Reliable Isolation of Central Nervous System Microvessels Across Five Vertebrate Groups
Summary
The goal of this protocol is to isolate microvessels from multiple regions of the central nervous system of lissencephalic and gyrencephalic vertebrates.
Abstract
Isolation of microvessels from the central nervous system (CNS) is commonly performed by combining cortical tissue from multiple animals, most often rodents. This approach limits the interrogation of blood-brain barrier (BBB) properties to the cortex and does not allow for individual comparison. This project focuses on the development of an isolation method that allows for the comparison of the neurovascular unit (NVU) from multiple CNS regions: cortex, cerebellum, optic lobe, hypothalamus, pituitary, brainstem, and spinal cord. Moreover, this protocol, originally developed for murine samples, was successfully adapted for use on CNS tissues from small and large vertebrate species from which we are also able to isolate microvessels from brain hemisphere white matter. This method, when paired with immunolabeling, allows for quantitation of protein expression and statistical comparison between individuals, tissue type, or treatment. We proved this applicability by evaluating changes in protein expression during experimental autoimmune encephalomyelitis (EAE), a murine model of a neuroinflammatory disease, multiple sclerosis. Additionally, microvessels isolated by this method could be used for downstream applications like qPCR, RNA-seq, and Western blot, among others. Even though this is not the first attempt to isolate CNS microvessels without the use of ultracentrifugation or enzymatic dissociation, it is unique in its adeptness for the comparison of single individuals and multiple CNS regions. Therefore, it allows for investigation of a range of differences that may otherwise remain obscure: CNS portions (cortex, cerebellum, optic lobe, brainstem, hypothalamus, pituitary, and spinal cord), CNS tissue type (gray or white matter), individuals, experimental treatment groups, and species.
Introduction
Our brain is the most important organ in our body. For this reason, keeping brain homeostasis despite external factors that may trigger a deviation from normalcy is a priority. According to some scholars, about 400–500 million years ago1, vertebrate animals developed what we now know as the blood-brain barrier (BBB)2,3. This protective "fence" exerts the greatest influence over central nervous system (CNS) homeostasis and functions by tightly regulating the transport of ions, molecules, and cells between blood and CNS parenchyma. When the BBB is disrupted, the brain becomes susceptible to toxic exposure, infection, and inflammation. Therefore, BBB dysfunction is associated with many, if not all, neurological and neurodevelopmental disorders4,5,6.
The sophisticated function of the BBB is attributed to the unique CNS microvasculature conformed by the neurovascular unit (NVU)2,3. Highly specialized endothelial cells, pericytes, and astrocytic end-feet are the cellular components of the NVU2,3. The extracellular matrix generated by these cells is also essential to the NVU and BBB physiology2,3. Although essential cellular and molecular components of the NVU are conserved among vertebrates, heterogeneity is reported among orders and species7,8. However, technical limitations impede our ability to fully consider these differences in neurobiology, biomedical, or translational research.
Because of this, we expanded a CNS region-specific microvessel-isolation method to make it applicable to numerous species from all five vertebrate groups: fish, amphibians, reptiles, birds, and mammals. The protocol is described for use on small-lissencephalic and large-gyrencephalic vertebrates, including species with translational relevance9. Additionally, we include other regions of the CNS not investigated before within this context, but relevant to neurophysiology and with tremendous clinical implications: the hypothalamus, pituitary, and white matter. Lastly, we tested the capacity of this isolation method as a reliable tool to identify changes in protein expression along the NVU and/or BBB9,10,11. As a proof-of-concept, we showed how to determine changes in VCAM-1 and JAM-B expression during EAE using the isolation method followed by immunofluorescence.
Protocol
All procedures of the present study are in accordance with the guidelines set by the University of California (UC), Davis Institutional Animal Care and Use Committee (IACUC). Animal care at UC Davis is regulated by several independent resources and has been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) since 1966. Porcine CNS tissues were obtained from UCD Department of Animal Sciences, Meat Sciences Laboratory. CNS tissues from rhesus macaques were obtained from the California National Primate Research Center Pathology Department (NIH P51OD011107). No anesthesia, euthanasia, or necropsy was performed by laboratory staff on pigs and macaques. Therefore, there are no particular recommendations regarding these matters.
NOTE: This method was validated for multiple species, but the provided protocol corresponds more directly to mouse and porcine tissues. Details pertaining to the optic lobe do not apply when using mammalian specimens. All biohazardous materials must be handled in an appropriate biosafety level (BSL) facility. All acutely toxic materials must be handled underneath a fume hood. All biohazardous medical waste and acutely toxic waste must be disposed of properly.
1. Preparation
- Prepare solutions (Table 1) at least one day ahead.
NOTE: It is very difficult to dissolve 70 kDa molecular weight (MW) dextran. It is more efficient to let the solution stir overnight covered with either paraffin film or foil. The protocol requires the use two different MV-2 solutions depending on the specimen under investigation. The 18% dextran efficiently separates the myelin from the microvessel pellet when performing the protocol on small lissencephalic specimens. However, it develops a smear of myelin within the interface of CNS tissues from large gyrencephalic specimens, as well as the hypothalamus and the pituitary from small specimens. This smear is prevented by using 20% dextran. - Prepare well-slides by loading 50 µL per well of poly-D-lysine and let dry for 2 h at room temperature (RT), preferably in a biological safety cabinet-class 2 (BSC-2). Do not overdry. Rinse twice with phosphate-buffered saline (PBS) and keep in the fridge until ready to use.
2. CNS Tissue Dissection from Small Lissencephalic Vertebrate Specimen
NOTE: The article demonstrates the application of the protocol on a C57BL6/J, 10 week-old, ~25 g, male mouse.
- Prepare two 15 mL conical tubes and a 1.7 mL microcentrifuge tube with 5 mL and 1 mL, respectively, of MV-1 solution per specimen. Keep tubes on ice.
- Anesthetization
- Anesthetize mice by intraperitoneal injection of a ketamine, xylazine, and acepromazine anesthetic cocktail at 100/10/3 mg per kg body weight and spray with 70% ethanol. Confirm anesthesia by assessing the absence of palpebral and pedal reflexes by touching an eye and pinching a foot, respectively.
- Anesthetize pigeons by inhalation of 5% isoflurane using an induction anesthesia box.
- Anesthetize fish and frogs in 1% Tricaine in either a fish holding water tank or amphibian Ringer's solution (Table of Materials), respectively.
- Anesthetize lizards by cooling at 4 °C prior to euthanasia.
- Decapitate the animal with surgical scissors, peel away the skin with forceps to expose the skull, and cut with LaGrange scissors through the foramen magnum (Figure 1A).
- Dissect the brain out with a spatula and transfer to a 15 mL conical tube with MV-1 solution. Keep tube on ice.
- Retrieve the pituitary from the skull's sella turcica with forceps and transfer to a 1.7 mL microcentrifuge tube with MV-1 solution. Keep tube on ice.
- Remove the skin and muscle to expose the vertebral column. Cut out the limbs, rib cage, and internal organs (Figure 1B).
- Dissect the spinal cord by one of the two methods described below. Then, transfer to a 5 mL conical tube with MV-1 solution. Keep tube on ice.
- Perform laminectomy by cutting in a rostral to caudal direction with LaGrange scissors, starting through the cervical vertebral foramen, until reaching the lumbar cord.
- Perform flushing in a caudal to rostral direction throughout the lumbar vertebral foramen with an 18 G needle and 10 mL syringe loaded with MV-1 solution (Figure 1C,D).
NOTE: This method only works with very small specimens, mostly rodents (<100 g).
- Using a dissecting scope, cut the dural sac from the spinal cord with spring scissors and remove the remaining meninges with a double-pronged pick (Figure 2).
- Transfer the brain to a Petri dish and, using a dissecting scope, remove the meninges with a double-pronged pick (Figure 2A–C).
- Excise the olfactory bulbs and thalamus with forceps, iris scissors, and spring scissors. Using the double-pronged pick, remove the choroid plexus from all the brain ventricles. Make sure to remove all the choroid plexus.
NOTE: The choroid plexus and olfactory bulbs are a massive source of contamination. In contrast to the BBB, these capillaries are highly fenestrated and the results of the subsequent experiments will be compromised if they are not removed. - Using forceps, separate the distinct CNS regions: cortex, cerebellum, brainstem, optic lobe (not on mammalian specimens), and hypothalamus.
3. CNS Tissue Dissection from a Large Gyrencephalic Vertebrate Specimen
NOTE: This protocol uses porcine CNS tissues obtained from an abattoir. Therefore, no anesthesia, euthanasia, or necropsy is described or shown here.
- Transport porcine CNS tissues in a 0.946 L (32 oz) histology container loaded with MV-1 solution inside an ice chest/bucket.
- Using a dissecting scope, remove the meninges with a double-pronged pick, forceps, iris scissors, and spring scissors from each CNS tissue. Make sure to remove any pieces of choroid plexus from the brain ventricles.
4. CNS Tissue Homogenization
NOTE: It is more efficient when two investigators engage in the homogenization process: one dissecting the meninges under the stereoscope and the other homogenizing the minced tissues. This way, the tissues are quickly returned to the ice bucket and kept cold.
- Place each CNS region on a Petri dish with ~1 mL of MV-1 solution. Using a single-edge blade, mince the tissue to obtain 1–2 mm pieces.
- For a small vertebrate specimen, use a 100 mm x 20 mm Petri dish.
- For a large vertebrate specimen, use a 150 mm x 15 mm Petri dish.
- Homogenization of a small vertebrate specimen (Table 2 and Table 3)
- Transfer the cortex, cerebellum, brainstem, optic lobe, and spinal cord to ~1 mL of MV-1 solution in an individual 10 mL Potter-Elvehjem glass tissue grinder with a PTFE pestle using a transfer pipette. Add 5 mL of MV-1 solution and homogenize each tissue (~10 strokes). Transfer to individual 15 mL conical tubes. Keep tubes on ice.
NOTE: For a small vertebrate, 5 or 4, with or without optic lobe, respectively, 10 mL Potter-Elvehjem grinders and 15 mL conical tubes are needed. - Transfer the hypothalamus and pituitary with forceps to ~100 µL of MV-1 solution in an individual 1.7 mL tube and carefully homogenize with a glass micropestle. Rinse each micropestle with ~1 mL of MV-1 solution. Keep tube on ice.
NOTE: For a small vertebrate, 2 glass micropestles and 1.7 mL tubes are needed.
- Transfer the cortex, cerebellum, brainstem, optic lobe, and spinal cord to ~1 mL of MV-1 solution in an individual 10 mL Potter-Elvehjem glass tissue grinder with a PTFE pestle using a transfer pipette. Add 5 mL of MV-1 solution and homogenize each tissue (~10 strokes). Transfer to individual 15 mL conical tubes. Keep tubes on ice.
- Homogenization of a large vertebrate specimen (Table 4 and Table 5)
- Using a transfer pipette, transfer the minced tissue to a 55 mL Potter-Elvehjem glass tissue grinder with a PTFE pestle and attach to overhead stirrer. Add half of the recommended volume of MV-1 solution, according to the specific CNS portion being homogenized (Table 5).
- Turn on the overhead stirrer at very low speed (~150 rpm) and carefully move the glass tube up and down for about 30 s.
- Turn off overhead stirrer, add more MV-1 solution, and repeat step 4.3.2 until obtaining a homogenous slurry.
- Transfer to a 50 mL conical tube. Keep tube on ice.
- Wash the grinder with deionized water between each CNS tissue homogenization.
NOTE: For a large vertebrate 5 or 4, with or without white matter, respectively, 50 mL conical tubes are needed. Likewise, two (2) 15 mL conical tubes are needed for the hypothalamus and pituitary.
5. Microvessel Purification
- Centrifuge tissue homogenates resulting from step 4.2.1, 4.2.2, or 4.3.4 at 2,000 x g for 10 min at 4 °C. A large white interface of myelin will form on the top of the reddish microvessels' pellets. Discard the supernatants.
- Small vertebrate specimen (Table 2 and Table 3)
- Resuspend the cortex, cerebellum, brainstem, optic lobe, and spinal cord pellets in 5 mL of ice-cold MV-2 solution with a 5 mL serological pipette (~10 flushes). Add 5 mL of ice-cold MV-2 solution to each suspension and carefully mix by flipping the tubes.
- Resuspend the hypothalamus and pituitary pellets with 1 mL of MV-2 solution.
- Large vertebrate specimen (Table 4 and Table 5)
- Add 20 mL of ice-cold MV-2 solution to the cortex, cerebellum, brainstem, and spinal cord microvessel pellets. Resuspend the pellets by mixing on a tube revolver shaker, stirring at 40 rpm for ~5 min. Balance the conical tubes' volumes of the cortex, cerebellum, brainstem, and spinal cord suspensions by adding more MV-2 solution.
- Resuspend the hypothalamus and pituitary microvessel pellets in 5 mL of MV-2 solution with a 5 mL serological pipette (~10 flushes). Add 5 mL of ice-cold MV-2 solution to the suspensions and carefully mix by flipping the tube.
- Small vertebrate specimen (Table 2 and Table 3)
- Centrifuge at 4,400 x g for 15 min at 4 °C.
- Carefully detach the thick and dense myelin layer on the liquid interface from each tube's walls by slowly rotating the tubes and allowing the supernatant to pass along the walls.
- Discard the myelin and liquid interface and blot the inside wall of each tube with a spatula wrapped with a low-lint paper wipe. For small vertebrate specimens, remove myelin layer from each hypothalamus and pituitary tube by suctioning carefully with a transfer pipette.
- Wipe out excess liquid with a twisted low-lint paper wipe. Resuspend each pellet with 1 mL of MV-3 solution using low-binding tips. Keep tubes on ice.
6. Microvessel Elution and Filtration
- Pour ice-cold MV-3 solution in individual beakers for each CNS region. Keep at 4 °C.
- For small vertebrate specimens, use 10 mL of MV-3 solution per 50 mL beaker. A total of 6–7 beakers is necessary depending on whether or not the optic lobe is included.
- For large vertebrate specimens, use 30 mL of MV-3 solution per 100 mL beaker. A total of 6–7 beakers is necessary depending on whether or not white matter is included.
- Place a cell strainer on top of a 50 mL conical tube. Use one per CNS region. Use a 100 µm strainer for the cortex, brainstem, optic lobe, spinal cord, and pituitary. Use a 70 µm cell strainer for the cerebellum and hypothalamus.
- Wet each strainer with 1 mL of ice-cold MV-3 solution.
- Add more MV-3 solution to the suspensions prepared in step 5.5 with a serological pipette while mixing to avoid aggregates. Carefully load microvessels on top of the strainer. Rinse with ice-cold MV-3 solution.
- For small vertebrate specimens (Table 2 and Table 3), add 10–15 mL of MV-3 solution with a serological pipette and rinse with 5 mL of MV-3 solution.
- For large vertebrate specimens, add 20 mL of MV-3 solution with a serological pipette and rinse with 10 mL of MV-3 solution.
- Assemble the filtration unit by placing a 20 µm nylon net filter on a modified filter holder (Figure 2D), one per CNS region.
- For small vertebrate specimens (Table 2 and Table 3), use 25 mm modified filter holders for the cortex, cerebellum, brainstem, optic lobe, and spinal cord. Use 13 mm modified filter holders for the hypothalamus and pituitary.
- For large vertebrate specimens (Table 4 and Table 5), use 47 mm modified filter holders for the cortex, cerebellum, brainstem, and spinal cord. Use 25 mm for the hypothalamus and pituitary.
- Place filter on top of a 50 mL conical tube and wet filter with 5 mL of ice-cold MV-3 solution making sure buffer pours down the filter holder to the conical tube.
- Transfer the eluted microvessels (from step 6.4) on top of the 20 µm nylon net filter and rinse the microvessels with 5–10 mL of ice-cold MV-3 solution.
- Recover the filter using clean forceps and immerse it in the beaker containing the ice-cold MV-3 solution prepared in step 6.1.
- Detach the microvessels from the filter by shaking it gently for about 30 s. For small vertebrate specimens, pour the beaker content in a 15 mL conical tube. For large vertebrate specimens, pour the beaker content in a 50 mL conical tube.
- Centrifuge at 2,000 x g for 5 min at 4 °C and resuspend the pellet in 1 mL of ice-cold MV-3 solution using a low-binding pipette tip.
- For small vertebrate specimens, transfer the suspension (from step 6.10) into a 1.7 mL microcentrifuge tube and centrifuge at 20,000 x g for 5 min at 4 °C.
NOTE: This spin is performed on a benchtop centrifuge at maximum speed (~20,000 x g) to ensure a robust yield. - For large vertebrate specimens, transfer the suspension from step 6.10 into a 5.0 mL microcentrifuge tube, add 4 mL of MV-3 solution, and centrifuge at 2,000 x g for 5 min at 4 °C.
- For small vertebrate specimens, transfer the suspension (from step 6.10) into a 1.7 mL microcentrifuge tube and centrifuge at 20,000 x g for 5 min at 4 °C.
7. Immunostaining
NOTE: Hematoxylin and eosin (H&E) staining was performed on reptile, amphibian, and fish specimens as a proof-of-concept of the protocol feasibility. Therefore, there is no recommendation for immunolabeling for these specimens.
- Remove the supernatant from the microcentrifuge tubes.
- Resuspend the pellet in 1x sterile PBS using a low-binding pipette tip (Table of Materials). Keep the microvessels from forming aggregates by pipetting multiple times. For small vertebrate specimens (Table 3), use ~100 µL–2,000 µL according to the pellet size. For large vertebrate specimens (Table 5), use ~1,000 µL–4,000 µL according to the pellet size.
- Using a low-binding pipette tip, transfer the microvessels to well slides, being careful to add them to the center of each well, avoiding the sidewalls.
- Set the well slides, uncovered, inside a BSC-2, and let dry for 20 to 30 min at RT.
NOTE: A relatively high volume of 1x PBS is used to avoid aggregate formation. Because of this, it is necessary to let the slides dry prior to fixation to make sure the microvessels are being held by the poly-D-lysine coating. - Remove residual 1x PBS by pipetting out with a transfer pipette, add 200 µL of 4% paraformaldehyde (PFA) and incubate for 30 min at RT.
- Pipette out the fixative solution and wash 3x with 200 µL of 1x PBS for 5 min at RT.
NOTE: H&E staining on fish, amphibian, and reptile CNS microvessels was performed at this point. - Add 200 µL of blocking buffer to the well slide and incubate for 60 min at 37 °C.
- Remove the blocking buffer with a transfer pipette and add 200 µL of the primary antibody cocktail in antibody diluent (Table of Materials). Incubate at 4 °C overnight.
- Pipette out the primary antibody cocktail and wash 3x with 200 µL of 1x PBS for 5 min at RT.
- Load the secondary antibody cocktail (Table of Materials) diluted in 1x PBS and incubate for 2 h at RT, protected from light.
- Pipette out the secondary antibody cocktail and wash 3x with 200 µL of 1x PBS for 5 min at RT, protected from light. After the last wash detach the well-slide's frame and blot-dry any excess 1x PBS with a low lint paper wipe.
- Add a coverslip, liquid antifade mountant medium, and 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining. Let dry overnight at RT protected from light.
- Once dry, keep protected from light on a slide box at 4 °C until ready for confocal microscopy and data acquisition.
Representative Results
Microvessels isolated from murine CNS showed all intrinsic cellular components of the neurovascular unit2,3. Using either platelet endothelial cell adhesion molecule-1 (PECAM, also known as CD31) or isolectin IB4 (a glycoprotein that binds the endothelial cell glycocalyx) for endothelial cells, platelet derived growth factor-β (PDGFRβ) or neuron-glial antigen 2 (NG2) for pericytes and aquaporin-4 (AQP4) for astrocytic end-feet (Figure 3A,B) demonstrates that these intrinsic components are present in isolated microvessels. CNS regions visible include the cortical microvessels (Figure 3B), cerebellum (Figure 3C), pituitary (Figure 3D), hypothalamus (Figure 3E), brainstem (Figure 3F), and spinal cord (Figure 3G). All showed expression of these canonical markers.
Likewise, the microvessels showed expression of adherens junction protein VE-cadherin (Figure 4A,C), tight junction proteins claudin-5 and zonula occludens-1 (CLDN5 and ZO-1, Figure 4A,D), tricellular junction protein angulin-1 (Figure 4A,E) and apicobasal markers C-X-C motif chemokine ligand 12 and gamma-glutamyltransferase 1 (CXCL12 and GGT1, Figure 4A,F). These findings are relevant because these proteins are indicators of pivotal BBB-specific properties9,12,13,14. A limited presence of astrocytes, oligodendrocytes, and neurons expressing glial fibrillary acidic protein (GFAP, Figure 4B,C,G), oligodendrocyte specific protein (OSP, Figure 4B,G), and neurofilament-medium (NFM, Figure 4B,H), respectively, demonstrates that isolated microvessels had negligible traces of unwanted non-neurovascular unit cells. Moreover, the majority of the microvessels were devoid of the expression of the α-smooth muscle actin (αSMA, Figure 5B,C). This is relevant because αSMA is a marker for smooth muscle cells associated with arterioles and venules (Figure 5A), indicating that this isolation protocol selectively targets small caliber microvessels15.
Microvessels obtained from other small lissencephalic vertebrates share some morphological features, as seen in fish (frog and lizard not shown) and avian microvessels. This suggests that this method is useful for further characterization of differences in NVU between species (Figure 6). Moreover, avian CNS microvessels (Figure 6H–N) showed crossreactivity with many of the antibodies developed for human and mouse. This encourages further investigation of avian specimens for biological and biomedical BBB studies. We observed similar results when we isolated microvessels from pigs and macaques, two gyrencephalic species (Figure 7). Only NVU canonical markers in porcine microvessels are shown. In addition to the aforementioned CNS regions, we were able to isolate microvessels from periventricular white matter (Figure 7B). This is relevant because white matter is implicated in neuroinflammatory conditions (e.g., multiple sclerosis16,17,18).
We wanted to find out if this method could be used as a reliable tool to determine changes in protein expression. Knowing that VCAM-1 and JAM-B have been implicated in the neuroinflammatory process at the spinal cord during EAE, we decided to quantitate the expression of these proteins at peak and chronic stages of EAE and sham-control mice (10 week-old C57BL/6J mice)9,10,11. To do this, we measured the arbitrary unit of intensity (AUI) along the microvessels' diameter. Then, using a threshold of ≤20 AUI for DAPI, we calculated the area under the curve (AUC) for VE-cadherin, VCAM-1, and JAM-B for 50 microvessels per CNS region (Figure 8A,B). Lastly, we performed two-way ANOVA followed by Sidak's post-hoc test to determine the statistical significance of changes in protein expression.
As anticipated, we observed an increase in VCAM-1 and JAM-B along spinal cord microvessels during the peak of EAE (Figure 8D,E). However, we observed changes in other CNS regions not previously reported for EAE. VCAM-1 significantly increased in pituitary microvessels and decreased in the hypothalamus and brainstem microvessels. There were changes during chronic EAE in all CNS tissues (Figure 8D). JAM-B significantly increased in the hypothalamus and pituitary microvessels during chronic EAE (Figure 8E). Interestingly, we observed changes in VE-cadherin along microvessels isolated from the hypothalamus and brainstem during the peak of EAE, the cerebellum during chronic EAE (Figure 8C), and a decrease in pituitary microvessel width during peak and chronic EAE. Overall, this data suggests that this method of microvessel isolation is a useful tool to characterize changes in regional protein expression patterns during health and disease.
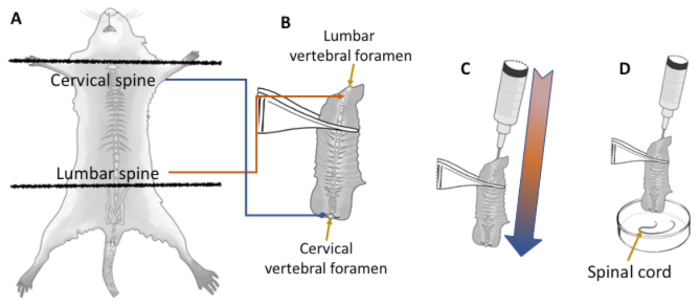
Figure 1: Efficient spinal cord dissection without laminectomy. Dissection of the spinal cord from small vertebrate specimens (up to 100 g) is performed faster and more efficiently by flushing the cord in a caudal to rostral direction throughout the vertebral foramen. Using dissecting scissors the head is removed by the atlas joint and the spine is cut out by the hip (A). All remaing organs are removed, leaving only the spine (B). The spinal cord within the lumbar vertebral foramen appears as a very small white circle (<1 mm diameter) compared to the width of the cervical cord (B, yellow arrows). Keeping a narrow opening at the lumbar vertebral foramen facilitates a pressure gradient from the lumbar to cervical spine when flushing with an 18 G needle and 10 cc syringe loaded with 1x PBS (C and D). Once flushed, the spinal cord (D, yellow arrow) is almost totally devoided of the dural sac, and only pia needs to be removed under the dissecting scope. Please click here to view a larger version of this figure.
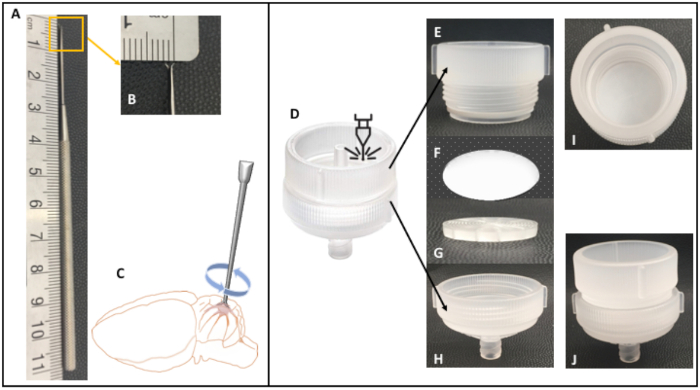
Figure 2: Double-pronged pick dissecting tool and modified filter holder. This 11 cm long dissecting tool (A) has two very sharp, nearly horizontally opposed points, 2 mm tip-to-tip (B). By applying gentle downward pressure and twisting slightly in a clockwise fashion (C), the points embed themselves horizontally into the meninges so that they can be lifted upward. This is especially useful when removing the meninges from the cerebellum, because it allows access into the depth of the cerebelli folia. It is also very useful when removing the meninges from cortical tissue, especially gyrencephalic. In this case, the pia is highly embedded with high caliber vasculature that will compromise the purity of the microsevessel isolation. Likewise, it facilitates the removal of the choroid plexus, which is the likeliest source of contamination. Filter holders of 47 mm, 25 mm, and 13 mm diameter were laser cut to remove the inlet-connector (D) from the top compartment (E), but keep the sieve component (G). This modification allowed for the assembly of a filter unit (I,J) by placing the 20 µm nylon filter net over the sieve and bottom part (G,H), securing the net in place when screwing the top part (E). Only the 25 mm filter holder is shown. Please click here to view a larger version of this figure.
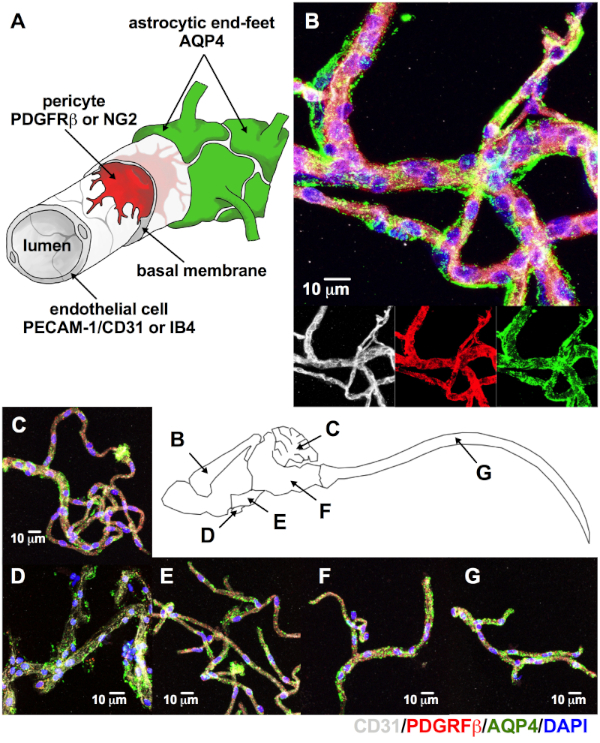
Figure 3: Microvessels isolated from multiple CNS regions expressed canonical neurovascular unit markers. (A) Using immunolabeling and confocal microscopy, intrinsic cellular components of the NVU are used to identify adult (8–14 week-old C57BL/6J) murine CNS microvessels. As seen on the merge image for cortical microvessels (B), above the individual confocal images for the endothelial cell marker CD31 (white), pericyte marker PDGFRβ (red), and astrocytic end-feet marker AQP4 (green) all these cellular components are retained. Notice that the intimate relationship between endothelial cells and pericytes make them appear magenta on the merged picture, while the astrocytic end-feet appear to have a halo surrounding them (B). The same expression pattern is observed for the cerebellum (C), pituitary (D), hypothalamus (E), brainstem (F), and spinal cord (G, DAPI, nuclear stain = blue; scale bar = 10 µm). Please click here to view a larger version of this figure.
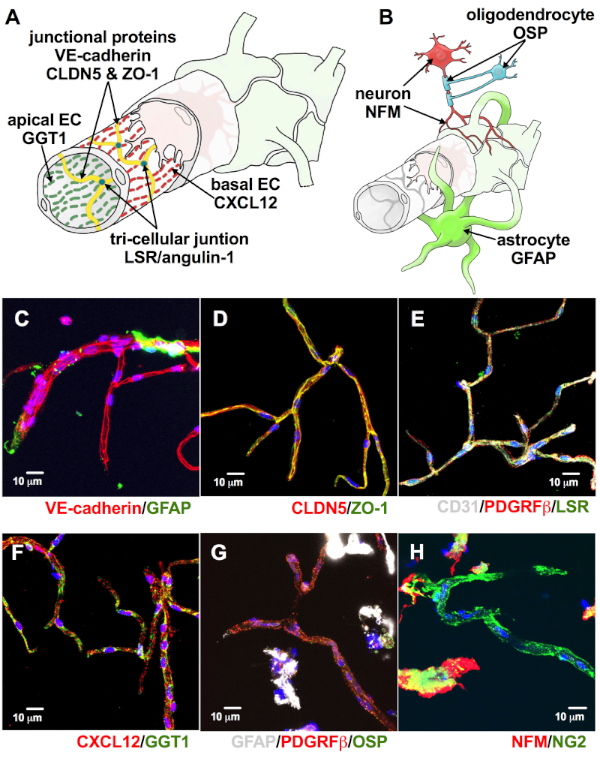
Figure 4: Microvessels expressed proteins associated with BBB-specific properties. Endothelial cells within the neurovascular unit (NVU) are also described by the proteins associated with their intrinsic barrier properties (A). As seen in the figure, endothelial cells have junctions made of VE-cadherin, CLDN5, ZO-1 (A, yellow), and angulin-1 (A, teal). They also exhibit apicobasal polarity to multiple proteins including GGT-1 and CXCL12 (A, green and red). Neurons, oligodendrocytes, and astrocyte-cell bodies may be included in the isolated microvessels, although they are not intrinsic to the NVU (B). These are identifiable by the expression of NFM (red), OSP (cyan), and GFAP (lime green), respectively (B). Consistent with these, our isolated microvessels expressed adherens protein VE-cadherin (C, red), tight junction proteins CLDN5 and ZO-1 (D, red and green, respectively, merge = yellow), tricellular junction protein LSR (E, green), and apicobasal markers CXCL12 and GGT1 (F, red and green, respectively). They also exhibited limited amounts of GFAP (A, green, G, white), OSP (G, green), and NFM (H, red), suggesting negligible retention of non-neurovascular unit cells (DAPI, nuclear stain = blue; scale bar = 10 µm). Please click here to view a larger version of this figure.
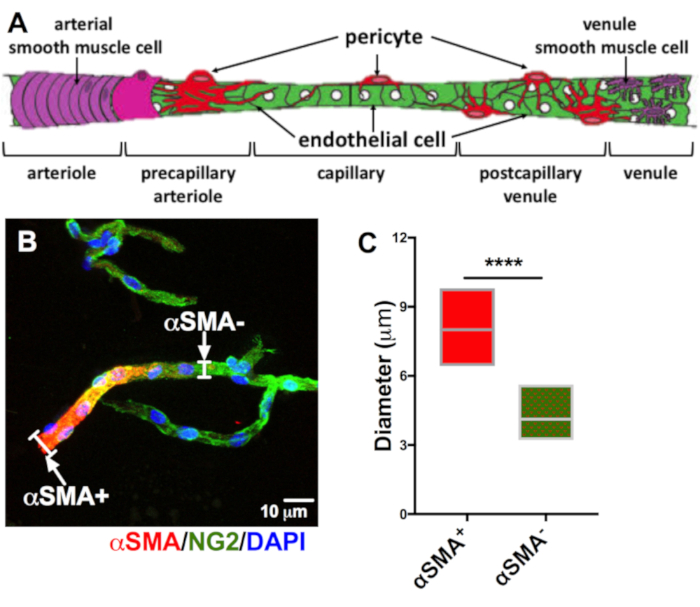
Figure 5: Most isolated microvessels do not express αSMA. The neurovascular unit exhibits a sophisticated transition from arteriole-capillary-venule (A). αSMA anular expression clearly identifies the arteriole, and as it becomes the capillary, αSMA (B, red) expression diminishes, exposing the mural marker NG2 (B, green). We measured the diameter of αSMA+ and αSMA- vessels (white brackets, n = 20), to distinguish them as arterioles or capillaries (DAPI, nuclear stain = blue, scale bar = 10 µm). Unpaired t-test analysis of diameter of αSMA+ and αSMA- vessels showed a high statistical significance (C, αSMA+ = red, αSMA- red/green, ****p < 0.0001). Please click here to view a larger version of this figure.
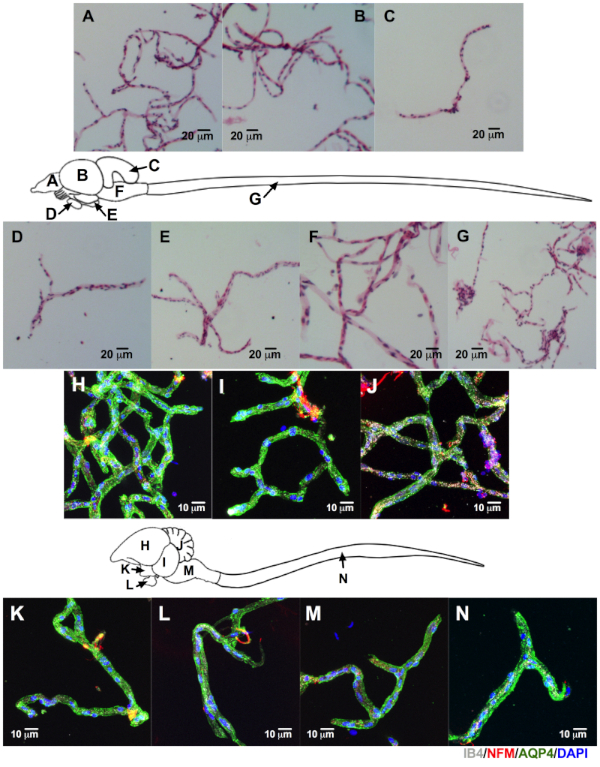
Figure 6: Successful isolation of CNS microvessels from other lissencephalic species. The CNS was harvested from a wild-caught frog (8.2 cm), lizard (12.7 cm), tank-raised rainbow trout (2 year old, 35.6 cm), and aviary-raised pigeon (9 month-old, ~300 g). The microvessels from the frog, fish, and lizard were stained by H&E (only microvessels from trout are shown, scale bar = 20 µm). Pigeon microvessels were immunolabeled. We were able to identify microvessels on all regions as labeled on the fish and pigeon CNS outlines: cortex (A and H), optic lobe (B and I), cerebellum (C and J), pituitary (D and L), hypothalamus (E and K), brainstem (F and M), and spinal cord (G and N). Additionally, we were able to identify endothelial cells with isolectin IB4 (white), astrocytic end-feet with AQP4 (green), and adjacent neurons with NFM (red) from the pigeon's isolated microvessels (DAPI, nuclear stain = blue, scale bar = 10 µm). Please click here to view a larger version of this figure.
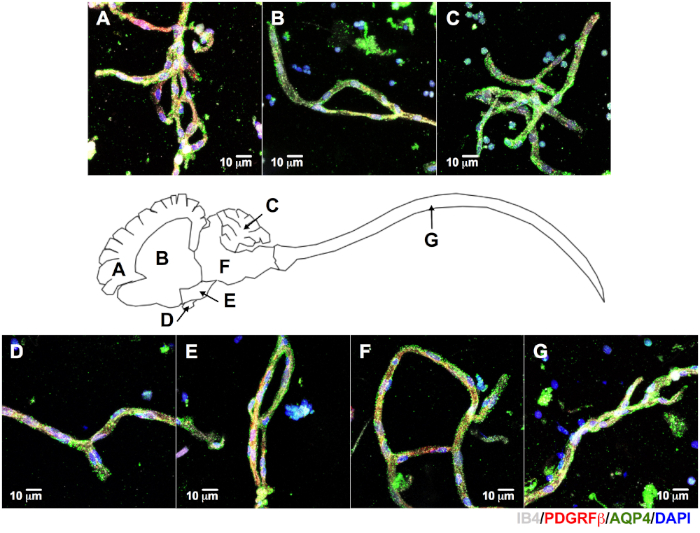
Figure 7: Isolation of CNS microvessels from gyrencephalic species. The brain and spinal cord were dissected from a farm-raised pig (~6 month-old, ~120 kg) for microvessel isolation and immunolabeling. We were able to identify microvessels positively labeled for IB4 (white), PDGFRβ (red), and AQP4 (green) on all regions of porcine CNS: cortex (A), periventricular white matter (B), cerebellum (C), pituitary (D), hypothalamus (E), brainstem (F), and spinal cord (G, DAPI, nuclear stain = blue, scale bar = 10 µm). Please click here to view a larger version of this figure.
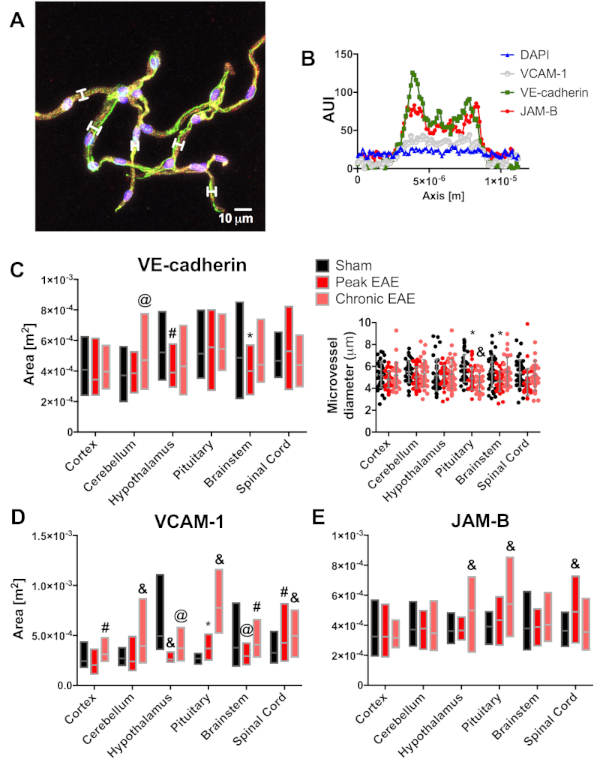
Figure 8: Example of other application of CNS microvessel isolation. Expression of JAB-B and VCAM-1 was quantified for murine EAE microvessels. Arbitrary units of intensity (AUI) were measured for the cortex, cerebellum, hypothalamus, pituitary, brainstem, and spinal cord of mice at peak and chronic stages of EAE, with sham as control (n = 4). For each CNS region, twelve microvessels were evaluated per mouse to determine the AUI per fluorochrome and the diameter of the microvessel (A). White brackets show examples of the confocal microscope software measuring tool used to determine AUI for VCAM-1 (white), VE-cadherin (green), JAM-B (red), and DAPI (blue, scale bar = 10 µm). Then, the resulting AUI were plotted against the diameter in microns (B) to calculate the area under the curve (AUC) for each immunolabeled protein as an estimate of protein expression. The mean AUC per group was then analyzed by two-way ANOVA, followed by Sidak's post hoc test, for a total of 48 microvessels per CNS region. Except for the pituitary microvessels, we observed no major differences between microvessel diameter associated with disease status (C, insert), but a significant decrease of VE-cadherin expression from the hypothalamus and brainstem in EAE mice (C). Similar statistical analysis showed a significant increase for VCAM-1 (D) and JAM-B (E) at the spinal cord, consistent with neuroinflammatory status during the peak of EAE. Interestingly, we also observed changes for VCAM-1 at the hypothalamus, pituitary, and brainstem. These results are under investigation (black bars and dots = sham, red bars and dots = peak EAE, salmon bars and dots = chronic EAE, *p<0.05, @p<0.01, #p<0.001, and &p<0.0001). Please click here to view a larger version of this figure.
| MV-1 | 10 mM HEPES |
| in 1x Hank's balanced salt solution (HBSS) with calcium and magnesium | |
| MV-2 | 18% 70 kDa molecular weight (MW) dextran |
| in 10 mM HEPES/HBSS (MV-1) | |
| MV-2 | 20% 70 kDa MW dextran |
| in 10 mM HEPES/HBSS (MV-1) | |
| MV-3 | 1% bovine serum albumin (BSA) |
| in 10 mM HEPES/HBSS (MV-1) | |
| Fixative | 4% paraformaldehyde (PFA) |
| in 1x phosphate-buffered saline (PBS) pH 7.4 | |
| Wash buffer | 1x PBS pH 7.4 |
| Permeabilization & blocking buffer | 10% BSA |
| 0.25% nonionic surfactant | |
| in 1x PBS pH 7.4 | |
| Antibody diluent | 5% BSA |
| 0.25% nonionic surfactant | |
| in 1x PBS pH 7.4 |
Table 1: Solutions used in the protocol.
| Step(s) | Action |
| 4.1 and 4.1.1 | Mince in MV-1 solution with a single-edge blade |
| 4.2 to 4.2.2 | Homogenize in MV-1 solution using a Potter-ELVJ PTFE or microtube pestle |
| 5.1 | Spin at 2,000 x g, 4 °C, 10 min |
| 5.1.1 to 5.1.1.2 | Resuspend the pellet in MV-2 solution |
| 5.2 | Spin at 4,400 x g, 4 °C, 15 min |
| 5.3 to 5.5 | Resuspend the pellet with 1 mL of MV-3 solution |
| 6.2 to 6.4.1 | Elute over a 50 mL conical tube through a cell strainer and rinse with MV-3 solution |
| 6.5, 6.5.1, 6.6, and 6.7 | Filter with a 20 μm nylon net on the modified filter holder |
| 6.8 | Load the nylon net into the 50 mL beaker with 10 mL of MV-3 solution and shake for 30 s |
| 6.9 to 6.10 | Decant into a 15 mL conical tube and spin at 2,000 x g, 4 °C, 5 min |
| 6.10 | Resuspend in 1 mL of MV-3 solution |
| 6.10.1 | Transfer to a 1.7 mL microtube and spin at 20,000 x g, 4 °C, 5 min |
| 7.1 to 7.3 | Resuspend in 1x PBS and load into well slides |
Table 2: Layout for small vertebrate microvessels isolation. This chart is an abridged version of the protocol when using a lissencephalic specimen.
| Step(s) | Solution | Action | CTX | CBL | OPT | BST | SC | HYP | PIT |
| 4.1 | MV-1 | Mince on dish | 100 mm x 20 mm Petri dish, 1 mL | n/a | |||||
| 4.2.1 and 4.2.2 | MV-1 | Homogenize | 5 mL, 10 mL Potter-Elvehjem with pestle | 1 mL, micropestle | |||||
| 5.1.1.1 and 5.1.1.2 | MV-2 | Resuspend | 5 mL + 5 mL, 18% Dextran | 1 mL, 20% dextran | |||||
| 5.5 and 6.4.1 | MV-3 | Resuspend | 1 mL + 10-15 mL | 1 mL | |||||
| 6.2 and 6.4.1 | MV-3 | Strainer size | 100 μm | 70 μm | 100 μm | 70 μm | 100 μm | ||
| Rinse | 5 mL | ||||||||
| 6.5, 6.5.1 and 6.7 | MV-3 | Filter size, rinse | 25 mm filter, 10 mL | 13 mm, 5 mL | |||||
| 6.8 | MV-3 | Shake on beaker | 10 mL | ||||||
| 6.10 | MV-3 | Resuspend | 1 mL | ||||||
| 7.2 | 1x PBS | Resuspend | 1.5 to 2.0 mL | 0.5 to 1.0 mL | 0.1 to 0.2 mL | ||||
| 7.3 | 1x PBS | Load per well | 200 μL | 100 μL | 25 to 50 μL | ||||
| CTX = cortex, CBL = cerebellum, BST = brainstem, OPT = optic lobe (not present in mammals), HYP = hypothalamus, PIT = pituitary, SC = spinal cord. Recommended volumes are for whole tissue from animals ranging in size from 20 g (young mouse) to 800 g (adult rat). | |||||||||
Table 3: Recommended volume/size. This outline has the recommended volume of solutions for using a small vertebrate specimen ranging from ~20–~800 g, or more specifically, the ~25 g mouse shown on the video. Final adjustment of the necessary volumes must be determined by the researcher according to the specific amount of wet tissue obtained after dissection. Practice and troubleshooting are highly recommended. CTX = cortex, CBL = cerebellum, BST = brainstem, OPT = optic lobe (not present in mammals), HYP = hypothalamus, PIT = pituitary, SC = spinal cord.
| Step(s) | Action |
| 4.1 and 4.1.2 | Mince in MV-1 with a single-edge blade |
| 4.3 to 4.3.4 | Homogenize in MV-1 solution using a Potter-Elvehjem grinder with a PTFE pestle |
| 5.1 | Spin at 2,000 x g, 4 °C, 10 min |
| 5.1.2 to 5.1.2.2 | Resuspend the pellet in MV-2 solution |
| 5.2 | Spin at 4,400 x g, 4 °C, 15 min |
| 5.3 to 5.5 | Resuspend the pellet with 1 mL of MV-3 solution |
| 6.2 to 6.4 and 6.4.2 | Elute over a 50 mL conical tube through a cell strainer and rinse with MV-3 solution |
| 6.5, 6.5.2, 6.6 and 6.7 | Filter with a 20 μm nylon net on the modified filter holder |
| 6.8 | Load the nylon net into a 50 mL beaker with 30 mL of MV-3 solution, and shake for 30 s |
| 6.9 and 6.10 | Decant into a 50 mL conical tube and spin at 2,000 x g, 4 °C, 5 min |
| 6.10 | Resuspend in 1 mL of MV-3 solution |
| 6.10.2 | Transfer to a 5 mL microtube and spin at 2,000 x g, 4 °C, 5 min |
| 7.1 to 7.3 | Resuspend in 1x PBS and load into well slides |
Table 4: Layout for large vertebrate microvessels isolation. This chart is an abridged version of the protocol when using a gyrencephalic specimen.
| Step(s) | Soution | Action | CTX | CBL | WM | BST | SC | HYP | PIT |
| 4.1 | MV-1 | Mince on dish | 3−5 mL | 1 mL | |||||
| 4.3 to 4.3.4 | MV-1 | Homogenize | 20−30 mL, 55 mL Potter-Elvehjem with pestle & overhead stirrer | 5 mL, 10 mL Potter-Elvehjem with pestle | |||||
| 5.1.2 to 5.1.2.2 | MV-2 | Resuspend | >20 mL, 20% dextran | 5 mL + 5 mL, 20% dextran | |||||
| 5.5, 6.4 and 6.4.2 | MV-3 | Resuspend | 1 mL + 20 mL | 1 mL + 10 mL | |||||
| 6.2 and 6.4.2 | MV-3 | Strainer size | 100 μm | 70 μm | 100 μm | 70 μm | 100 μm | ||
| Rinse | 10 mL | ||||||||
| 6.5, 6.5.2 and 6.7 | MV-3 | Filter size, rinse | 47 mm filter, 10 mL | 25 mm, 10 mL | |||||
| 6.8 | MV-3 | Shake on beaker | 30 mL | ||||||
| 6.10 and 6.10.2 | MV-3 | Resuspend | 1 mL + 4 mL | ||||||
| 7.2 | 1x PBS | Resuspend | 2.0 to 4.0 mL | 1.0 to 2.0 mL | |||||
| 7.3 | 1x PBS | Load per well | 200 μL | 100 μL | |||||
| CTX = cortex, CBL = cerebellum, WM = white matter, BST = brainstem, HYP = hypothalamus, PIT = pituitary, SC = spinal cord. Recommended volumes are for samples of ~45 cm3 for CTX, CBL, BST and SC whole HYP and PIT. | |||||||||
Table 5: Recommended volume/size. This outline has the recommended volume of solutions for using a large vertebrate specimen. More specifically, it applies to CNS tissue biopsies of ~45 cm3 for cortex, cerebellum, white matter, brainstem, spinal cord, and the whole hypothalamus and pituitary, as shown on the video. Final adjustment of the necessary volumes must be determined by the researcher according to the specific amount of wet tissue obtained after dissection. Practice and troubleshooting are highly recommended. CTX = cortex, CBL = cerebellum, WM = white matter, BST = brainstem, HYP = hypothalamus, PIT = pituitary, SC = spinal cord.
Discussion
The BBB includes the unique properties of the brain microvasculature endothelial cells coupled by a sophisticated architecture of tight-, adherens-, "peg-socket"- junctions, and adhesion plaques critical for CNS homeostasis2,3,19. Endothelial cells properties are induced and maintained by pericytes and the surrounding astroglia end-foot processes2,3,19. BBB microvasculature displays polarity (i.e., the asymmetrical expression pattern of proteins localized on luminal or abluminal endothelial cell surfaces)20. While this may briefly define the BBB, in reality it remains one of the most enigmatic notions in neuroscience. For example, regional, sex, and age differences within the NVU may explain CNS regional vulnerabilities to trauma, toxicity, infection, and inflammation, which can have major clinical consequences3,5,6. Another obstacle in the understanding of the BBB is the differences observed among multiple taxa7,8. One of the major hurdles of understanding and investigating the BBB is precisely the difficulty related to the isolation of the NVU that encompasses the BBB, while still keeping its barrier-specific properties14.
In an attempt to accelerate our understanding of the BBB, CNS-regional differences, as well as species differences, we adapted previously published methods. We successfully adapted these, in particular Boulay et al.21, to obtain microvessels from single cortical, cerebellar, hypothalamic, pituitary, brainstem, and spinal tissues on a myriad of lissencephalic small vertebrates: fish, amphibians, reptiles, birds, and mammals (Figure 3, Figure 4, Figure 5, and Figure 6). One advantage of this method is that its adaptations are based on the overall size of CNS tissue: the researcher chooses the amount of solution, size of tissue grinder, conical tube, filter holders, etc. according to the wet tissue, regardless of species, sex, and age. In our experience with immunolabeling, a ~20 g specimen yields enough microvessels for an 8 well slide per cortex, cerebellum, optic lobe, brainstem, and spinal cord and half an 8 well slide per hypothalamus and pituitary. However, yield of cortical and optic lobe is much higher in comparison to cerebellum, brainstem, and spinal cord.
As shown, we performed the necessary adaptations for isolation and immunolabeling of CNS microvessels of pig and macaque, larger gyrencephalic mammals that are more relevant for translational research (Figure 7). Notably, we were able to include periventricular white matter on these species only. Since the CNS in these animals is larger, we collected enough white matter to allow us to separate a microvessel pellet from the myelin layer during the second centrifugation (step 5.3, MV-2 with 20% dextran). We speculate that a critical mass is needed to be able to achive this separation and we are actively seeking how to obtain a similar result with murine CNS tissue.
Although omitted for the sake of simplicity, we did perform immunolabeling beyond the NVU canonical markers on avian, porcine, and macaque microvessels. Notably, all species shared cellular and molecular markers previously identified on murine samples as relevant for BBB function (junctional proteins VE-cadherin, CLDN5, ZO-1, and LSR and apicobasal markers CXCL12 and GGT1) or in proximity to NVU (NFM, OSP, and GFAP). Again, these findings encourage the use of this method on other species to further identify NVU orthology and divergence among species. It also opens the opportunity for further investigation into NVU strain, sex, and age differences within the same species and the feasibility of using other organisms for BBB biomedical research. We also show evidence of a successful use of this microvessel-isolation method to quantitate changes in protein expression levels during neuroinflammation (Figure 8). Even though we performed this experiment as a proof-of-concept, the approach used here is currently extensively exploited in our laboratory. We favor this approach over other quantitative methods (e.g., Western blot) because we want to focus not only on the expression level but relative protein abundance, relocation of CXCL12 and other apicobasal proteins, linkage of junctional proteins, etc., within the complicated appearance of CNS microvessels9,13,22. Likewise, we are presently troubleshooting our method for other applications, such as further isolation of NVU cellular components (endothelial cells and pericytes), RNA-seq, and proteomics23,24,25,26.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Dr. Cruz-Orengo was supported by the University of California, Davis, School of Veterinary Medicine Start Up Funds.
Materials
| 10X PBS | ThermoFisher | BP39920 | Used for blocking and antibody diluent. |
| 20% PFA | Electron Microscopy Sciences | 15713-S | Used as fixative (4% PFA) |
| 70,000 MW Dextran | Millipore Sigma | 9004-54-0 | Used for MV-2 solution |
| Adson Forceps | Fine Science Tools (FST) | 11006-12 | Used for removal of muscle and skin |
| Adson Forceps, student quality | FST | 91106-12 | Same as above but cheaper |
| Bovine serum albumin (BSA) | Millipore Sigma | A7906-100G | Used for MV-3 solution, blocking and antibody diluent |
| Corning 100 μm Cell strainer | Millipore Sigma | CLS431752-50EA | |
| Corning 70 μm Cell strainer | Millipore Sigma | CLS431751-50EA | |
| Corning Deskwork low-binding tips | Millipore Sigma | CLS4151 | Same as below but cheaper. |
| Cultrex Poly-D-Lysine | R&D | 3439-100-01 | Used for slide coating |
| Donkey anti-Goat IgG-ALEXA 555 | Thermo | A21432 | Used as secondary antibody. Recommended dilution of 1:200. |
| Donkey anti-Mouse IgG-ALEXA 488 | Thermo | A21202 | Used as secondary antibody. Recommended dilution of 1:200. |
| Donkey anti-Rabbit IgG-ALEXA 488 | Thermo | A21206 | Used as secondary antibody. Recommended dilution of 1:200. |
| Donkey anti-Rabbit IgG-ALEXA 647 | Thermo | A31573 | Used as secondary antibody. Recommended dilution of 1:200. |
| Donkey anti-Rat IgG-DyLight 650 | Thermo | SA5-10029 | Used as secondary antibody. Recommended dilution of 1:200. |
| Double-Pronged Tissue Pick | FST | 18067-11 | Used for removal of meninges and choroid plexus |
| Dumont #3c Forceps | FST | 11231-20 | Used for more delicate and/or small CNS tissue handling (like pituitary) |
| Dumont #7 Forceps | FST | 11274-20 | Used for CNS tisssue dissection and handling |
| Dumont #7 Forceps, student | FST | 91197-00 | Same as above but cheaper |
| ep Dualfilter T.I.P.S. LoRetention Tips | Eppendorf | 22493008 | Better quality than the tips above (more expensive). |
| Extra Fine Graefe Forceps, serrated | FST | 11151-10 | Used for bone removal |
| Fine Scissors, sharp | FST | 14060-09 | Used for removal of pig and macaque dural sac |
| Glass Pestle 1.5 mL Microcentrifuge Tube Tissue Grinder Homogenizer, Pack of 10 | Chang Bioscience Inc. (eBay) | GP1.5_10 | Used for small vetebrate hypothalus and pituitary. |
| Goat anti-CXCL12, biotinylated | PeproTech | 500-P87BGBT | Used as primary antibody on CNS microvessels from all specimens. Recommended dilution: 1:20. |
| Goat anti-JAM-B | R&D | AF1074 | Used as primary antibody to assess neuroinflammation. Recommended concentration: 5 μg/mL. |
| Goat anti-Mouse IgG-ALEXA 488 | Thermo | A11001 | Used as secondary antibody. Recommended dilution of 1:200. |
| Goat anti-Mouse IgG-ALEXA 555 | Thermo | A21424 | Used as secondary antibody. Recommended dilution of 1:200. |
| Goat anti-PDGFRβ | R&D | AF1042 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Goat anti-Rabbit IgG-ALEXA 555 | Thermo | A21249 | Used as secondary antibody. Recommended dilution of 1:200. |
| Goat anti-Rabbit IgG-DyLight 488 | Thermo | 35552 | Used as secondary antibody. Recommended dilution of 1:200. |
| Goat anti-Rat IgG-DyLight 650 | Thermo | SA5-10021 | Used as secondary antibody. Recommended dilution of 1:200. |
| Graefe Forceps, curved tip, 1X2 teeth | FST | 11054-10 | Use for nylon filter net holding and shaking |
| HBSS, 1X buffer with calcium and magnesium | Corning | 21-022-CM | Used for MV-1 solution |
| HEPES, 1M liquid buffer | Corning | 25-060-CI | Used for MV-1 solution |
| Isolectin GS-IB4-Biotin-XX | ThermoFisher Scientific (Thermo) | I21414 | Glycoprotein isolated from legume Griffonia simplicifolia that binds D-galactosyl residues of endothelial cell glycocalysx. Used for avian and porcine CNS microvessels. Recommended concentration: 5 μg/mL. |
| LaGrange Scissors, serrated | FST | 14173-12 | Used for skull dissection and laminectomy (except pig and macaque) |
| Millicell EZ slide 8-well unit | Millipore Sigma | PEZGS0816 | |
| Mouse anti-CLDN5 | Thermo | 35-2500 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Mouse anti-GGT1 | Abcam | ab55138 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Mouse anti-Human CD31 | R&D | BBA7 | Used as primary antibody on primate CNS microvessels. Recommended concentration: 16.5 μg/mL. |
| Mouse anti-NFM | Thermo | RMO-270 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Mouse anti-αSMA | Thermo | MA5-11547 | Used as primary antibody on CNS microvessels from all specimens. Recommended dilution of 1:200. |
| Nylon Filter Net, roll | Millipore Sigma | NY6000010 | Laser-cut to 13 mm diameter filter net discs. Used for small vetebrate hypothalus and pituitary. |
| Nylon Filter Nets, 25 mm | Millipore Sigma | NY2002500 | Used on most small vertebrates CNS tissues, except hypothalamus and pituitary. Used for macaque and pig hypothalamus and pituitary. |
| Nylon Filter Nets, 47 mm | Millipore Sigma | NY2004700 | Used for macaque and pig CNS tissues, except hypothalamus and pituitary. |
| ProLong Gold antifade reagent with DAPI | ThermoFisher | P36935 | Used to coverslip slides. |
| Rabbit anti-AQP4 | Millipore Sigma | A5971 | Used as primary antibody on CNS microvessels from all specimens. Recommended dilution of 1:200. |
| Rabbit anti-LSR | Millipore Sigma | SAB2107967 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Rabbit anti-NG2 | Millipore Sigma | AB5320 | Used as primary antibody on CNS microvessels from all specimens. Recommended dilution of 1:200. |
| Rabbit anti-OSP | Abcam | ab53041 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 1 μg/mL. |
| Rabbit anti-VE-Cadherin | Abcam | ab33168 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Rabbit anti-ZO-1 | Thermo | 61-7300 | Used as primary antibody on CNS microvessels from all specimens. Recommended concentration: 5 μg/mL. |
| Rat anti-CD31 | Becton Dickinson | BD 550274 | Used as primary antibody for murine CNS microvessels. Recommended concentration: 5 μg/mL. |
| Rat anti-GFAP | Thermo | 13-0300 | Used as primary antibody on CNS microvessels from all specimens. Recommended dilution of 1:200. |
| Rat anti-VCAM-1 | Becton Dickinson | BD 553329 | Used as primary antibody to assess neuroinflammation. Recommended concentration: 5 μg/mL. |
| Sterile Ringer's Solution, Frog | Aldon Corporation | IS5066 | Used for amfibian anesthesia |
| Streptavidin-ALEXA 555 | Thermo | S32355 | Used as secondary antibody to label biotinylated primary antibodies. Recommended dilution of 1:500. |
| Streptavidin-ALEXA 647 | Thermo | S32357 | Used as secondary antibody to label biotinylated primary antibodies. Recommended dilution of 1:500. |
| Surgical Scissors, sharp | FST | 14002-12 | Used for removal of muscle and skin |
| Surgical Scissors, sharp-blunt | FST | 14001-16 | Used for decapitation (except pig and macaque) |
| Swinnex Filter Holder, 13 mm | Millipore Sigma | SX0001300 | Modified by laser-cut. Used for small vetebrate hypothalus and pituitary. |
| Swinnex Filter Holder, 25 mm | Millipore Sigma | SX0002500 | Modified by laser-cut. Used on most small vertebrates CNS tissues, except hypothalamus and pituitary. Used for macaque and pig hypothalamus and pituitary. |
| Swinnex Filter Holder, 47 mm | Millipore Sigma | SX0004700 | Modified by laser-cut. Used for macaque and pig CNS tissues, except hypothalamus and pituitary. |
| Triton X-100 | ThermoFisher | 50-165-7277 | Used for blocking and antibody diluent. |
| Wheaton 120 Vac Overhead Stirrer | VWR (Supplier DWK Life Sciences) | 62400-904 (DWK #903475) | Used for macaque and pig CNS tissues with 55 mL tissue grinder, except hypothalamus and pituitary. |
| Wheaton Potter-Elvehjem tissue grinder with PTFE pestle, 10 mL | VWR (Supplier DWK Life Sciences) | 14231-384 (DWK #357979) | Used on most small vertebrates CNS tissues, except hypothalamus and pituitary. Used for macaque and pig hypothalamus and pituitary. |
| Wheaton Potter-Elvehjem tissue grinder with PTFE pestle, 55 mL | VWR (Supplier DWK Life Sciences) | 14231-372 (DWK #357994) | Used for macaque and pig CNS tissues, except hypothalamus and pituitary. |
References
- Bundgaard, M., Abbott, N. J. All vertebrates started out with a glial blood-brain barrier 4-500 million years ago. Glia. 56, 699-708 (2008).
- Daneman, R., Prat, A. The blood-brain barrier. Cold Spring Harbor Perspectives in Biology. 7, a020412 (2015).
- Obermeier, B., Verma, A., Ransohoff, R. M. The blood-brain barrier. Handbook of Clinical Neurology. 133, 39-59 (2016).
- Kealy, J., Greene, C., Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neuroscience Letters. , (2018).
- Sweeney, M. D., Kisler, K., Montagne, A., Toga, A. W., Zlokovic, B. V. The role of brain vasculature in neurodegenerative disorders. Nature Neuroscience. 21, 1318-1331 (2018).
- Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., Zlokovic, B. V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiological Reviews. 99, 21-78 (2019).
- Wilhelm, I., Nyul-Toth, A., Suciu, M., Hermenean, A., Krizbai, I. A. Heterogeneity of the blood-brain barrier. Tissue Barriers. 4, e1143544 (2016).
- O’Brown, N. M., Pfau, S. J., Gu, C. Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes & Development. 32, 466-478 (2018).
- Cruz-Orengo, L., et al. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. Journal of Experimental Medicine. 208, 327-339 (2011).
- Serres, S., et al. VCAM-1-targeted magnetic resonance imaging reveals subclinical disease in a mouse model of multiple sclerosis. FASEB Journal. 25, 4415-4422 (2011).
- Tietz, S., Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. Journal of Cell Biology. 209, 493-506 (2015).
- Sohet, F., et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. Journal of Cell Biology. 208, 703-711 (2015).
- Cruz-Orengo, L., et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. Journal of Clinical Investigation. 124, 2571-2584 (2014).
- Dayton, J. R., Franke, M. C., Yuan, Y., Cruz-Orengo, L. Straightforward method for singularized and region-specific CNS microvessels isolation. Journal of Neuroscience Methods. 318, 17-33 (2019).
- Smyth, L. C. D., et al. Markers for human brain pericytes and smooth muscle cells. Journal of Chemical Neuroanatomy. 92, 48-60 (2018).
- Granberg, T., et al. In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain. 140, 2912-2926 (2017).
- Datta, G., et al. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain. 140, 2927-2938 (2017).
- Tommasin, S., Gianni, C., De Giglio, L., Pantano, P. Neuroimaging Techniques to Assess Inflammation in Multiple Sclerosis. 신경과학. 403, 4-16 (2019).
- Liebner, S., et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathologica. 135, 311-336 (2018).
- Cornford, E., Hyman, S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx. 2, 27-43 (2005).
- Boulay, A. C., Saubamea, B., Decleves, X., Cohen-Salmon, M. Purification of Mouse Brain Vessels. Journal of Visualized Experiments. , 53208 (2015).
- Paul, D., Cowan, A. E., Ge, S., Pachter, J. S. Novel 3D analysis of Claudin-5 reveals significant endothelial heterogeneity among CNS microvessels. Microvascular Research. , (2012).
- Munikoti, V. V., Hoang-Minh, L. B., Ormerod, B. K. Enzymatic digestion improves the purity of harvested cerebral microvessels. Journal of Neuroscience Methods. 207, 80-85 (2012).
- Yousif, S., Marie-Claire, C., Roux, F., Scherrmann, J. M., Decleves, X. Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Research. 1134, 1-11 (2007).
- Bourassa, P., Tremblay, C., Schneider, J. A., Bennett, D. A., Calon, F. Beta-amyloid pathology in human brain microvessel extracts from the parietal cortex: relation with cerebral amyloid angiopathy and Alzheimer’s disease. Acta Neuropathologica. 137, 801-823 (2019).
- Porte, B., et al. Proteomic and transcriptomic study of brain microvessels in neonatal and adult mice. PLoS One. 12, e0171048 (2017).

