirds of the total cells for ILC2 isolation according to the ILC2 enrichment protocol.
- Add antibody cocktail (50 µL/mL) to the cell suspension and incubate for 5 min at RT.
- Vortex rapid spheres for 30 s and add to the sample at a rate of 75 µL/mL of cell suspension. Gently mix and incubate for 5 min at RT.
- Top the tube up to 3 mL total volume with separation buffer and place in the 8-chamber easy separation magnet. Incubate for 3 min at RT.
- Tip the magnet forward (away from the sphere-antibody-cell complexes adhered to the back of the tube) and pipette off the cell suspension into a clean 5 mL tube.
- Add 1.5 mL of complete RPMI to the tubes and centrifuge at 378 x g for 5 min at RT.
- Pour off the media from the cell pellet and resuspend the ILC2 at 1 x 107 cells per mL.
- Place 100 uL of male and female ILC2 per well in a U-bottom, 96-well plate and add 100 µL of ILC2 Expansion Media to each well.
- Incubate the cells for 4–5 days to expand the ILC2.
- Collect the ILC2 into a 5 mL tube and add up to 4.5 mL of serum-free RPMI. Centrifuge the cells at 378 x g for 5 min at RT.
- Count cells using a hemacytometer and resuspend at 1 x 107 ILC2 per mL in serum-free RPMI.
- Use the remaining cells for the CD4+ T cell isolation procedure, which is conducted according to the mouse CD4+ T cell isolation protocol with few modifications.
- Add rat serum (50 µL/mL) to the CD4 T cell enrichment suspension.
- Add Isolation cocktail (50 µL/mL) to the sample and incubate for 10 min at RT.
- Vortex rapid spheres for 30 s and add to the sample at a rate of 75 µL/mL.
- Gently mix the cell suspension and incubate for 2.5 min and RT
- Top the samples up to 3 mL and place the 5 mL tubes into the 8-chamber easy separation magnet and incubate for 5 min at RT.
- Tip the magnet forward and pipette the cell suspension into a clean 5 mL tube.
- Add 1.5 mL of serum-free RPMI to the tubes and centrifuge at 378 x g for 5 min at RT.
- Pour off the media from the cell pellet and resuspend the CD4+ T cells at 1 x 107 cells per mL in serum-free RPMI.
4. Determine CCR4 Expression on CD4+ T Cells and Group 2 Innate Lymphoid Cells (ILC2) from OVA-challenged Animals by Flow Cytometry
NOTE: The following steps may be performed on an open bench top as they are non-sterile techniques.
- Acquire approximately 1–2.5 x 105 ILC2 cells from step 3.11.10 and 1–2.5 x 106 CD4+ T cells from step 3.12.8 in separate 5 mL tubes.
- Keep an additional tube of at least 5.0 x 104 CD4+ T cells as an unstained control.
- Suspend the cells in 100–200 µL of FACS buffer and add 1 µL of Fc Block to every tube, then incubate on ice (or in a 4 °C refrigerator) for 10 min.
- Add 1–2 mL of FACS Buffer to every tube and centrifuge at 378 x g for 5 min at RT.
- Pour off the supernatant and resuspend the cells in 100–200 µL of FACS Buffer.
- Add 37.5 µL of the CCR4 Antibody Staining Cocktail to the cell suspension in every tube except the tube containing the ‘unstained’ cells.
- Incubate the tubes on ice, or in a 4 °C refrigerator, for 20–30 min.
- Add 1–2 mL of FACS Buffer to every tube and centrifuge at 378 x g for 5 min at RT.
- Pour off the supernatant.
- Repeat steps 4.7 and 4.8.
- Add 250–300 µL of 1x stabilizing fixative (see Table of Materials) to every tube.
- Prepare single-colored bead controls for each antibody in the CCR4 antibody cocktail according to the protocol provided with the compensation beads.
- Vortex the compensation beads.
- Add one drop of the beads to each single-colored control tube.
- Add 1 µL of each antibody in the CCR4 Antibody cocktail to its own labeled tube.
- Gently mix and incubate for 10 min in a 4 °C refrigerator or on ice.
- Add 1–2 mL of FACS Buffer to all the tubes and centrifuge at 378 x g for 5 min at RT.
- Pour off the supernatant and resuspend the beads in 200 µL of FACS buffer.
- Refrigerate the single-colored controls until all the samples are stained and ready to be analyzed on the flow cytometer.
- Analyze the unstained cells, single-colored controls and the experimental samples on the flow cytometer within 24 h of fixation.
5. Ex Vivo Transmigration Procedure
NOTE: The following steps should be performed in a Biological Safety Cabinet, as they require sterile technique.
- Acquire the ILC2 from step 3.11.10 and CD4 T cells from step 3.12.8 and determine the number of transwell inserts needed for the experiment.
NOTE: Example: For 1.2 mL of enriched CD4 T cells from step 3.12.8, multiply 1.2 x 1,000 µL = 1,200 µL; then divide 1,200 uL by 100 µL = 12, the number of inserts needed for CD4 T transmigration. - Gently move the 3 µm transwell inserts from the middle rows of a 24-well plate.
- Add 500 µL of migration media with CCL17 to approximately one-third of the wells.
- Add 500 µL of migration media with CCL22 to another one-third of the wells.
- Add 500 µL of serum-free RPMI containing no chemokine to the last one-third of the wells.
- Clearly label the lid on the plate with the appropriate transmigration media placed in the bottom wells.
- Place the transwell inserts back into the wells containing the various treatments.
- Gently add 100 µL of CD4 T cells or ILC2 to the top well of each insert. Do not mix or pipette the cell suspension up and down in the transwells, as this may confound the results of the experiment.
- Clearly label the lid of the plate with the cell type placed in each well and write the date and time of day that the cells were added.
- Repeat steps 5.2 to 5.9 until all the cells have been placed in transwell inserts with media.
- Gently place the plate in a 37 °C incubator with 5% CO2 for 48 h. Minimize contact with the plate over the incubation period.
6. Quantification of Ex Vivo Transmigration
- Gently remove the plate from the incubator and remove all the transwell inserts from the middle rows into the empty wells just above or below.
- Collect the cells from the bottom and top wells of the transwell inserts into tubes labeled with TOP or BOTTOM, with CCL17, CCL22, or media, with the cell type, and with the replicate number (at least 3 replicates per experiment).
- Rinse the bottom wells with 500 µL of 1x PBS and collect this rinse into the corresponding tube.
- Rinse the top wells with 250 µL of 1x PBS and collect this rinse into the corresponding tube.
- Pellet the cells by centrifugation at 378 x g for 5 min at RT.
- Gently pipette off all supernatant from the cell pellet.
- Resuspend T cells and ILC2 into 50 µL of 1x PBS.
- Take 10 µL of the cell suspensions and add to 90 µL of 0.4% trypan blue.
- Count the cells on the automated cell counter.
- Record %viability.
- Record the cell count per mL for each sample.
- Determine the total number of cells per treatment in the top and the bottom chamber; record the cell counts.
Representative Results
CCR4 expression on CD4+ T cells and ILC2.
For the success of the ex vivo transmigration experiment, it is imperative to determine whether the lymphocytes are responsive to CCL17 and CCL22 through CCR4; therefore, we determined CCR4 expression on both CD4+ T cells and ILC2 by flow cytometry. While it is well known that OVA-specific CD4+ helper T cells express CCR4, less is known of the expression of CCR4 on ILC2. Figure 1 shows representative results of CCR4 expression, comparatively, on CD4+ T cells (Figure 1A,C) and ILC2 (Figure 1B,D) from male and female, OVA-challenged BALB/c mice. Flow cytometry was used to detect CCR4 using a monoclonal antibody conjugated to allophycocyanin (APC). Using One-Way Analysis of Variance (ANOVA), we determined there were no differences in CCR4 expression between male and female hosts (Figure 1A–D), however, the expression of CCR4 on a per cell basis (MFI) on ILC2 was higher in comparison to CD4+ T cells (Figure 1C compared to Figure 1D). These results are important in showing that the ILC2 and CD4+ T cells should respond to CCL17 and CCL22 in the following experiment.
Responsiveness of CD4+ T cells to CCR4 ligands in the top and bottom chambers of a transmigration system.
CD4+ T cells from male, OVA-challenged BALB/c mice were isolated from the lungs and spleens and placed in the top chamber of a transmigration apparatus separated by a 3 µM porous membrane (Figure 2). A summary of the in vivo preparation of OVA-treated mice (Figure 2A) and the transmigration procedure (Figure 2B) are shown for reference. A combination of CCL17 (25 ng/mL) and CCL22 (25 ng/mL) were placed in the top chamber, the bottom chamber or both the top and bottom chambers to confirm (Figure 2C), (1) that the CD4+ T cells from OVA-challenged animals were responsive to CCR4 ligands, and (2) that chemokine-induced migration was an active process by which T cells were moving through the pores in response to the chemokine gradient, and that the lymphocytes were not moving through the pores independent of chemokine. A media (No Chemokine) control was included to show that CD4+ T cells could not migrate through the 3 µM pores without stimulation. In this condition, the highest percentage of cells remained in the top chamber. When the chemokines were placed in the top and bottom chamber simultaneously, we detected 52% of the total T cells in the bottom chamber and 48% of the cells in the top chamber (TOP/BOTTOM treatment). As expected, the distribution of cells moved in response to chemokine placed only in the top or only in the bottom chamber, as we detected the highest percentage of cells in the compartment where chemokine was present.
Responsiveness of CD4+ T cells and ILC2 to CCL17 and CCL22 in an ex vivo transmigration apparatus.
CD4 T cells and ILC2 from male and female, OVA-challenged mice were isolated from lungs and spleens then placed in the top chamber of a transwell transmigration apparatus (Figure 3). The bottom chamber of the apparatus was filled with untreated cell culture media, media containing CCL17, or media containing CCL22. The representative results show that less than 14% (13.37 + 6.5%) of the cells migrated in media control conditions (Figure 3A–D). In response to CCL22, both cell types, regardless of whether they were from male or female hosts, responded to CCL22 (Figure 3A–D), however, the results for CCL17 were less consistent. CCL17 only induced significant migration for the female CD4 T cells and ILC2 in comparison to media alone (Figure 3C,D). CCL17 treatment was not different than media for male CD4+ T cells or male ILC2 (Figure 3A,B), and CCL22 induced greater migration than CCL17 in male ILC2 (Figure 2B).
Suboptimal transmigration results for CD4+ T cells with low viability.
Suboptimal results were generated to provide the researcher an example of what to expect when the transmigration experiment does not work properly (Figure 4). We isolated male CD4+ T cells from animals according to this protocol and placed them in the top well of transmigration system. After the CD4+ T cells were added, however, the plate remained at room temperature for the first 24 h, then the plate was moved into the incubator for the remaining 24 h of the incubation period. Not surprisingly, we detected no migration towards CCL17 and CCL22 (Figure 4A) and the viability of the cells was notably low (<15%) for the cells in the top (Figure 4B). These flawed results highlight the importance of using the correct temperatures and conditions detailed in this protocol to achieve optimum results.
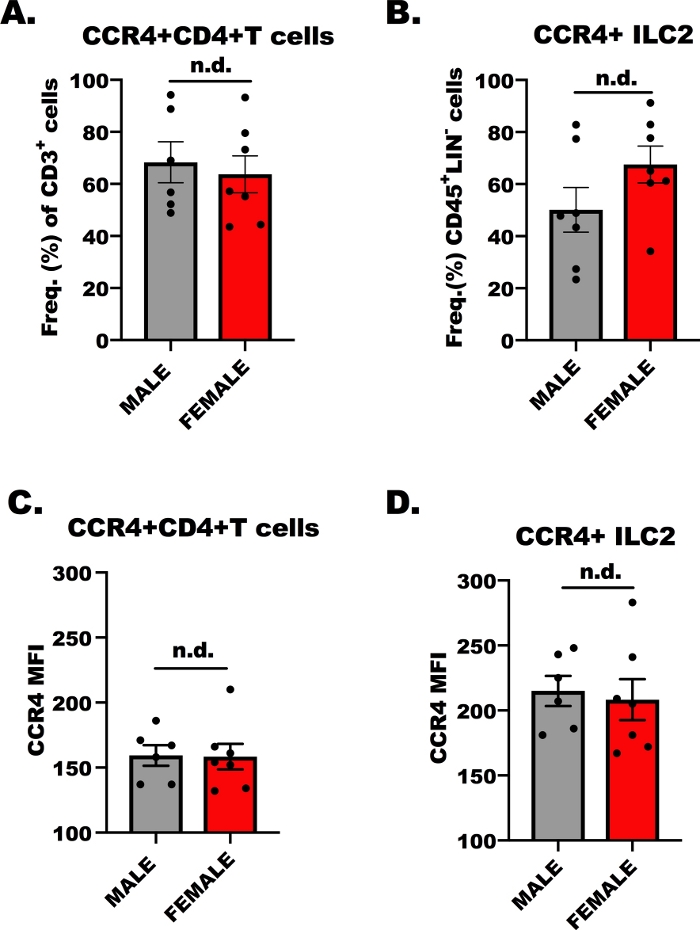
Figure 1: CCR4 expression on CD4+ T cells and ILC2. 7 to 9 week old, male and female, BALB/c mice were injected once with 100 µL of OVA-adsorbed to aluminum hydroxide (500 µg/mL; OVA and 20 mg/mL aluminum hydroxide) 7 days prior to the first of 5, repetitive, daily airway challenges with 1.5% OVA in saline. Allergen-challenged animals were humanely euthanized, and lung and spleen tissue was collected for ILC2 and CD4+ T cells isolation. A small aliquot of cells was then stained and analyzed by flow cytometry to determine the level of CCR4 on each cell type. (A) Frequency of CD4+ T cells that are CCR4+ from OVA+ mice, where the error bars represent the standard error of the mean (+ SEM). (B) Frequency of ILC2 that were CCR4+ (+SEM). (C, D) Mean fluorescence intensity (+SEM) of CCR4 on (C) CD4+ T cells and (D) ILC2. A total of 13 mice were used to generate these data, and the flow experiment was repeated two times, with 3 replicates of each treatment per experiment. Significance was determined by One-Way ANOVA; n.d. indicates there were no differences between groups. Please click here to view a larger version of this figure.
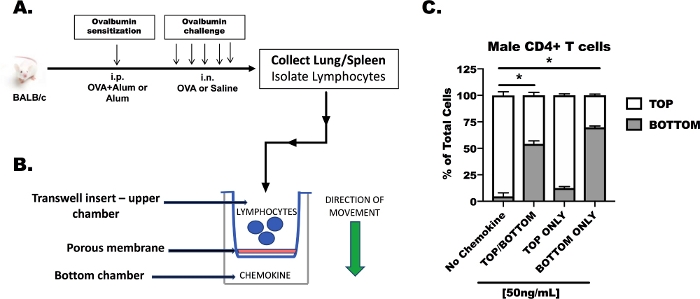
Figure 2: Responsiveness of CD4+ T cells to CCR4 ligands in the top and bottom chambers of a transmigration system. Male BALB/c mice sensitized and challenged with chicken egg ovalbumin (OVA) and CD4+ T cells were isolated from the spleens and lungs (A, B). For this transmigration experiment, CD4+ T cells were suspended in serum-free media at 1 x 107 cells/mL. CCL17 and CCL22 were added to serum-free media at a concentration of 50 ng/mL (25 ng/mL of each chemokine to achieve a total of 50 ng/mL). Chemokine-containing media was added to the top chamber only, to the bottom chamber only, or to both the top and bottom chambers. A total volume of 500 µL of transmigration media was added to the bottom wells and 100 µL of cellular suspension (1 x 106 cells/well) was added to the top well. Transmigration was measured after 48 h in culture (C). These data were generated from a single experiment, 3 OVA-treated, male mice were used for tissue collection, and 3 replicates were made per treatment. Statistical significance was determined by One-Way ANOVA; *p < 0.05. Please click here to view a larger version of this figure.
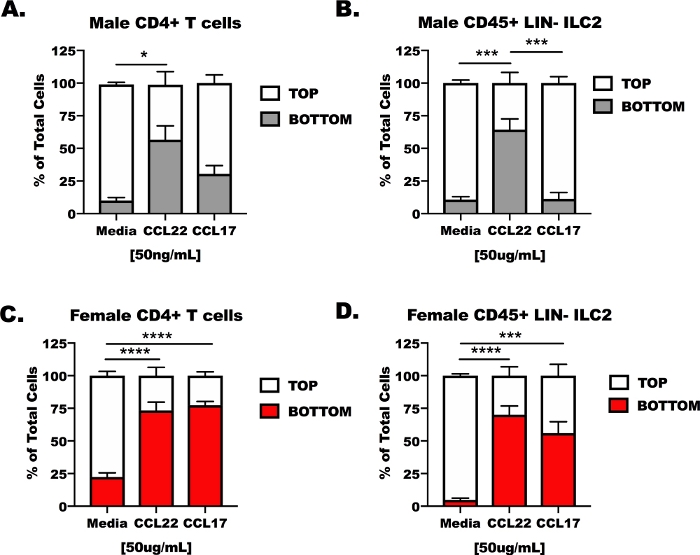
Figure 3: Responsiveness of CD4+ T cells and ILC2 to CCL17 and CCL22 in an ex vivo transmigration apparatus. Mice were prepared as in Figure 1 for CD4+ T cell and ILC2 isolation from spleens and lungs. CD4+ T cells and ILC2 were suspended in serum-free media at 1 x 107 cells/mL. CCL17 or CCL22 were added to serum-free media at a concentration of 50 ng/mL. 500 µL of transmigration media was added to the bottom wells and 100 µL of cellular suspension (1 x 106 cells/well) was added to the top well. Transmigration was measured after 48 h in culture. (A) CD4+ T cells and (B) ILC2 from male hosts were treated with media as control, CCL17, or CCL22. Similarly, (C) female CD4+ T cells and (D) female ILC2 were treated with media, CCL17, or CCL22. A total of 14 mice were used to generate these data. The transmigration experiment was repeated 4 times, with 3–6 replicates of each treatment per experiment. Significance was determined by One-Way ANOVA; *p < 0.05, ***p < 0.001, ****p < 0.0001. Please click here to view a larger version of this figure.
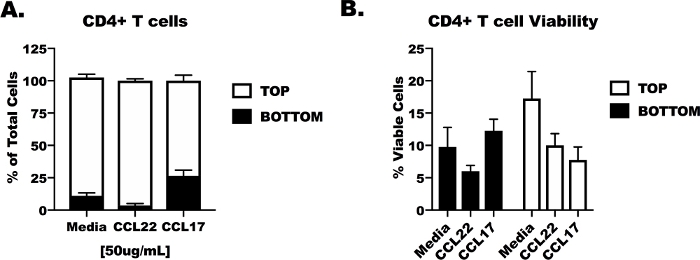
Figure 4: Suboptimal transmigration results for CD4 T cells with low viability. Naïve male BALB/c mice were acquired for lung and spleen tissue collection and CD4+ T cell isolation as in Figure 1, Figure 2, and Figure 3. CD4 T cells were added to the top chamber of the transmigration apparatus and serum-free media containing CCL17, CCL22 or no chemokine (media control) were added to the bottom well. For the first 24 h of the experiment the plate was left at room temperature, then it was moved to a 37 °C incubator with 5% CO2 for an additional 24 h. (A) Percentage of cells remaining in the top and bottom wells after 24 h. (B) Viability of the CD4+ T cells in the top and bottom chamber following poor incubation conditions. These data were generated from a single experiment, 3 naïve male mice were used for tissue collection, and 3 replicates were made per treatment. Statistical significance was not determined. Please click here to view a larger version of this figure.
Discussion
Herein, we present a well-established method for assessing chemokine-induced migration of lymphocytes in an ex vivo transmigration system. There are several critical steps in the protocol, the first of which is verifying the expression of the correct chemokine receptor on the immune cells in the experiment. In our hands, we chose CCR4 because of the body of literature that highlights the importance of CCR4 on Th2 helper T cells in allergic inflammation. Ovalbumin-induced inflammation was shown previously to be limited by at least two CCR4 antagonists24,25; however, this was prior to the discovery of the group 2 innate lymphoid cells (ILC2)26,27. We generated novel data showing that ILC2 cells express higher CCR4 than CD4+ T cells and showed that these cells were consistently responsive to CCL22.
A second critical step to follow in the protocol is to ensure that cells are kept in optimum medium for culture prior to beginning the transmigration part of the protocol. In the case of ILC2, we had to culture these cells in ILC2 Expansion Media that contains both IL-2 and IL-33. IL-2 and IL-7 are both reported in the literature to support ILC2 in culture for up to 14 days28,29. If viability becomes an issue for CD4+ T cells and ILC2 in future experiments, the addition of IL-2 or IL-7 would likely improve survival of the lymphocytes until the endpoint of the experiment. Each of the media presented herein were defined over the course of several experiments and were optimized for use in this protocol14,30,31. In Figure 4, we presented faulty results to demonstrate the importance of using an incubator with proper temperature and 5% CO2. Keeping the transmigration plates in the incubator where they will not be disturbed is another critical step for the success of the protocol.
As stated previously, there are advantages to using the in vivo microscopy available at most institutions, however, in vivo imaging can be time consuming and costly. An alternative experimental procedure that is less costly uses microfluidics in combination with chemokine gradients to understand leukocyte extravasation and tissue migration32,33,34. These systems hold scientific value because they assess the complexities of cell kinetics that involves endothelial cells, which can be grown onto the capillaries of the microfluidic systems. Furthermore, these microfluidic systems assess the importance of adherences proteins (e.g., E-cadherin) on the endothelial cells and integrins on the immune cells in the process of cell adherence under blood flow. Nonetheless these systems require specialized equipment and complex computational programming and statistics to determine the importance of each treatment conditions. Therefore, although the limitation of the transmigration method presented here is that it is artificial in nature, it can be used as an important screening tool to limit the waste of unnecessary reagents in subsequent in vivo methods. The significance of the method is that as new cells are discovered, as is the case for ILC2, we can screen these cells for their responsiveness to known chemokines. This is one of the future applications involving ILC2 and potential therapies that may inhibit their migration into the lungs during asthma exacerbation. This transmigration protocol will be used to screen various inhibitors that may be used to limit CCR4 or other chemotactic mediators involved in recruiting ILC2. Altogether, this ex vivo transmigration protocol will lead to the generation of critical data that can be verified with future in vivo experiments.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by the American Lung Association (K.J.W.), the Memorial Eugene Kenney fund awarded to T.A.W. and K.J.W., generous start-up support from the University of Utah for K.J.W., and a Department of Veterans Affairs award to T.A.W. (VA I01BX0003635). T.A.W. is the recipient of a Research Career Scientist Award (IK6 BX003781) from the Department of Veterans Affairs. The authors wish to acknowledge editorial assistance from Ms. Lisa Chudomelka. The authors thank the UNMC Flow Cytometry core for their support in collecting the flow cytometry data generated for this manuscript.
Materials
| 0.4% Trypan Blue | Sigma-Aldrich | 15250061 | |
| 1 mL syringe | BD Bioscience | 329424 | U-100 Syringes Micro-Fine 28G 1/2" 1cc |
| 100x Penicillin-Streptomycin, L-Glutamine | Gibco | 10378-016 | Dilute to 1x in RPMI media |
| 15 mL conical tubes | Olympus Plastics | 28-101 | polypropylene tubes |
| 3 um transwell inserts | Genesee Scientific | 25-288 | 24-well plate containing 12 transwell inserts |
| 3x stabilizing fixative | BD Pharmigen | 338036 | Prepare 1x solution according to manufacturers protocol |
| 5 mL polystyrene tubes | STEM Cell Technologies | 38007 | |
| 50 mL conical tubes | Olympus Plastics | 28-106 | polypropylene tubes |
| 8-chamber easy separation magnet | STEM Cell Technologies | 18103 | |
| ACK Lysing Buffer | Life Technologies Corporation | A1049201 | |
| Advanced cell strainer, 40 um | Genesee Scientific | 25-375 | nylon mesh, 40 micron strainers |
| Aluminum Hydroxide, Reagent Grade | Sigma-Aldrich | 239186-25G | 20 mg/mL |
| anti- mouse CCR4; APC-conjugated | Biolegend | 131211 | 0.5 ug/test |
| anti-mouse CD11b | BD Pharmigen | 557396 | 0.5 ug/test |
| anti-mouse CD11c; PE eFluor 610 | Thermo-Fischer Scientific | 61-0114-82 | 0.25 ug/test |
| anti-mouse CD16/32, Fc block | BD Pharmigen | 553141 | 0.5 ug/test |
| anti-mouse CD19; APC-eFluor 780 conjugated | Thermo-Fischer Scientific | 47-0193-82 | 0.5 ug/test |
| anti-mouse CD3; PE Cy 7-conjugated | BD Pharmigen | 552774 | 0.25 ug/test |
| anti-mouse CD45; PE conjugated | BD Pharmigen | 56087 | 0.5 ug/test |
| anti-mouse ICOS (CD278) | BD Pharmigen | 564070 | 0.5 ug/test |
| anti-mouse NK1.1 (CD161); FITC-conjugated | BD Pharmigen | 553164 | 0.25 ug/test |
| anti-mouse ST2 (IL-33R); PerCP Cy5.5 conjugated | Biolegend | 145311 | 0.5 ug/test |
| Automated Cell Counter | BIORAD | 1450102 | |
| Automated Dissociator | MACS Miltenyi Biotec | 130-093-235 | |
| Bovine Serum Albumin, Lyophilized Powder | Sigma-Aldrich | A2153-10G | 0.5% in serum-free RPMI |
| Cell Counter Clides | BIORAD | 1450015 | |
| Chicken Egg Ovalbumin, Grade V | Sigma-Aldrich | A5503-10G | 500 ug/mL |
| Collagenase, Type 1, Filtered | Worthington Biochemical Corporation | CLSS-1, purchase as 5 X 50 mg vials (LS004216) | 25 U/mL in RPMI |
| compensation beads | Affymetrix | 01-1111-41 | 1 drop per contol tube |
| Dissociation Tubes | MACS Miltenyi Biotec | 130-096-335 | |
| FACS Buffer | BD Pharmigen | 554657 | 1x PBS + 2% FBS, w/ sodium azide; stored at 4 °C |
| Heat Inactivated-FBS | Genesee Scientific | 25-525H | 10% in complete RPMI & ILC2 Expansion Media |
| mouse CCL17 | GenScript | Z02954-20 | 50 ng/mL |
| mouse CCL22 | GenScript | Z02856-20 | 50 ng/mL |
| mouse CD4+ T cell enrichment kit | STEM Cell Technologies | 19852 | |
| mouse IL-2 | GenScript | Z02764-20 | 20 ng/mL |
| mouse ILC2 enrichment kit | STEM Cell Technologies | 19842 | |
| mouse recombinant IL-33 | STEM Cell Technologies | 78044 | 20 ng/mL |
| RPMI | Life Technologies Corporation | 22400071 | |
| Separation Buffer | STEM Cell Technologies | 20144 | 1 X PBS + 2% FBS; stored at 4C |
| small animal nebulizer and chamber | Data Sciences International | ||
| sterile saline | Baxter | 2F7124; NDC 0338-0048-04 | 0.9% Sodium Chloride |
References
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. The Journal of Experimental Medicine. 115, 453-466 (1962).
- Moser, B. Editorial: History of Chemoattractant Research. Frontiers in Immunology. 6, 548 (2015).
- Borroni, E. M., Savino, B., Bonecchi, R., Locati, M. Chemokines sound the alarmin: The role of atypical chemokine in inflammation and cancer. Seminars in Immunology. 38, 63-71 (2018).
- Charo, I. F., Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. The New England Journal of Medicine. 354 (6), 610-621 (2006).
- Abboud, D., Hanson, J. Chemokine neutralization as an innovative therapeutic strategy for atopic dermatitis. Drug Discovery Today. 22 (4), 702-711 (2017).
- Aldinucci, D., Casagrande, N. Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer. International Journal of Molecular Sciences. 19 (5), E1477 (2018).
- Chonco, L., et al. Novel DNA Aptamers Against CCL21 Protein: Characterization and Biomedical Applications for Targeted Drug Delivery to T Cell-Rich Zones. Nucleic Acid Therapy. 28 (4), 242-251 (2018).
- Trivedi, P. J., Adams, D. H. Chemokines and Chemokine Receptors as Therapeutic Targets in Inflammatory Bowel Disease; Pitfalls and Promise. Journal of Crohn’s and Colitis. 12 (12), 1508 (2018).
- Pietrosimone, K. M., Bhandari, S., Lemieux, M. G., Knecht, D. A., Lynes, M. A. In vitro assays of chemotaxis as a window into mechanisms of toxicant-induced immunomodulation. Current Protocols in Toxicology. 58 (Unit 18.17), (2013).
- Davis, D. M. How studying the immune system leads us to new medicines. Lancet. 391 (10136), 2205-2206 (2018).
- Culley, F. J., Pennycook, A. M., Tregoning, J. S., Hussell, T., Openshaw, P. J. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. Journal of Virology. 80 (9), 4521-4527 (2006).
- Denney, H., Clench, M. R., Woodroofe, M. N. Cleavage of chemokines CCL2 and CXCL10 by matrix metalloproteinases-2 and -9: implications for chemotaxis. Biochemical and Biophysics Research Communications. 382 (2), 341-347 (2009).
- Burrell, B. E., et al. Lymph Node Stromal Fiber ER-TR7 Modulates CD4+ T Cell Lymph Node Trafficking and Transplant Tolerance. Transplantation. 99 (6), 1119-1125 (2015).
- Warren, K. J., Iwami, D., Harris, D. G., Bromberg, J. S., Burrell, B. E. Laminins affect T cell trafficking and allograft fate. The Journal of Clinical Investigation. 124 (5), 2204-2218 (2014).
- Hirata, H., et al. Th2 cell differentiation from naive CD4(+) T cells is enhanced by autocrine CC chemokines in atopic diseases. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. , (2018).
- Lin, R., Choi, Y. H., Zidar, D. A., Walker, J. K. L. beta-Arrestin-2-Dependent Signaling Promotes CCR4-mediated Chemotaxis of Murine T-Helper Type 2 Cells. American Journal of Respiratory Cell and Molecular Biology. 58 (6), 745-755 (2018).
- Zhang, Y., et al. A new antagonist for CCR4 attenuates allergic lung inflammation in a mouse model of asthma. Science Reports. 7 (1), 15038 (2017).
- Lu, Y., et al. Dynamics of helper CD4 T cells during acute and stable allergic asthma. Mucosal Immunology. 11 (6), 1640-1652 (2018).
- Li, B. W. S., Beerens, D., Brem, M. D., Hendriks, R. W. Characterization of Group 2 Innate Lymphoid Cells in Allergic Airway Inflammation Models in the Mouse. Methods in Molecular Biology. 1559, 169-183 (2017).
- Li, B. W. S., et al. Group 2 Innate Lymphoid Cells Exhibit a Dynamic Phenotype in Allergic Airway Inflammation. Frontiers in Immunology. 8, 1684 (2017).
- Warren, K. J., et al. Sex differences in activation of lung-related type-2 innate lymphoid cells in experimental asthma. Annals of Allergy, Asthma, Immunology: Official Publication of the American College of Allergy, Asthma, Immunology. , (2016).
- Warren, K. J., et al. Ovalbumin-sensitized mice have altered airway inflammation to agriculture organic dust. Respiratory Research. 20 (1), 51 (2019).
- Poole, J. A., et al. alphabeta T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease. Annals of Allergy, Asthma, Immunology: Official Publication of the American College of Allergy, Asthma, Immunology. 109 (4), 266-273 (2012).
- Matsuo, K., et al. A CCR4 antagonist ameliorates atopic dermatitis-like skin lesions induced by dibutyl phthalate and a hydrogel patch containing ovalbumin. Biomedical Pharmacotherapy. 109, 1437-1444 (2019).
- Mikhak, Z., et al. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. The Journal of Allergy and Clinical Immunology. 123 (1), 67-73 (2009).
- Monticelli, L. A., et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunology. 12 (11), 1045-1054 (2011).
- Saenz, S. A., et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 464 (7293), 1362-1366 (2010).
- Hoyler, T., et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 37 (4), 634-648 (2012).
- Nakajima, H., Shores, E. W., Noguchi, M., Leonard, W. J. The common cytokine receptor gamma chain plays an essential role in regulating lymphoid homeostasis. The Journal of Experimental Medicine. 185 (2), 189-195 (1997).
- Warren, K. J., et al. RSV-specific anti-viral immunity is disrupted by chronic ethanol consumption. Alcohol. , (2016).
- Warren, K. J., Poole, J. A., Sweeter, J. M., DeVasure, J. M., Wyatt, T. A. An association between MMP-9 and impaired T cell migration in ethanol-fed BALB/c mice infected with Respiratory Syncytial Virus-2A. Alcohol. , (2018).
- Molteni, R., et al. A novel device to concurrently assess leukocyte extravasation and interstitial migration within a defined 3D environment. Lab on a Chip. 15 (1), 195-207 (2015).
- Bersini, S., et al. Human in vitro 3D co-culture model to engineer vascularized bone-mimicking tissues combining computational tools and statistical experimental approach. Biomaterials. 76, 157-172 (2016).
- Jeon, J. S., et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proceedings of the National Academy of Sciences of the United States of America. 112 (1), 214-219 (2015).

