3D Culturing of Organoids from the Intestinal Villi Epithelium Undergoing Dedifferentiation
Summary
The procedure describes isolation of the villi from the mouse intestinal epithelium undergoing dedifferentiation to determine their organoid forming potential.
Abstract
Clonogenicity of organoids from the intestinal epithelium is attributed to the presence of stem cells therein. The mouse small intestinal epithelium is compartmentalized into crypts and villi: the stem and proliferating cells are confined to the crypts, whereas the villi epithelium contains only differentiated cells. Hence, the normal intestinal crypts, but not the villi, can give rise to organoids in 3D cultures. The procedure described here is applicable only to villus epithelium undergoing dedifferentiation leading to stemness. The method described uses the Smad4-loss-of-function:β-catenin gain-of-function (Smad4KO:β-cateninGOF) conditional mutant mouse. The mutation causes the intestinal villi to dedifferentiate and generate stem cells in the villi. Intestinal villi undergoing dedifferentiation are scraped off the intestine using glass slides, placed in a 70 µm strainer and washed several times to filter out any loose cells or crypts prior to plating in BME-R1 matrix to determine their organoid-forming potential. Two main criteria were used to ensure that the resulting organoids were developed from the dedifferentiating villus compartment and not from the crypts: 1) microscopically evaluating the isolated villi to ensure absence of any tethered crypts, both before and after plating in the 3D matrix, and 2) monitoring the time course of organoid development from the villi. Organoid initiation from the villi occurs only two to five days after plating and appears irregularly shaped, whereas the crypt-derived organoids from the same intestinal epithelium are apparent within sixteen hours of plating and appear spherical. The limitation of the method, however, is that the number of organoids formed, and the time required for organoid initiation from the villi vary depending on the degree of dedifferentiation. Hence, depending upon the specificity of the mutation or the insult causing the dedifferentiation, the optimal stage at which villi can be harvested to assay their organoid forming potential, must be determined empirically.
Introduction
The intestinal crypts but not villi, form organoids when cultured in Matrigel or BME-R1 matrix. These organoids are self-organizing structures, often referred to as the "mini-gut" owing to the presence of the various differentiated lineages, progenitor, and stem cells present in the intestinal epithelium in vivo. The potential to form organoids from crypts is attributed to the presence of stem cells1. The intestinal villi on the other hand consist only of differentiated cells, and hence cannot form organoids. However, mutations2 or conditions that permit dedifferentiation of the villus epithelium may lead to stem cells in the villi2,3. This fate change resulting in stemness in the villi epithelium can be confirmed by plating the dedifferentiating villus epithelium in 3D matrix to determine their organoid-forming potential as an indicator of de-novo stemness in the villus epithelium. Hence, the critical aspect of this procedure is to ensure absence of crypt contamination.
The Smad4KO:β-cateninGOF conditional mutation causes dedifferentiation in the intestinal epithelium marked by the expression of proliferation and stem cell markers in the villi, and eventually the formation of crypt-like structures in the villi referred to as ectopic crypts The presence of stem cells these dedifferentiated villi was determined by the expression of stem cell markers in the ectopic crypts (in vivo) and the ability of the mutant villi to form organoids when plated Matrigel3. The below mentioned procedure elaborates the methodology used to confirm the stemness of the dedifferentiating intestinal epithelium in the Smad4KO:β-cateninGOF mutant mice. A key feature of this methodology for isolating villi was the use of scraping of the intestinal lumen, as opposed to the EDTA chelation method4. Unlike in the EDTA chelation method, villi isolation by scraping retains majority of the underlying mesenchyme and allows adjust the pressure of scraping to yield villi without tethered crypts. The pressure of scraping is subjective to the operator, hence, the optimal pressure to yield villi without crypts tethered must be determined empirically by the operator. The critical aspect of this procedure is to ensure the absence of crypt contamination by microscopic examination of the villi both before and after plating in the BME-R1 matrix.
Intestinal villi are scraped off the intestinal lumen with glass slides and placed in a 70 µm filter and washed with PBS to get rid of loose cells or crypts, if any, prior to plating in BME-R1 matrix. The method stresses on the following criteria to avoid crypt contamination: a) confining the villi harvest the proximal half of the duodenum where the villi are the longest, b) minimizing the number of villi-yielding scrapes, c) washing the filter containing the villi through a series of PBS in a six-well dish, and d) confirming the absence of crypt contamination by microscopic examination prior to and after plating in BME-R1 matrix. Villi isolation by scraping, rather than by EDTA chelation, prevents the complete loss of the underlying mesenchyme that may provide the niche signals5, 6, 7, 8, if required, for organoid initiation from the villus epithelium.
Protocol
All the mouse experiments conducted, including the use of Tamoxifen and euthanasia by cervical dislocation, had the approval of the Institutional Animal Care and Use Committee at Stevens Institute of echnology.
1. Mice
NOTE: The generation of Smad4f/f; Catnblox(ex3)/+; Villin-CreERT2 mice have been previously described3. Adult female mice between eight to twelve weeks of agewere used.
- Induce the Smad4KO:β-cateninGOF mutation in the Smad4f/f; Catnblox(ex3)/+; Villin-CreERT2 with intraperitoneal injection of Tamoxifen in corn oil for four consecutive days.
- Sacrifice the miceby cervical dislocation ten days after the first tamoxifen injection.
- Spray the abdomen with 70% ethanol to prevent mouse fur getting into the peritoneal cavity.
- Open the abdominal cavity with dissection scissors to expose the intestine. Isolate the intestine with the aid of the scissors and forceps.
NOTE: Concomitant loss of Smad4 along with gain of function mutation of β-catenin (Smad4KO;β-cateninGOF) in intestinal epithelium was attained by injectingSmad4f/f; Catnblox(ex3)/+; Villin-CreERT2 mice every day for four consecutive days with 0.05 g Tamoxifen/kg body weight in corn oil. These mice are harvested ten days after the first tamoxifen injection to ensure the presence of cells expressing stem cell-associated markers in the dedifferentiating villi.
2. Duodenum isolation and preparation
- Dissect out the proximal half of the duodenum.
- Flush the duodenum with 5 mL of ice-cold PBS in a 10 mL syringe to clear the luminal content.
- Open the duodenum longitudinally with an angled scissor and lay the duodenum flat on a 15 cm Petri plate on ice with the lumen of the duodenum facing the operator.
3. Villi isolation by scraping
- Prior to beginning the scraping, place a 70 µm mesh strainer in one of the wells of a 6-well tissue culture plate. Fill all the wells with 4 mL of 1x PBS and place the 6-well tissue culture plate on ice (Figure 1).
- Scrape the villi as follows using two microscopic glass slides: one to hold the duodenum down and the other to scrape (Figure 1B1).
- Scrape the luminal side of the duodenum superficially to-and-fro twice to remove the mucus. Apply pressure such that this step removes the mucus layer, but not the villi.
- Scrape the duodenum again, to-and-fro twice applying the same pressure as in 3..1, during which villi can be seen collecting on the slides (Figure 1B2). This is the optimal pressure (which must be determined empirically by the operator) to yield the villi without the crypts tethered.
- Use a 1 mL transfer pipet containing PBS to transfer the villi (that are collected on the slide from step 3.2.2.) to a 70 µm mesh strainer placed in a 6-well dish. The villi are collected thus, after every scrape (Figure 1B2).
- Wash the villi collected in the 70 µm strainer (from step 3.3) by transferring the strainer (with the villi) through a series of wells in a 6-well dish containing cold PBS (~ 4 mL/well). This is to remove loose crypts, if any.
- Using a p1000 pipet, transfer the villi suspension in PBS (~ 3 mL) from the 70 µm strainer to a new 15 mL tube on ice.
- Use a 0.1% BSA coated blunt-ended p200 pipet tip to aspirate 50 µL volume of the villi suspension onto a glass slide. Count the number of villi in the 50 µL droplet under 4x magnification to determine the concentration of villi in the PBS suspension. For example, if there are 10 villi in the 50 µL suspension examined, then the villi concentration is 0.2 villi/µL. This is also the time to confirm the absence of tethered crypts.
- Calculate the volume of villi suspension required to plate at a concentration of 0.5 villi/µL of BME-R1 matrix. Add an extra 100 µL to account for pipetting error and the volume required for microscopic examination required to ensure the purity of the villi.
CAUTION: The pressure used in the first two scrapes, that removes the mucus but not the villi, should be used in the subsequent scrapes during which villi will be released. Limit the number of to-and-fro scrapings to two after the villi release is first observed. This measure avoids release of villi with the crypts tethered. It is essential to microscopically evaluate the villi to ensure the absence of the tethered crypts.
4. Plating of villi on BME-R1 matrix
- Using a 0.1% BSA coated p200 blunt-ended pipet tip, transfer to a microcentrifuge tube the volume of villi (from step 3.6) required to plate at a density of ~ 6 villi per well in 12.5 µL of BME-R1 matrix. The use of BSA-coated blunt-ended tip at this point ensures that the villi, which are too large for a pointed tip are aspirated without being blocked or lost from being stuck to the sides of the tip.
- Spin down the villi for 2 min at 200 x g in a refrigerated centrifuge (4 °C) and remove the supernatant.
- Repeat step 4.2 to remove any residual PBS and proceed to the next step under a laminar flow-hood.
- Resuspend the villi pellet gently in the required amount of cold BME-R1 thawed on ice.
- Using a p20 pipet, plate 12.5 µL/well of the villi in BME-R1 matrix in 3D in a prewarmed 96-well U-bottom plate.
- Incubate the plate in a tissue culture incubator at 37 °C for 15 minutes to allow solidification of the BME-R1 matrix.
- Add 125 µL/well of pre-warmed ENR (Epidermal growth factor/Noggin/R-spondin1) media: advanced DMEM F-12 media, supplemented with 1x Penicillin: Streptomycin, 10 mM HEPES, Glutamax, 50 ng/mL EGF, 100 ng/mL Noggin, 5% conditioned media from HEK293-T cells expressing R-Spondin1, 1x N2, 1x B27, 1 mM N-acetyl cysteine, and 0.05 mg/mL Primocin.
- Incubate the plated villi in a tissue culture incubator maintained at 37 °C with 5% CO2, and change the media every other day.
- Discard any well in which organoids appear before two days of plating, since the earliest time point at which villi-derived organoids are expected is after two days.
NOTE: Any villi that has half or more than half the length of villi are counted as one villus. Unlike crypt-derived organoids, villi-derived organoids are not expected within 24 hours after plating. The organoid-initiating villi appear to be darkening and shrinking prior to formation of organoids. Hence, any wells with organoids developing within a day of plating should be discarded to avoid the plausibility of having derived from plausible crypt contamination. The methodology used to produce the conditioned media is available upon request.
Representative Results
The determining factor for the success of the procedure is preventing crypt contamination. Organoid development from the villi (and not from any contaminating crypts) is ensured by confirming four major criteria: 1) ensuring the purity of the harvested villi by microscopic examination before and after plating the villi in BME-R1, 2) plating limited number of villi per well to allow visualization of all the plated villi individually, 3) onitoring the development of organoid daily; images of the time course show development of organoid from villi (Figure 3), and 4) onitoring the kinetics and morphological appearance of the organoids initiating from villi; the organoids initiating from villi appear irregularly shaped at first and take two to five days before they can be seen under 4x magnification as opposed to the crypt-derived organoids (cultured by isolating crypts using the EDTA/PBS chelation method1,4) that appear overnight as spherical structures with well-defined borders (Figure 2).
The optimal time for sacrificing the mice for testing the organoid-forming potential of the villi is after the dedifferentiating villi-epithelium express stem cell markers and begin the development of ectopic crypts in the villi in vivo (around 10 days after Tamoxifen injection of the Smad4f/f; Catnblox(ex3)/+; Villin-CreERT2 mice). These mice have a tamoxifen inducible cre-recombinase that causes Smad4 loss concomitant with β-catenin activation with tamoxifen. The ectopic crypts develop fully within two weeks of induction of the mutation, while the stem cells in the villus epithelium appear within a week after the induction of mutation in vivo. The number of villi that form organoids when plated in Matrigel has been reported to vary between 2% to 12%3. Harvesting the mutant mouse for plating the villi around the time when stem cells are present (around a week after the cre-recombinase induction) will prolong the time required for villi-derived organoids appear, whereas delaying the harvest to later than ten days after the cre-induction increases the risk crypt of contamination.
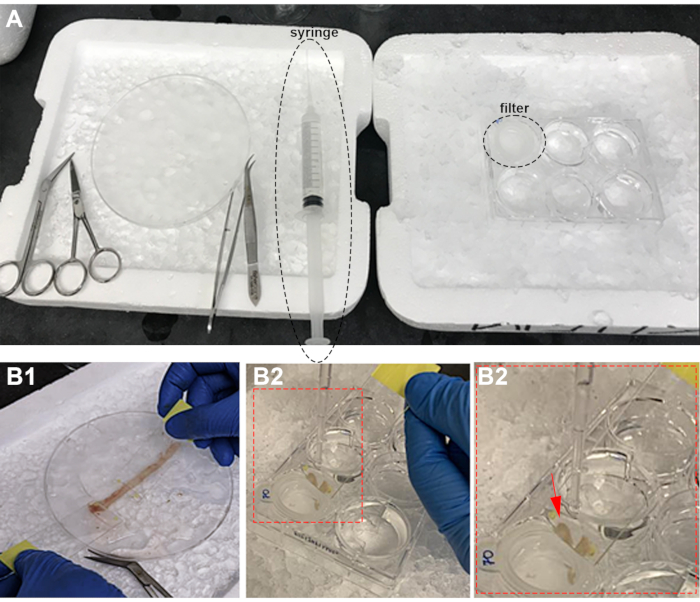
Figure 1: Experimental set-up and villi scraping from intestinal epithelium. A) PBS-filled syringe (syrg) with tubing and filter (Fltr) in PBS. B) Villi isolation by scraping (B1) and transferring to a filter (B2). The arrow points the villi collected on the slide. Please click here to view a larger version of this figure.
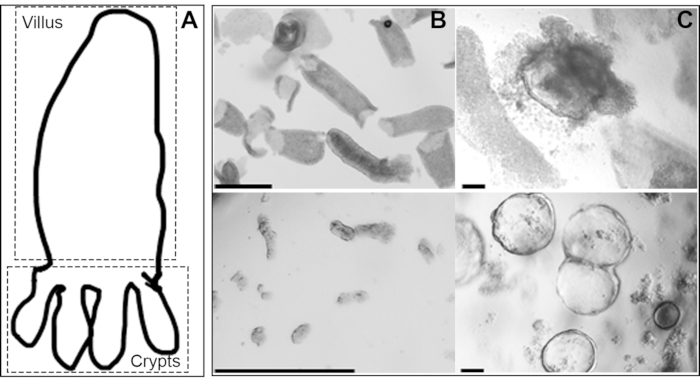
Figure 2: Distinction in the appearance of the organoids emerging from the crypts versus the dedifferentiated villi epithelium from the same mouse intestine: A) Cartoon showing villus and crypts. B) Whole mount (in PBS) of villi prepared by scraping (top panel) and crypts prepared by EDTA-chelation (lower panel). C) Villi-derived (top panel) and crypt-derived (lower panel) organoids from the same mouse intestinal epithelium in BME-R1 (scale bars, 500 um). Please click here to view a larger version of this figure.
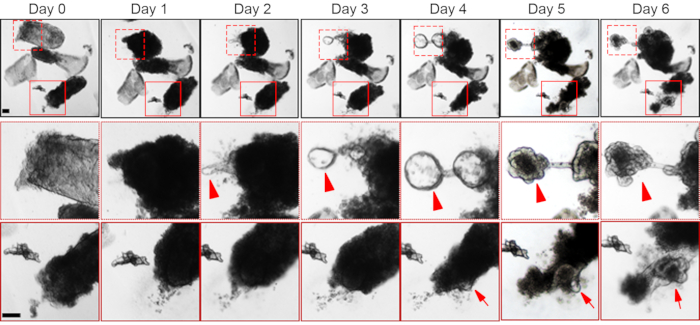
Figure 3: Time course of organoid formation from the dedifferentiating villi. Organoid initiation from two different villi is shown (the boxed regions are enlarged in the middle and bottom panels). The villi with organoid-forming potential appears dense, possibly due to the retention of the underlying mesenchyme. Organoid initiation from the villus is apparent at day two (solid arrow) from one of the villi (box with broken boundary), while in the other villus (box with solid boundary) the organoid appears at day four (arrow). The upper box in the day 6 panel shows a developed villi-derived organoid. Scale bar, 100 µm. Please click here to view a larger version of this figure.
Discussion
This method can confirm the self-renewal capacity of dedifferentiating villi epithelium that acquire stem cell markers in vivo. The normal intestinal epithelium can give rise to organoids from the crypt but not the villi compartment, when cultured in 3D because of the presence of stem cells in the crypts1. Thus, organoid formation from the dedifferentiated villi epithelium cultured in 3D cultures confirms stem cell formation from cell fate reversal. Reports on cell fate reversal in the intestinal epithelium arising from mutations and/or injury are increasing2, 3, 9, 10, 11, 12. Hence, this method is applicable in similar scenarios where the dedifferentiating villi epithelium express stem cell markers in vivo.
We confirmed the development of the organoids from the villi of the Smad4KO:β-cateninGOF mutant through the time course showing development of organoid from the villi (Figure 3). The most critical aspect is taking precautionary measures against crypt contamination, which would lead to false positives. The following measures were taken to ensure the purity of the villi preparation before and after plating: a) isolation of villi from the proximal half of the duodenum where the villi are the longest, b) minimizing the number of scrapes and adjusting the pressure of scraping to yield villi without tethered crypts, c) washing the villi with PBS after placing them in a 70 µm mesh strainer to exclude any loose crypts or cells d) microscopic examination of the harvested villi prior to and after plating, and e) minimizing the number of villi plated per well so that they can be visualized individually in the plated matrix.
Several considerations were made to optimize the procedure. Firstly, we harvested the villi from the proximal duodenum where the villi are the longest. The physical delineation between the differentiation and the proliferative compartments in the small intestine allows us to adopt scraping to mechanically separate the villi from the crypts. This method can thus be adopted only in the cases where the compartmentalization between the differentiation and proliferative compartment is strictly delineated – as is the case in the small intestine but not in the colon. Secondly, we optimized the time of villi isolation after induction of the mutation based on the degree of dedifferentiation observed in vivo. Dedifferentiation in the conditional Smad4KO:β-cateninGOF mutant is marked by the appearance in vivo of stem cell markers and ectopic crypts in the villus epithelium within seven and ten days respectively3 . Thus, the time required to initiate organoids from the mutant villi decreases as the time elapsed after induction of the mutation increases. Therefore, while adapting this method to other dedifferentiation models of the intestine, the optimal time for sacrificing the mice for villi isolation should be determined empirically depending upon the kind of mutation or injury and the degree of ensuing dedifferentiation in vivo. Thirdly, we opted to isolate the villi by scraping rather than by the EDTA-chelation method in order to retain the underlying mesenchyme, as epithelial-mesenchymal cross-talks have been implicated in intestinal stem cell function in the intestine5, 6, 7, 8, 13. Villi that initiate the organoids mainly appear dark possibly due to the presence of the underlying mesenchyme (Figure 2B and 3). We verified the expression of the stem cell associated marker CD4414, 15, and the intestine specific transcription factor Cdx216, 17, 18, 19, 20 confirming the epithelial origin of the villi-derived organoids (Figure S1).
Compared to in vivo approaches, organoids are more amenable to genetic manipulation and screening. Since we observe phenotypic differences between the organoids emerging from the crypts and the villi of the same Smad4KO:β-cateninGOF intestinal epithelium (Figure 2B), we speculate molecular differences between the two, which is currently being investigated. Thus, this method is useful in investigating the implications of dedifferentiation-causing mutations and its differential effects in the progenitor versus differentiation compartment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This publication was supported by Award Number K22 CA218462-03 from the NIH National Cancer Institute. The HEK293-T cells expressing R-Spondin1 was a generous gift from Dr. Michael P. Verzi.
Materials
| Advanced DMEM F-12 media | Gibco | 12634010 | |
| 3,3-diaminobenzidine | Vector Labs | SK-4105 | |
| 96 well U-bottom plate | Fisher Scientific | FB012932 | |
| ABC kit | Vector Labs | PK4001 | |
| Angled scissor | Fisher Scientific | 11-999 | |
| Animal-Free Recombinant Human EGF | Peprotech | AF-100-15 | |
| B-27 Supplement (50X), minus vitamin A | Gibco | 12587010 | |
| Bovine Serum Albumin (BSA) Protease-free Powder | Fisher Scientific | BP9703100 | |
| CD44 antibody | BioLegend | 1030001 | |
| Cdx2 antibody | Cell Signaling | 12306 | |
| Corn oil | Sigma-Aldrich | C8267-500ML | |
| Corning 70-micron cell strainer | Life Sciences | 431751 | |
| Cultrex Reduced Growth Factor Basement Membrane Extract, Type R1 | R&D | 3433-005-R1 | |
| Dissection scissors | Fisher Scientific | 22-079-747 | |
| Forceps | Fisher Scientific | 17-456-209 | |
| Glutamax (100X) | Gibco | 35050-061 | |
| HEK 293-T cells expressing RSPO-1 | Gift from Dr. Michael Verzi | ||
| HEPES (1M) | Gibco | 15630-080 | |
| Histogel | Thermoscientific | HG-4000-012 | |
| Mesh filter | Fisher Scientific | 07-201-431 | |
| Micrscope glass slide | VWR | 89218-844 | |
| N-2 Supplement (100X) | Gibco | 17502048 | |
| N-acetyl cysteine | Sigma-Aldrich | A9165 | |
| p200 Blunt tips | VWR | 46620-642 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140-122 | |
| Primocin (50mg/mL) | Invivogen | ant-pm-1 | |
| Quality Biological Inc PBS (10X) | Fisher Scientific | 50-146-770 | |
| Recombinant Murine Noggin | Peprotech | 250-38 | |
| Signal diluent | Cell Signaling | 8112L | |
| Tamoxifen | Sigma-Aldrich | T5648-1G | |
| 6-well tissue culture plate | Fisher Scientific | 50-146-770 |
References
- Sato, T., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244), 262-265 (2009).
- Schwitalla, S., et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 152 (1-2), 25-38 (2013).
- Perekatt, A. O., et al. SMAD4 Suppresses WNT-Driven Dedifferentiation and Oncogenesis in the Differentiated Gut Epithelium. 암 연구학. 78 (17), 4878-4890 (2018).
- Roche, J. K. Isolation of a purified epithelial cell population from human colon. Methods in Molecular Medicine. 50, 15-20 (2001).
- Aoki, R., et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cellular and Molecular Gastroenterology and Hepatology. 2 (2), 175-188 (2016).
- Seiler, K. M., et al. Tissue underlying the intestinal epithelium elicits proliferation of intestinal stem cells following cytotoxic damage. Cell and Tissue Research. 361 (2), 427-438 (2015).
- Stzepourginski, I., et al. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proceedings of the National Academy of Sciences of the United States of America. 114 (4), 506-513 (2017).
- Roulis, M., et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 580 (7804), 524-529 (2020).
- Haramis, A. P., et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 303 (5664), 1684-1686 (2004).
- Madison, B. B., et al. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 132 (2), 279-289 (2005).
- van Es, J. H., et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature Cell Biology. 14 (10), 1099-1104 (2012).
- Tetteh, P. W., et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 18 (2), 203-213 (2016).
- Baulies, A., et al. The Transcription Co-Repressors MTG8 and MTG16 Regulate Exit of Intestinal Stem Cells From Their Niche and Differentiation Into Enterocyte vs Secretory Lineages. Gastroenterology. 159 (4), 1328-1341 (2020).
- Zeilstra, J., et al. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene. 33 (5), 665-670 (2014).
- Gracz, A. D., et al. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells. 31 (9), 2024-2030 (2013).
- Gao, N., White, P., Kaestner, K. H. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Developmental Cell. 16 (4), 588-599 (2009).
- Verzi, M. P., Shin, H., Ho, L. L., Liu, X. S., Shivdasani, R. A. Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Molecular and Cellular Biology. 31 (10), 2026-2039 (2011).
- Verzi, M. P., Shin, H., San Roman, A. K., Liu, X. S., Shivdasani, R. A. Intestinal master transcription factor CDX2 controls chromatin access for partner transcription factor binding. Molecular and Cellular Biology. 33 (2), 281-292 (2013).
- Grainger, S., Hryniuk, A., Lohnes, D. Cdx1 and Cdx2 exhibit transcriptional specificity in the intestine. PLoS One. 8 (1), 54757 (2013).
- Stringer, E. J., et al. Cdx2 determines the fate of postnatal intestinal endoderm. Development. 139 (3), 465-474 (2012).

