Robotic Lateral Pancreaticojejunostomy for Chronic Pancreatitis
Summary
Robotic lateral pancreaticojejunostomy (RLPJ) may be used in patients with painful, morphine dependent, chronic pancreatitis and a dilated main pancreatic duct. We describe a standardized and reproducible technique for RLPJ, which includes transection of the gastroduodenal artery.
Abstract
Lateral pancreaticojejunostomy (LPJ) has shown good postoperative outcomes in patients with painful, morphine dependent, chronic pancreatitis (CP). The recent rise of robotic and laparoscopic pancreatic surgery has found benefits such as reduced time to functional recovery. Few studies have reported on the feasibility, technique and outcome of robotic LPJ, especially including transection of the gastroduodenal artery. The present study describes the main steps for robotic LPJ in a patient with painful chronic pancreatitis with a dilated main pancreatic duct. The patient underwent robotic LPJ. The LPJ anastomosis is performed using a running suture technique in a longitudinal side-to-side manner. Routinely, the gastroduodenal artery is transected to drain the entire length of the main pancreatic duct. The patient is in French position; 7 trocars are placed (4 robotic, 2 laparoscopic assistants, 1 liver-retractor). After docking of the robot system, the omental bursa is opened, and the right gastroepiploic artery and vein are ligated at their base at the lower border of the pancreas. Intraoperative ultrasonography is performed in order to determine trajectory of the dilated main pancreatic duct which is opened for its entire length after the gastroduodenal artery has been suture ligated both cranially and caudally from the main pancreatic duct. A Roux limb is created, and a latero-lateral PJ is fashioned using several 3-0 barbed sutures. A stapled jejuno-jejunostomy is created at sufficient distance from the pancreatic anastomosis, aided by a 50 cm suture. The described technique for robotic LPJ is a complex but feasible operation for patients with treatment refractory CP and a dilated main pancreatic duct. Due to its complexity, implementation in high volume centers with extensive experience with CP surgery may improve outcomes.
Introduction
Surgery is highly effective in selected patients with painful chronic pancreatitis1,2,3. Surgical intervention for chronic pancreatitis (CP) carries high postoperative risks4. Yet, in selected patients it might be the only available treatment, especially for painful CP that is non-responsive to medical therapy5,6. Pain and impaired quality of life are the most common indications for surgery in patients affected by CP3,7. The extension of pancreatic gland involvement and the location of the disease are the main preoperative parameters necessary to assess the best surgical strategy. Both resectional and decompressive techniques have proven to be successful for the treatment of this condition8. Decompressive techniques are preferred since they allow to preserve the pancreatic parenchyma, hence they lower the risk of postoperative endocrine and exocrine insufficiency9. Longitudinal pancreatojejunostomy was fist described in 1958 by Puestow and Gilesby10 and is nowadays one of the most common surgical techniques to treat CP11.
The debate of the best surgical technique and surgical approach, i.e., open or minimally invasive, is unresolved. In the few reported series in literature, laparoscopic LPJ shows advantages regarding postoperative pain and length of hospital stay9. Additionally, robotic surgery augments the minimally invasive approach with enhanced optics (three-dimensional vision) and extended degrees of freedom in the articulated instruments. This allows for very precise movements during suturing for pancreatic bleeding and the reconstruction phase of the operation5,7,9,12,13.
Here, we describe our robotic technique of LPJ which involves transection of the gastroduodenal artery. Patients are selected on the basis of symptoms related to CP (mostly continuous pain), and dilatation of main pancreatic duct (MPD) on preoperative imaging of at least 5 mm. A contrast-enhanced computed tomography (CT) scan is obtained to confirm the diagnosis and to exclude any neoplastic disease, distal biliary duct strictures, the presence of active acute pancreatitis (peripancreatic fluid and alteration in pancreatic parenchyma texture), and to measure the dilatation and the extension of the MPD dilatation. After these considerations, if the patient is deemed suitable for robotic LPJ, schedule a preoperative anesthesiology evaluation14,15.
A 45-year-old male presented with refractory CP non-responsive to conservative (pain) management for three years on the basis of episodes of heavy alcohol consumption. The patient ceased smoking (60-pack year) and drinking prior to surgery, and underwent endoscopic treatment (a 10 Fr, 7 cm stent was positioned in the MPD). Preoperative bilirubin- and lipase level were in the normal range. There was no pancreatic endocrine and exocrine insufficiency. The preoperative CT scan showed a dilated MPD in the body and tail of the pancreas (Figure 1).
Protocol
The present protocol follows the ethics guidelines of the Amsterdam UMC.
1. Operative setting and trocar placement
- Place the patient in French position with a heating device, laying on a short grain mattress with the right arm tucked in and slightly lowered, and the left arm abducted to 90°.
- After all safety procedures (hood, sterile glove and sterile scrub) are ascertained, create a sterile exposition. Have the first surgeon at the robot console and the table-side surgeon between the patient’s legs. Perform the procedure using a 7-port technique.
- Create pneumoperitoneum by placing a Veress needle in the left hypochondrium, then place seven trocars as shown in Figure 2 (4 robotic, 2 laparoscopic assistants, and 1 trocar for the liver snake-retractor).
NOTE: The sequence of instruments (Table of Materials) during each operative step is seen in Table 1. - Place the robot on the right side next to the patient and dock the arms to the previously placed trocars.
- Retract the falciform ligament ventrally using a percutaneous suture by using a straight needle.
2. Surgical technique
- Mobilization of the stomach
- Open the greater omentum 2 cm distal from the gastroepiploic vessels using a sealing device by the table-side surgeon.
- Use the snake-retractor to retract the stomach and the left liver ventrally and cranially. Expose the anterior surface of the pancreas by dissecting the adhesions between the pancreas and the stomach and duodenum.
- Identify the right gastroepiploic vein and artery at the lower border of the pancreas and transect these using either a sealing device, clips or an endo-stapler using a vascular cartridge.
- Ultrasound of the pancreas
NOTE: Now, the entire pancreas can be exposed.- Perform an intraoperative ultra-sound to locate the MPD.
NOTE: The ultrasound is integrated in the robotic console and controlled trough a grabbing point intended for a fenestrated robotic grasper.
- Perform an intraoperative ultra-sound to locate the MPD.
- Opening the MPD, ligation of the gastroduodenal artery
- Determine the trajectory of the MPD and open the pancreas with the robotic monopolar diathermia hook. Insert a 5−7 Fr, 10 cm stent in the duct which prevents cautery damage to the dorsal surface of the MPD during dissection.
- Start the pancreatic duct incision at the pancreatic neck or body; then follow the duct towards the pancreatic tail.
- Finalize by proceeding from the pancreatic body to the pancreatic head which includes crossing the gastroduodenal artery. Expose and ligate the gastroduodenal artery on both sides, just below and above the pancreatic duct with stitches and subsequently transected.
- Identification of the Roux limb
NOTE: Pulling up the small bowel to identify the Roux limb.- Reflect the transverse colon cranially to identify the ligament of Treitz and open the mesocolon in the avascular plane to the (patient’s) left of the middle mesocolonic vessels.
- Approximately 30 cm from Treitz ligament, divide the jejunum using an endo-stapler. Mark the proximal end using a long suture which is retained. Pass the Roux limb through the defect and placed in close proximity to the MPD. Place the stapled end/stump of the bowel towards the splenic hilum.
- Mark the future lateral jejuno-jejunostomy site by a single suture after measuring a distance of 50 cm between the pancreatic anastomosis and the lateral jejuno-jejunostomy site.
- Pancreaticojejunostomy anastomosis
NOTE: The Roux limb is opened with monopolar electrocautery.- First, complete the caudal part of the anastomosis (‘inferior border’) with a single row running suture until the medial end is reached, then complete the anterior or cranial wall in the same fashion with a new running suture.
NOTE: Both the layers are made using a 3-0 barbed suture (9” 23 cm, V-20, ½ circle 26 mm needle). - Examine the lateral pancreatojejunostomy for leaks.
NOTE: A single stitch with a monofilament 5-0 suture (5” 13 cm, RB, ½ circle 17-26 mm needle), can be placed over any suspected wall defects. If one single barbed suture is not enough, use a second which can be tied to the first suture.
- First, complete the caudal part of the anastomosis (‘inferior border’) with a single row running suture until the medial end is reached, then complete the anterior or cranial wall in the same fashion with a new running suture.
- Jejunojejunostomy anastomosis
- Aid the side-to-side lateral, isoperistaltic jejuno-jejunostomy anastomosis by measuring 60 cm between the pancreatic anastomosis and the site of this anastomosis.
- Align both small bowel loops and perform the enterotomies with monopolar electrocautery in each loop.
- Then, create an anastomosis using an endo-stapler. Close the remaining opening using a 3-0 barbed suture (9” 23 cm, V-20, ½ 22-26 mm needle) single layer.
- Drain placement
- Place a single tubular drain next to the LPJ site through the right trocar opening at the anterior axillary line and fix it with a single stitch to the external abdominal wall.
Representative Results
Representative results are shown in Table 2. The operative time was 388 min with 200 mL estimated intraoperative blood loss. The postoperative course was uncomplicated, thus no postoperative pancreatic fistula (POPF) was detected; on postoperative 3, the drain fluid amylase level measured was low and the drain was removed that day. The patient was discharged the next day. Pathology assessment confirmed the preoperative diagnosis of CP without malignancy.
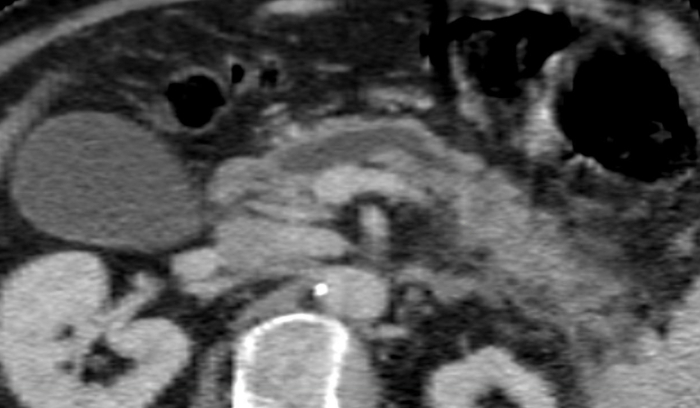
Figure 1: The appearance of the main pancreatic duct on CT scan. Diameter of the main pancreatic duct: 8.4 mm. Please click here to view a larger version of this figure.
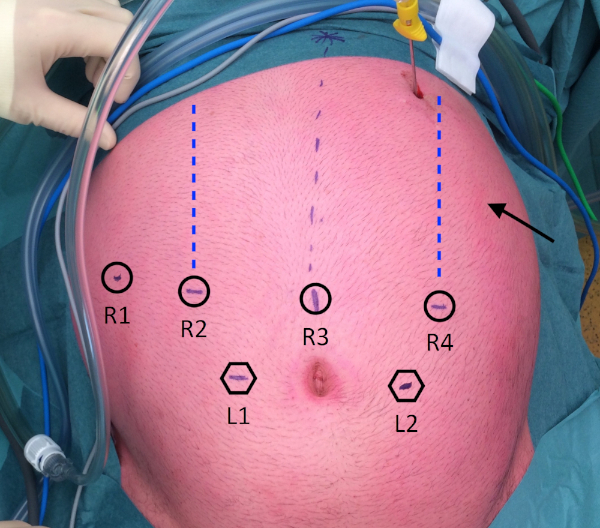
Figure 2: Trocar placement. R1: placed at the right anterior axillary line; R2: placed at the right mid-clavicular line; R3: placed on, or just right of, the midline; R4: placed at the left mid-clavicular line. L1: placed at a distance of 8 cm caudally from R2 and R3; L2: placed at a distance of 8 cm caudally from R3 and R4; Arrow: Stomach retractor, placed at the left anterior axillary line. Please click here to view a larger version of this figure.
| Instruments Used | |||||||
| Robotic | Laparoscopic | ||||||
| (Console surgeon) | (Table-side surgeon) | ||||||
| Operative steps | Dominant | Non-dominant | 3rd arm | L | R | ||
| 2.1. Mobilize stomach | Permanent cautery hook | Fenestrated bipolar forceps | Prograsp forceps or cadiere forceps | Sealing device, suction, curved scissors, clip-applier | |||
| 2.2. US pancreas | Permanent cautery hook | Fenestrated bipolar forceps | Prograsp forceps or cadiere forceps | Suction, endo-echo device | |||
| 2.3. Open pancreas | Permanent cautery hook | Fenestrated bipolar forceps | Prograsp forceps or cadiere forceps | Sealing device, suction, curved scissors, clip-applier | |||
| 2.4. Roux limb | Prograsp forceps or cadiere forceps | Fenestrated bipolar forceps | – | Sealing device, stapler | Suction | ||
| 2.5. LPJ | Large needle driver | Cadiere forceps | Prograsp forceps | Fenestrated grasper, suction, clip-applier, curved scissors | |||
| 2.6. JJ | Positioning anastomosis site | Prograsp forceps | Cadiere forceps | Fenestrated bipolar forceps | Fenestrated graspers | ||
| Enterotomies for stapler | Permanent cautery hook | Cadiere forceps | Fenestrated bipolar forceps | Fenestrated grasper | |||
| Stapling | Prograsp forceps | Cadiere forceps | Fenestrated bipolar forceps | Stapler | Fenestrated grasper | ||
| Closing enterotomies | Large needle driver | Cadiere forceps | Prograsp forceps | Fenestrated grasper, suction, clip-applier, curved scissors | |||
| Positioning anastomosis site | Prograsp forceps | cadiere forceps | Fenestrated bipolar forceps | Fenestrated grasper | Prograsp forceps | ||
| Fixating roux-loop to the mesentery | Large needle driver | Cadiere forceps | Prograsp forceps | Fenestrated, grasper, Suction | Large needle driver | ||
| clip-applier, curved scissors | |||||||
| The configuration for instrument arms is so that the 3rd arm is on the most lateral port controlled by the robotic (console) surgeon. | |||||||
Table 1: Sequence of instruments during each operative step.
| Variable | Outcome |
| Intraoperative | |
| Operative time (min) | 388 |
| Dissection (min) | 161 |
| Reconstruction (min) | 113 |
| Estimated intraoperative blood loss (mL) | 200 |
| Postoperative | |
| Clavien/Dindo complication grade | 0 |
| Drain removal, postoperative day | 3 |
| Postoperative hospital stay, days | 4 |
| Pathological diagnosis | Chronic pancreatitis without malignancy |
| Operative time comprises of steps 1.3−2.7.1, dissection comprises of steps 2.1−2.4.2, reconstruction comprises of steps 2.5−2.6.3. | |
Table 2: Representative results of the surgery.
Discussion
Robotic LPJ can be used in selected patients with painful chronic pancreatitis and a dilated MPD. Robotic LPJ combines the advantages of the minimally invasive approach and the freedom of articulating wrists as known from open surgery. Generally, a minimally invasive approach offers enhanced postoperative recovery, a lower postoperative pain, and a shorter length of hospital stay9,16,17,18. The robotic approach has benefits over the standard laparoscopic approach. First, enhanced vision owing to the three-dimensional, high definition imaging allows for better visualization of anatomical structures for the surgeon during both the dissection and the reconstruction phase5,13,19. Secondly, the needle drivers augmented with wristed articulation allow for easy suturing to control bleeding while opening of the MPD. Thirdly, opening of the MPD is not limited by the direction of the laparoscopic instruments, since the monopolar diathermia has wristed articulation.
As observed by Khan et al.8, the intraoperative ultrasound is a useful tool to identify the MPD. Adding the color Doppler, ultrasound may also be very useful in identifying the trajectory of the gastroduodenal artery. Measuring the length of the Roux-loop using a long suture is important. Determining the length of a bowel loop using the robotic view can be particularly challenging. This may be relevant in case of a pancreatic fistula with risk of reflux of bowel content in case of a short Roux loop.
The robotic procedure is a lengthier, more costly, and more challenging procedure than the open approach13. Moreover, the use of this technology requires high experienced surgeons both in open and laparoscopic surgery, especially because tactile feedback is lacking20. The RLPJ is challenging and encompasses many critical steps during the dissection and the reconstruction phases.
The robotic LPJ with double transection of gastroduodenal artery is a complex but feasible operation for patients with painful CP and a dilated MPD unresponsive to conservative treatments. Due to the possible complication of this procedure and concerns on the threshold for annual case volume for minimally invasive pancreatic surgery, we believe it should be performed only in high-volume centers by surgeons with extensive experience in both open and minimally invasive pancreatic surgery21.
Due to the limited indications for surgery, currently, all published series are based on a low number of patients, varying from 6 to 17 inclusions7,16,17,18. Further studies should investigate the long-term outcomes for patients undergoing robotic LPJ to affirm the robotic approach as beneficial and safe.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge Melissa Hogg, MD who supported and trained us in robotic pancreatic surgery.
Materials
| Systems | |||
| Arietta Ultrasound | Hitachi | L43K / arietta v70 | Used for intraoperative laparoscopic ultrasonography. |
| da Vinci Surgeon Console | IS | SS999 | Used to control the surgical robot. |
| da Vinci Vision Cart | IS | VS999 | The vision cart houses advanced vision and energy technologies and provides communications across da Vinci system components. |
| da Vinci Xi | IS | K131861 | The surgical robot: ‘patient side-cart’ |
| Instruments | |||
| Cobra Liver Retractor Diamond-Flex | CareFusion | 89-6216 | Retracting the liver for optimal exposure of the surgical site. |
| da Vinci Xi Endoscope with Camera, 8 mm, 30° | IS | 470027 | The camera of the da Vinci robot. |
| ENDOEYE Rigid Video Laparoscope, 10 mm, 30° | Olympus | WA50042A | To see within the intra-abdominal cavity. |
| ENDOWRIST Fenestrated Bipolar Forceps | IS | 470205 | Used for dissection and coagulation. |
| ENDOWRIST HOT SHEARS | IS | 470179 | Used for cutting and coagulation. |
| ENDOWRIST Mega SutureCut Needle Driver | IS | 470309 | Used as a needle driver. |
| ENDOWRIST Permanent Cautery Hook | IS | 470183 | Used for coagulation. |
| ENDOWRIST PROGrasp Forceps | IS | 470093 | Used for dissection. |
| LigaSure Dolphin Tip 37cm | Medtronic | LS1500 | Used for vessel sealing and dividing. |
References
- Van Der Gaag, N. A., et al. Functional and medical outcomes after tailored surgery for pain due to chronic pancreatitis. Annals of Surgery. 255 (4), 763-770 (2012).
- Cahen, D. L., et al. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology. 141 (5), 1690-1695 (2011).
- Cahen, D. L., et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. New England Journal of Medicine. 356, 676-684 (2007).
- Zhao, X., Cui, N., Wang, X., Cui, Y. Surgical strategies in the treatment of chronic pancreatitis: An updated systematic review and meta-analysis of randomized controlled trials. Medicine (United States). 96 (9), 6220 (2017).
- Meehan, J. J., Sawin, R. Robotic lateral pancreaticojejunostomy (Puestow). Journal of Pediatric Surgery. 46 (6), 5-8 (2011).
- Kempeneers, M. A., et al. Multidisciplinary treatment of chronic pancreatitis: an overview of current step-up approach and new options. Nederlands Tijdschrift voor Geneeskunde. 161, (2017).
- Kim, E. Y., Hong, T. H. Laparoscopic Longitudinal Pancreaticojejunostomy Using Barbed Sutures: an Efficient and Secure Solution for Pancreatic Duct Obstructions in Patients with Chronic Pancreatitis. Journal of Gastrointestinal Surgery. 20 (4), 861-866 (2016).
- Khan, A. S., Siddiqui, I., Vrochides, D., Martinie, J. B. Robotic pancreas drainage procedure for chronic pancreatitis: robotic lateral pancreaticojejunostomy (Puestow procedure). Journal of Visceral Surgery. 4 (4), 72 (2018).
- Khaled, Y. S., Ammori, M. B., Ammori, B. J. Laparoscopic lateral pancreaticojejunostomy for chronic pancreatitis: A case report and review of the literature. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 21 (1), 36-40 (2011).
- Puestow, C. B., Gillesby, W. J. Retrograde Surgical Drainage of Pancreas for Chronic Relapsing Pancreatitis. A.M.A Archives of Surgery. 76 (6), 898-907 (1958).
- Adams, D. B. The Puestow Procedure: How I Do It. Journal of Gastrointestinal Surgery. 17 (6), 1138-1142 (2013).
- Eid, G. M., Entabi, F., Watson, A. R., Zuckerbraun, B. S., Wilson, M. A. Robotic-Assisted Laparoscopic Side-to-Side Lateral Pancreaticojejunostomy. Journal of Gastrointestinal Surgery. 15, 1243 (2011).
- Wright, G. P., Zureikat, A. H. Development of Minimally Invasive Pancreatic Surgery: an Evidence-Based Systematic Review of Laparoscopic Versus Robotic Approaches. Journal of Gastrointestinal Surgery. 20 (9), 1658-1665 (2016).
- D’Haese, J. G., Ceyhan, G. O., Demir, I. E., Tieftrunk, E., Friess, H. Treatment options in painful chronic pancreatitis: A systematic review. HPB. 16 (6), 512-521 (2014).
- Bachmann, K., Izbicki, J. R., Yekebas, E. F. Chronic pancreatitis: Modern surgical management. Langenbeck’s Archives of Surgery. 396, 139-149 (2011).
- Khaled, Y., Ammori, B. Laparoscopic lateral pancreaticojejunostomy and laparoscopic berne modification of beger procedure for the treatment of chronic pancreatitis: The first UK experience. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 24 (5), 178-182 (2014).
- Palanivelu, C., et al. Laparoscopic lateral pancreaticojejunostomy: A new remedy for an old ailment. Surgical Endoscopy and other Interventional Techniques. 20 (3), 458-461 (2006).
- Tantia, O., Jindal, M. K., Khanna, S., Sen, B. Laparoscopic lateral pancreaticojejunostomy: Our experience of 17 cases. Surgical Endoscopy and Other Interventional Techiques. 18 (7), 1054-1057 (2004).
- Eid, G. M., Entabi, F., Watson, A. R., Zuckerbraun, B. S., Wilson, M. A. Robotic-Assisted Laparoscopic Side-to-Side Lateral Pancreaticojejunostomy. Journal of Gastrointestinal Surgery. 15 (7), 1243-1243 (2011).
- Hamad, A., Zureikat, A. H., Zeh, H. J., Boggi, U. Minimally Invasive Drainage Procedures for Chronic Pancreatitis. Minimally Invasive Surgery of the Pancreas. , 115-121 (2018).
- Wright, G. P., Zureikat, A. H. Development of Minimally Invasive Pancreatic Surgery: an Evidence-Based Systematic Review of Laparoscopic Versus Robotic Approaches. Journal of Gastrointestinal Surgery. 20, 1658-1665 (2016).

