Multimodal 3D Printing of Phantoms to Simulate Biological Tissue
Summary
Spin coating, polyjet printing, and fused deposition modeling are integrated to produce multilayered heterogeneous phantoms that simulate structural and functional properties of biological tissue.
Abstract
Biomedical optical imaging is playing an important role in diagnosis and treatment of various diseases. However, the accuracy and the reproducibility of an optical imaging device are greatly affected by the performance characteristics of its components, the test environment, and the operations. Therefore, it is necessary to calibrate these devices by traceable phantom standards. However, most of the currently available phantoms are homogeneous phantoms that cannot simulate multimodal and dynamic characteristics of biological tissue. Here, we show the fabrication of heterogeneous tissue-simulating phantoms using a production line integrating a spin coating module, a polyjet module, a fused deposition modeling (FDM) module, and an automatic control framework. The structural information and the optical parameters of a "digital optical phantom" are defined in a prototype file, imported to the production line, and fabricated layer-by-layer with sequential switch between different printing modalities. Technical capability of such a production line is exemplified by the automatic printing of skin-simulating phantoms that comprise the epidermis, dermis, subcutaneous tissue, and an embedded tumor.
Introduction
Biomedical optical imaging represents a family of medical imaging tools that detect diseases and tissue anomalies based on light interactions with biological tissue. In comparison with other imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT), biomedical optical imaging takes the advantage of noninvasive measurement of tissue structural, functional, and molecular characteristics using low-cost and portable devices1,2,3,4. However, despite its superiority in cost and portability, optical imaging has not been widely accepted for clinical diagnosis and therapeutic guidance, partially due to its poor reproducibility and lack of quantitative mapping between optical and biological parameters. The main reason for this limitation is the lack of traceable standards for quantitative calibration and validation of biomedical optical imaging devices.
In the past, a variety of tissue-simulating phantoms were developed for biomedical optical imaging research in various tissue types, such as brain5,6,7, skin8,9,10,11,12, bladder13, and breast tissues14,15,16,17. These phantoms are primarily produced by one of the following fabrication processes: 1) spin coating10,18 (for simulating homogenous and thin-layered tissue); 2) molding19 (for simulating bulky tissue with geometric features); and 3) three-dimensional (3D) printing20,21,22 (for simulating multilayered heterogeneous tissue). Skin phantoms produced by molding are able to mimic the bulk optical properties of skin tissue but cannot simulate the lateral optical heterogeneities19. Bentz et al. used a two-channel FDM 3D printing method to mimic different optical properties of biological tissue23. However, using two materials cannot sufficiently simulate tissue optical heterogeneity and anisotropy. Lurie et al. created a bladder phantom for optical coherence tomography (OCT) and cystoscopy by combining 3D printing and spin coating13. However, heterogeneous features of the phantom, such as blood vessels, had to be hand painted.
Among the above phantom fabrication processes, 3D printing provides the most flexibility for simulating the structural and functional heterogeneities of biological tissue. However, many biological tissue types, such as skin tissue, consist of multilayered and multiscaled components that cannot be effectively duplicated by a single 3D printing process. Therefore, integration of multiple manufacturing processes is necessary. We propose a 3D printing production line that integrates multiple manufacturing processes for automatic production of multilayered and multiscaled tissue simulating phantoms as a traceable standard for biomedical optical imaging (Figure 1). Although spin coating, polyjet printing, and FDM are automated in our 3D printing production line, each modality retains the same functional characteristics as the established processes. Therefore, this paper provides a general guideline for producing multiscaled, multilayered, and heterogeneous tissue-simulation phantoms without the need for physical integration of multiple processes in a single apparatus.
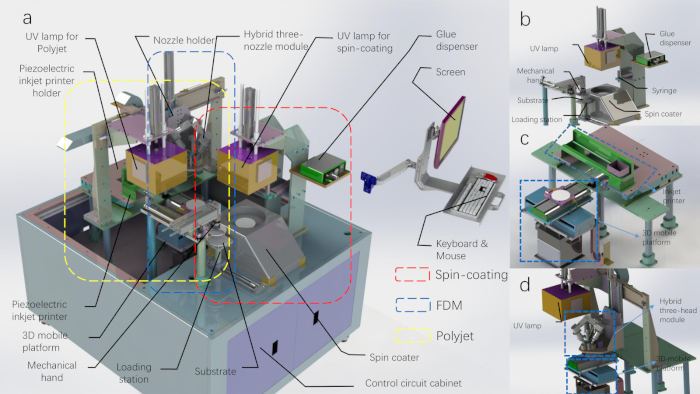
Figure 1: The CAD diagram of the 3D printing production line. (A) The 3D printing production line with the top shell removed. (B) The schematic of the spin coating module and the mechanical hand module. (C) The schematic of the polyjet printing module. (D) The schematic of the FDM printing module (the UV lamp belongs to the polyjet printing module). Please click here to view a larger version of this figure.
Protocol
1. Preparing materials for 3D printing
NOTE: Our optical phantom production line uses a variety of printing materials to simulate the structural and functional heterogeneities of biological tissue. The selection of the printing materials also depends on the manufacturing processes.
- Material preparation for spin coating printing
- Add 100 mg of titanium dioxide (TiO2) powder into a beaker containing 100 mL of stereolithography (SLA) photopolymer resin.
- Stir the mixture in the beaker for 30 min on a magnetic stirrer.
- Seal the beaker with tinfoil and sonicate it in an ultrasonic machine for 15 min.
- Vacuum the material for 10 min and load it into the storage syringe of the device.
- Material preparation for polyjet printing
- Add 17.56 g of 2-hydroxy-2-methylpropiophenone (1-hydroxycyclohexyl phenyl ketone) into the beaker containing 80 g of triethylene glycol dimethacrylate to get 18% (w/w) material.
- Seal the beaker with tinfoil and sonicate it in an ultrasonic machine for 15 min.
- Take out 20 mL of the mixture and add 5 mg of the oil-soluble Chinese red dye into it. Repeat step 1.2.2.
- Vacuum all the materials, load the solution with dye into the cartridges for the Y (Yellow) channel, and load the pure solution into the cartridges for the K (Black) channel.
- Material preparation for FDM printing
- Load 200 g of gel wax into each of three beakers and then heat them to 60 °C on a magnetic stirrer.
- Add 600 mg TiO2 powder into the first beaker. Add 80 mg of graphite powder into the second one.
- Stir the gel wax mixed with TiO2 and gel wax mixed with graphite powder in different beakers for 30 min on the magnetic stirrer.
- Vacuum the three different materials for 2 min and load them into the extruder of the hybrid-three-nozzle module before solidification.
2. Preparing computer models for multimodal 3D printing
NOTE: The heterogeneous skin tissue is simplified into three layers: epidermis, dermis, and subcutaneous tissue. The epidermis layer is produced by spin coating using the material introduced in step 1.1. The dermis layer is produced by polyjet printing using the photosensitive polymer introduced in step 1.2. The subcutaneous tissue layer is produced by FDM using the material introduced in step 1.3. A prototype computer aided design (CAD) file of different printing parameters is generated to guide the aforementioned fabrication processes.
- Design of a digital optical phantom for skin
- Design the skin phantom with the following three layers: an epidermis layer of 100 µm thick, a dermis layer of 400 µm thick, and a subcutaneous tissue of 1 cm thick.
- Draw a tumor model using a 3D modeling software package (e.g., Solidworks) (Figure 5A).
- Parameter setting for spin coating
- Set the parameters of rotating speed and duration in the control software of the printing device. The first-stage spin coating speed used in this demonstration is 200 revolutions per min (rpm), the spin coating time is 20 s, the speed in the second-stage spin coating is 1,000 rpm, and the spin coating time is 40 s.
- Set the amount of spin coating material as 3 mL and the time of light-curing as 180 s in the control software.
- Preparation of the source file for polyjet printing
- Import the blood vessel image to be printed into the AcroRIP Color software package and set the parameters (printing position and inkjet amount) according to the relationship between the optical parameters of the printed phantoms and the image properties. In this printed blood vessel picture, the K channel is loaded with a transparent photocurable material, and the Y channel is loaded with a photocurable material mixed with Chinese red dye.
- Generate a ".prn" file with parameters defined for 3D printing.
- Preparation of G code for FDM printing
- Draw a frustum model with a 3D mapping software package (e.g., Solidworks) to simulate a tumor.
- Import the ".stl" file of the tumor model into a Cura software package installed with an all-in-one nozzle slicing script.
- Slice the model to generate the G code required for printing.
- Loading of the documents to the printing control software
- Click the "File" menu item in the menu bar, select the "Import UV Print File" submenu item, and load the UV printing ".prn" files introduced in step 2.3.
- Load the G code generated in step 2.4 into the print control software as in step 2.5.1.
- Click the Start Printing button to start the automated 3D printing procedure.
3. Printing the skin epidermis layer phantom component by spin coating
NOTE: The spin coating module is mainly comprised of three parts: 1) a spin coater; 2) a glue dispenser; and 3) a UV lamp.
- Move the substrate on the loading station to the sample stage of the spin coater with a mechanical hand. Start the vacuum pump to fix the substrate by adsorption.
- The glue dispenser controls the syringe to drip the material introduced in step 2.2.2 at the center of the substrate.
- The spin coater starts to work following the set speed and time parameters.
- Put down the UV lamp (wavelength: 395 nm) and turn it on for 180 s.
- Lift the UV lamp, turn off the spin-coater, and print the skin epidermis layer.
4. Printing the skin dermis layer phantom component by polyjetting
NOTE: The polyjet printing module consists of a piezoelectric inkjet nozzle, a three-dimensional mobile platform, a control panel, and a UV lamp (mercury lamp). The solvent-based photocurable material, absorption material, and scattering material are used as a matrix. Different optical parameters are obtained by spraying materials in different proportions in different regions. Finally, the dermis layer phantom is printed and cured layer-by-layer.
- Move the substrate to the 3D mobile platform and open the suction valve to adsorb the substrate on the platform.
- The 3D mobile platform holds the substrate to the initial position of the UV printer.
- Push the inkjet printer to the working position by the cylinder, and the inkjet printer works the time specified in the ".prn" file sent by the host computer. Here, the paper feed signal of the inkjet printer is used to drive the movement of the Y-axis mobile platform.
- The inkjet printer prints the layer designed in step 2.5.1 and the cylinder pushes the inkjet printer back to the original position. The Y-axis of the 3D moving platform placed with the substrate is initialized by moving to its initial position.
- The substrate moves 50 mm in the positive direction of the Y-axis. The UV lamp is pushed down by the cylinder (10 mm above the substrate).
- Turn on the UV lamp for 180 s according to the curing time setting.
- Push the UV lamp to the initial position with the cylinder. The Y-axis of the 3D mobile platform placed with the substrate is initialized and returned to its initial position.
- Move the 3D mobile platform placed with substrate down by 0.1 mm along the Z-axis.
- Repeat steps 4.1–4.8 to print the next layer until the multilayer printing is completed.
5. Printing the subcutaneous tissue phantom component by FDM
NOTE: The FDM module is comprised of a hybrid-three-head module, a single-head module, and a 3D mobile platform. The gel wax, the absorbing material, and the scattering material are used as raw materials to prepare a phantom simulating subcutaneous tissue/tumor. The gel wax is heated and melted in the feeder. Uniformly stirred by the extrusion head, it is extruded to print the final phantoms with the desired optical parameters.
- Turn on the heating power of the nozzle module and set the temperature to 60 °C.
- Move the mixing nozzle to the working position by pushing the cylinder.
- The FDM module receives the G code commands sent by the host computer, and the mixing nozzle is heated up to 68 °C.
- Turn on the agitation motor and mix the materials well.
- Initialize the 3D mobile platform and the XYZ axes move to the initial position.
- The printing process is executed following the G code commands. In a layer-by-layer printing procedure, the materials are extruded in proportion to the mixing ratio that determine the optical parameters of the phantom in each layer. The printing continues until the subcutaneous tissue portion or the tumor portion is fully printed.
- Move the mixing nozzle module to the initial position by pushing the cylinder.
CAUTION: Because graphite powder has strong light absorption, it needs to be mixed as uniformly as possible to avoid changes in the optical parameters induced by aggregation. TiO2 powder of large particle size easily precipitates and affects material placement accuracy, so it is necessary to fully mix it. TiO2 should be replaced if stored for a long time.
6. Moving the substrate back to the loading station
- Initialize the 3D mobile platform and move the XYZ axis to the initial position. Move the 3D mobile platform to the handover location.
- Move the mechanical hand to the position above the substrate by pushing the cylinder.
- Pick up the substrate and move it over the loading station with the mechanical hand. Place the substrate on the loading station and complete the automated printing.
7. Casting the subcutaneous tissue layer phantom component by molding
NOTE: If the tumor model for the phantom is designed, it will be necessary to cast the entire phantom by pouring the polydimethylsiloxane (PDMS) outside the tumor. Steps 7.1–7.3 are not required for the FDM module to print subcutaneous tissue layer without a tumor.
- Press on a substrate with a 3D printed rectangular mold.
- Pour liquid PDMS into the mold.
- Place the substrate in an incubator and store at 60 °C for 2 h.
- Remove the phantom from the substrate.
Representative Results
Phantom fabricated by spin coating
The spin coating evenly distributes the droplets on the substrate by rotating the turntable, and a single layer of the original body is fabricated after curing. The rotational speed of the substrate and the time of rotation not only affect the surface quality of the phantom, but also determine the thickness of each layer of the phantom. Phantoms of different thicknesses can be fabricated by repetitive spin coating layer-by-layer. The optical parameters of the phantoms can be determined by changing the proportion of scattering and absorption materials, as described in our previous publication24. Increasing the TiO2 concentration in the photocurable resin will increase the scattering coefficient of the phantom. Considering that spin coating has a precision of 0.01 mm and the skin epidermis is between 0.04–1.6 mm thick, the process satisfies the requirement for simulating the skin epidermis (Figure 2).
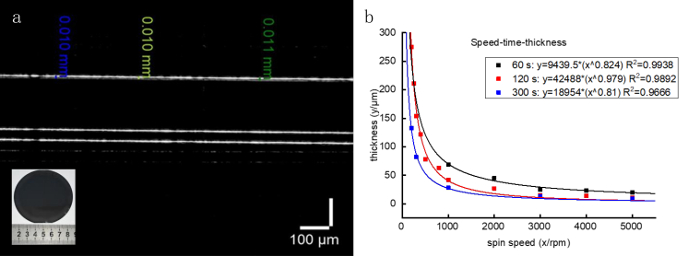
Figure 2: A single layer phantom fabricated by spin coating. (A) The PDMS material is added to 50% proportional tert-butyl alcohol and spin-coated at 3,000 rpm for 40 s to form the single layer phantom. The thickness of the phantom is 10 ± 1 μm as measured by OCT. (B) Correlations between the achievable thickness of the PDMS film and the spin speed at different spinning times. Please click here to view a larger version of this figure.
Phantom fabricated by polyjet printing12
Light-curable materials from different channels are mixed with different optical particles and printed by piezoelectric inkjets on a substrate according to the ".prn" file. A single layer of the phantom is obtained after curing. The resolution of the polyjet printer is 18 μm x 18 μm x 10 μm (length x width x height), the positional resolution of the mobile platform is 1 μm, and the nozzle supports four different types of printing materials. The accuracy of the printing plane is 50 μm, and the thickness of each layer is determined by the amount of the ejected materials. As the ejection amount of a single channel is set at 60%, the mean thickness of each layer is 100 ± 10 μm. The dermis layer of skin tissue is typically between 0.4–2.4 mm thick, and the inkjet printing module is able to reach a thickness resolution of 100 μm. The epidermal blood vessels are simulated by mixing the printing materials with Chinese red dye (Figure 3).
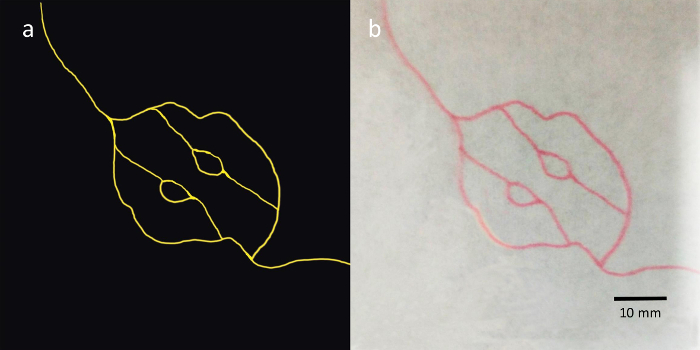
Figure 3: Blood vessel simulations printed by polyjet printing. (A) Blood vessel picture for printing lines mimicking blood vessels. (B) The lines mimicking blood vessels printed on a white paper, where the paper is fixed on the substrate of the 3D mobile platform in the printing process. Please click here to view a larger version of this figure.
Phantom fabricated by FDM printing
Gel wax is mixed with graphite powder and TiO2 powder and printed in a desired shape by FDM printing. The dimensional error in the horizontal direction of the phantom is less than 1%. The lateral length of the phantom exceeds 20 mm, the minimally printable feature is 1 mm, and the printable range is 100 mm x 100 mm x 20 mm. The absorption and scattering parameters of a phantom depend on the ratio of the TiO2 and graphite powder inside. Figure 4 presents phantoms of different feature sizes printed by the FDM printing using the gel wax without TiO2 and graphite powder. We can change the ratio of TiO2 to graphite powder during printing, and thus fabricate phantoms of different absorption and scattering parameters, including gradients (Figure 4B). The correlation of absorption and scattering parameters with the ratio of TiO2 to graphite powder can be found in the references24.
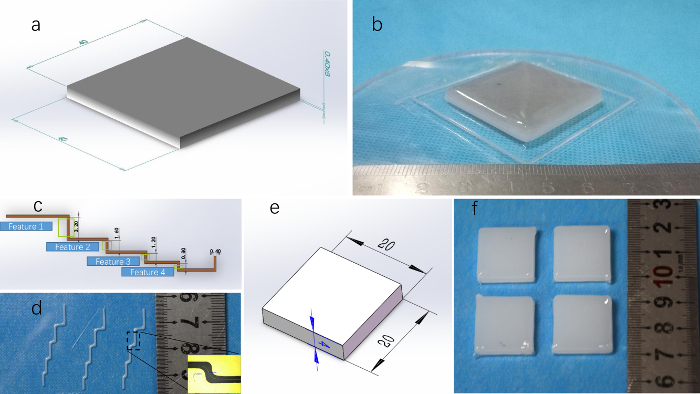
Figure 4: Results of FDM printing. (A) An eight-layer 40 mm x 40 mm x 0.4 mm cuboid model with gradient color. (B) Gradient phantom obtained by printing the gel wax mixed with TiO2 and graphite powder in a gradual scale. (C) CAD model in multi-corner shape. (D) Multi-corner model printed. The bottom right of the picture is the result measured under a front view microscope. The minimum print feature of FDM is 1 mm. (E) Cuboid phantoms printed in the FDM module. (F) The measured results indicate that the variation in size is less than 1% when the lateral dimension is above 20 mm. Please click here to view a larger version of this figure.
Phantom fabricated by automated printing production line
By integrating the above three printing methods and following the aforementioned protocol, the production line system is able to produce a tumor-simulating phantom. Taking a simplified skin model as an example, the epidermis layer, the dermis layer, and the subcutaneous tissue layer with different thicknesses and optical properties are fabricated by spin coating, polyjet printing, and FDM printing, respectively. Therefore, the possibility of combining spin coating, polyjet printing, and FDM printing to produce optical phantoms was verified, and the system was able to produce tissue optical phantoms with the simulated optical and structural characteristics (Figure 5, Figure 6).
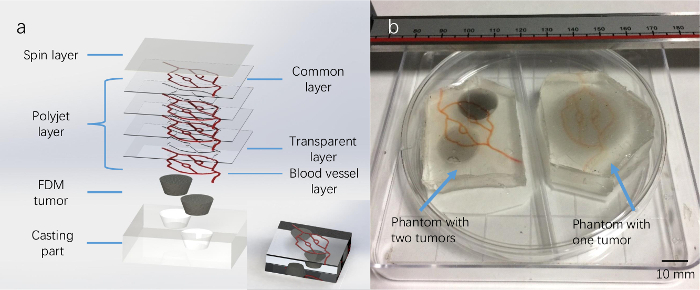
Figure 5: Fabricated multilayer skin phantoms with an embedded tumor. (A) A schematic diagram of a multilayered structure of a tumor phantom, including one spin-coated layer, seven polyjet printed layers (including three transparent layers and three layers of blood vessel layers, and one common layer, and one FDM printed tumor). The bottom right of the picture is a schematic rendering of the phantom. (B) The phantom on the left has two embedded tumors and the right one has one embedded tumor. Please click here to view a larger version of this figure.
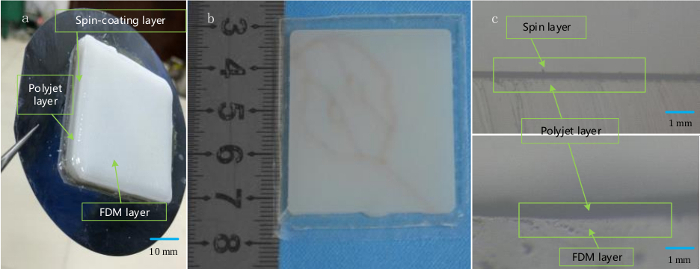
Figure 6: Fabricating multilayered skin-mimicking phantoms. (A) A multilayered skin phantom printed on a silicon wafer consists of a spin coating layer, a polyjet printing layer, and an FDM printing layer from bottom to top. (B) Front view of the phantom embedded with blood vessel-like grooves on its surface. (C) Microscopic image of a cross-section of the phantom showing the different layers. Please click here to view a larger version of this figure.
Discussion
In the fabrication of the multilayered phantom, the material used for spin coating is a kind of light-curable material instead of PDMS. The intermediate layer is printed with the polyjet printing method, which uses the light-curable resin as raw material. Although thin PDMS phantoms can be made by spin coating after adding tert-butyl alcohol, a PDMS layer cannot effectively bind to the light-curable material during polyjet printing. Therefore, we chose the light-curable resin for spin coating.
Currently, only two materials are available for polyjet printing. The addition of TiO2 powder and Indian ink to the light-curable material simulates the optical properties of the dermis layer, which can be added into the system in future work.
For FDM printing, the materials should be thoroughly mixed before extrusion. Therefore, the process delay due to mixing may be longer than for the traditional FDM printing process. The movement of the substrate on the 3D mobile platform is also delayed for the corresponding time during printing. To print phantoms with complex shapes, control of the delay needs to be improved.
The last step in the fabrication of the tumor-simulating phantom is casting. In fact, in the design of the nozzle assembly, an added nozzle is used to inject a fourth material. However, the control of the movement process of the 3D mobile platform is complicated, and the nozzle may destroy the original tumor model. This can be improved by redesigning the motion control program.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant Nos. 11002139 and 81327803) and the Fundamental Research Funds for the Central Universities. We thank Zachary J. Smith of the University of Science and Technology for providing the audio voiceover.
Materials
| 2-Hydroxy-2-methylpropiophenone | aladdin | H110280-500g | Light initiator http://www.aladdin-e.com/ |
| 3D printing control system | USTC | USTC-3DPrinter_control1.0 | custom-made github: https://github.com/macanzhen/ |
| 3D printing system | USTC | USTC-3DPrinter1.0 | custom-made |
| AcroRip color | Human Plus | AcroRip v8.2.6 | |
| All-in-one nozzle slicing script | Shenzhen CBD Technology Co.,Ltd. | github: https://github.com/macanzhen/ |
|
| Chinese Red Dye | Juents | Oil-soluble | |
| Cura | Ultimaker | Cura_15.04.6 | |
| Gel Wax | Shanghai Lida Industry Co.,ltd. | LP | melting point: 56 °C |
| Graphite | aladdin | G103922-100g | Change object optical absorption parameters http://www.aladdin-e.com/ |
| PDMS | Dow Corning | 184 | |
| Titanium dioxide | ALDRICH | 24858-100G | 347 nm |
| Triethylene glycol dimethacrylate | aladdin | T101642-250ml | Photocured monomer http://www.aladdin-e.com/ |
| UV ink SLA Photopolymer Resin | time80s | RESIN-A | http://www.time80s.com/zlxz |
References
- Lu, G., Fei, B. Medical hyperspectral imaging: a review. Journal of Biomedical Optics. 19 (1), 010901 (2014).
- Wang, K., et al. Development of a non-uniform discrete Fourier transform based high speed spectral domain optical coherence tomography system. Optics Express. 17 (14), 12121-12131 (2009).
- Zhao, H., Gao, F., Tanikawa, Y., Homma, K., Yamada, Y. Time-resolved diffuse optical tomographic imaging for the provision of both anatomical and functional information about biological tissue. Applied Optics. 44 (10), 1905-1916 (2005).
- Ding, Z., Ren, H., Zhao, Y., Nelson, J. S., Chen, Z. High-resolution optical coherence tomography over a large depth range with an axicon lens. Optics Letters. 27 (4), 243-245 (2002).
- Iida, H., et al. Three-dimensional brain phantom containing bone and grey matter structures with a realistic head contour. Annals of Nuclear Medicine. 27 (1), 25-36 (2013).
- Mobashsher, A. T., Abbosh, A. Three-dimensional human head phantom with realistic electrical properties and anatomy. IEEE Antennas and Wireless Propagation Letters. 13, 1401-1404 (2014).
- Li, J. B., et al. A new head phantom with realistic shape and spatially varying skull resistivity distribution. IEEE Transactions on Biomedical Engineering. 61 (2), 254-263 (2013).
- Bykov, A., et al. Multilayer tissue phantoms with embedded capillary system for OCT and DOCT imaging. Life Sciences. (International Society for Optics and Photonics). , 73760 (2011).
- Bykov, A. V., Popov, A. P., Priezzhev, A. V., Myllylä, R. Skin phantoms with realistic vessel structure for OCT measurements in Laser Applications. European Conference on Biomedical Optics. , 80911 (2010).
- Park, J., et al. Fabrication of double layer optical tissue phantom by spin coating method: mimicking epidermal and dermal layer. Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue V. , 85830 (2013).
- Wróbel, M. S., et al. Use of optical skin phantoms for preclinical evaluation of laser efficiency for skin lesion therapy. Journal of Biomedical Optics. 20 (8), 085003 (2015).
- Sheng, S., Wu, Q., Han, Y., Dong, E., Xu, R. Fabricating optical phantoms to simulate skin tissue properties and microvasculature. Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue Vii. , 932507 (2015).
- Lurie, K. L., Smith, G. T., Khan, S. A., Liao, J. C., Ellerbee, A. K. Three-dimensional, distendable bladder phantom for optical coherence tomography and white light cystoscopy. Journal of Biomedical Optics. 19 (3), 36009 (2014).
- Hahn, C., Noghanian, S. Heterogeneous breast phantom development for microwave imaging using regression models. Journal of Biomedical Imaging. 2012, 6 (2012).
- Ansari, M. A., Mohajerani, E. Estimation of optical abnormalities in breast phantom by diffuse equation. Optik-International Journal for Light and Electron Optics. 125 (20), 5978-5981 (2014).
- Roman, M., Gonzalez, J., Carrasquilla, J., Erickson, S. J., Godavarty, A. A Gen-2 Hand-Held Optical Imager: Phantom and Preliminary in-vivo Breast Imaging Studies. 29th Southern Biomedical Engineering Conference. , 103-104 (2013).
- Michaelsen, K. E., et al. Anthropomorphic breast phantoms with physiological water, lipid, and hemoglobin content for near-infrared spectral tomography. Journal of Biomedical Optics. 19 (2), 026012 (2014).
- Park, J., et al. Optical tissue phantoms based on spin coating method. Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue VII. , 93250 (2015).
- Mustari, A., et al. Agarose-based tissue mimicking optical phantoms for diffuse reflectance spectroscopy. Journal of Visualized Experiments. (138), e57578 (2018).
- Luciano, N. J., et al. Utilizing 3D printing technology to merge MRI with histology: A protocol for brain sectioning. Journal of Visualized Experiments. (118), e54780 (2016).
- Dong, E., et al. Three-dimensional fuse deposition modeling of tissue-simulating phantom for biomedical optical imaging. Journal of Biomedical Optics. 20 (12), 121311 (2015).
- Beltrame, E. D. V., et al. 3D Printing of Biomolecular Models for Research and Pedagogy. Journal of Visualized Experiments. (121), e55427 (2017).
- Bentz, B. Z., Chavan, A. V., Lin, D., Tsai, E. H., Webb, K. J. Fabrication and application of heterogeneous printed mouse phantoms for whole animal optical imaging. Applied Optics. 55 (2), 280-287 (2016).
- Liu, G., et al. Fabrication of a multilayer tissue-mimicking phantom with tunable optical properties to simulate vascular oxygenation and perfusion for optical imaging technology. Applied Optics. 57 (23), 6772-6780 (2018).

