Observing Islet Function and Islet-Immune Cell Interactions in Live Pancreatic Tissue Slices
Summary
This study presents the application of live pancreatic tissue slices to the study of islet physiology and islet-immune cell interactions.
Abstract
Live pancreatic tissue slices allow for the study of islet physiology and function in the context of an intact islet microenvironment. Slices are prepared from live human and mouse pancreatic tissue embedded in agarose and cut using a vibratome. This method allows for the tissue to maintain viability and function in addition to preserving underlying pathologies such as type 1 (T1D) and type 2 diabetes (T2D). The slice method enables new directions in the study of the pancreas through the maintenance of the complex structures and various intercellular interactions that comprise the endocrine and exocrine tissues of the pancreas. This protocol demonstrates how to perform staining and time-lapse microscopy of live endogenous immune cells within pancreatic slices along with assessments of islet physiology. Further, this approach can be refined to discern immune cell populations specific for islet cell antigens using major histocompatibility complex-multimer reagents.
Introduction
Involvement of the pancreas is pathognomonic to diseases such as pancreatitis, T1D, and T2D1,2,3. The study of function in isolated islets usually involves removal of the islets from their surrounding environment4. The live pancreatic tissue slice method was developed to allow for the study of pancreatic tissue while maintaining intact islet microenvironments and avoiding the use of stressful islet isolation procedures5,6,7. Pancreatic tissue slices from human donor tissue have been successfully used to study T1D and have demonstrated processes of beta cell loss and dysfunction in addition to immune cell infiltration8,9,10,11,12,13. The live pancreatic tissue slice method can be applied to both mouse and human pancreatic tissue5,6,8. Human pancreatic tissue slices from organ donor tissues are obtained through a collaboration with the Network for Pancreatic Organ Donors with Diabetes (nPOD). Mouse slices can be generated from a variety of different mouse strains.
This protocol will focus on non-obese diabetic-recombination activating gene-1-null (NOD.Rag1-/-) and T cell receptor transgenic (AI4) (NOD.Rag1-/-.AI4α/β) mouse strains. NOD.Rag1-/- mice are unable to develop T and B cells due to a disruption in the recombination-activating gene 1 (Rag1)14. NOD.Rag1-/-.AI4α/β mice are used as a model for accelerated type 1 diabetes because they produce a single T cell clone that targets an epitope of insulin, resulting in consistent islet infiltration and rapid disease development15. The protocol featured here describes procedures for functional and immunological studies using live human and mouse pancreatic slices through the application of confocal microscopy approaches. The techniques described herein include viability assessments, islet identification and location, cytosolic Ca2+ recordings, as well as staining and identification of immune cell populations.
Protocol
NOTES: All experimental protocols using mice were approved by the University of Florida Animal Care and Use Committee (201808642). Human pancreatic sections from tissue donors of both sexes were obtained via the Network for Pancreatic Organ Donors with Diabetes (nPOD) tissue bank, University of Florida. Human pancreata were harvested from cadaveric organ donors by certified organ procurement organizations partnering with nPOD in accordance with organ donation laws and regulations and classified as "Non-Human Subjects" by the University of Florida Institutional Review Board (IRB) (IRB no. 392-2008), waiving the need for consent. nPOD tissues specifically used for this project were approved as nonhuman by the University of Florida IRB (IRB20140093). The objectives of sections 1-3 of this protocol are to explain how to successfully dissect a mouse, prepare and process the pancreas, and generate live pancreatic tissue slices. Solutions should be prepared ahead of time, and the recipes can be found in Supplemental Table 1. Time is the most critical factor during these protocol steps. Once the mouse has been sacrificed, tissue viability will begin to decline. All three parts of this protocol need to be completed as quickly as possible until all the necessary slices are generated.
1. Preparation for generation of mouse pancreas slices
- Clamp the blade onto the vibratome blade holder, but do not attach it to the vibratome yet.
- Melt 100 mL of 1.25% w/v low melting-point agarose in a microwave. Operate the microwave in 1 min intervals, and stop the microwave for 10 s if the agarose solution begins to boil. Repeat this process until the agarose is melted, and a homogeneous solution is produced. Place the bottle in a 37 °C water bath.
NOTE: The low agarose concentration is to account for the lower density of the mouse pancreas. - Fill a 10 mL Luer lock syringe with 3 mL of warm agarose. Fit a 27 G 25 mm needle onto the syringe. Keep the syringe with the capped attached needle in a 37 °C water bath until the solution is to be injected.
NOTE: A 27 G needle is preferred as it fits securely into the common bile duct of mice between 10-25 g in body weight and allows for the flow of the highly viscous agarose solution. While larger bore needles may be selected for use, smaller (larger gauge) needles are not recommended as these may be more easily clogged with the agarose solution. - Add 20 mL of chilled extracellular solution (ECS) to a 10 cm Petri dish.
NOTE: The ECS does not need to be bubbled at any point. - Fill two 10 cm untreated Petri dishes with 15 mL of Krebs-Ringer bicarbonate buffer (KRBH) containing 3 mM D-glucose and soybean trypsin inhibitor at a concentration of 0.1 mg/mL per dish.
NOTE: Throughout this protocol, it is essential that all solutions used for maintaining slices contain soybean trypsin inhibitor to prevent tissue damage caused by pancreatic digestive proteases.
2. Mouse pancreas excision and tissue processing
NOTE: The protocol for excising the pancreas, processing the tissue, and generating slices is modified from Marciniak et al5. To ensure tissue viability, minimize the amount of time between pancreas removal and slice generation. All necessary equipment should be prepared in advance and oriented in a manner to allow for rapid progression through the steps below. Bile duct canulation and injection as well as pancreas excision are best performed under a stereoscope.
- The mouse is deeply anesthetized with isoflurane and sacrificed by cervical dislocation.
NOTE: Isoflurane is the preferred anesthetization method. A concentration of 5% isoflurane should be used. For example, 0.26 mL should be used with a 1 L chamber16. A decrease in pancreatic tissue viability was observed when CO2 was used. - Spray the mouse with 70% v/v ethanol liberally to reduce tissue contamination with fur during the dissection and excision. Place the mouse in a dorsal down, ventral up orientation with the anterior side to the left.
- Using scissors, open the peritoneum and remove the rib cage, taking care not to puncture the heart or adjacent vessels. Use forceps to flip the liver into the chest cavity, and to move the intestines out of the body cavity to expose the common bile duct. Use a Johns Hopkins bulldog clamp to occlude the ampulla of Vater.
- Retrieve a 10 mL Luer lock syringe preloaded with 3 mL of warm agarose solution from the 37 °C water bath.
NOTE: Once the syringe with the agarose is removed from the water bath, the pancreas injection needs to be performed quickly before the agarose cools and sets in the syringe. - Holding the forceps in the left hand, use them to gently support and stabilize the bile duct for the injection.
- Hold the syringe in the right hand, insert the needle bevel-side up into the bile duct. Slowly and steadily inject the pancreas. Once the injection starts, the flow cannot be stopped without the agarose hardening in the syringe and in the pancreas.
NOTE: The volume used will depend on the weight of the mouse. Based on experience, it is recommended that 1 mL of agarose solution be used per 10 g of mouse body weight with a maximum volume of 2 mL. The pancreas should look slightly inflated with a more definitive structure, but not overextended. Over-injection results in islets that become separated from the exocrine tissue and that have a "blown-out" appearance in the slices. - Excise the agarose-filled pancreas from the mouse. Using forceps and scissors, cut the pancreas away, starting at the stomach, moving to the intestines, and ending at the spleen. Once cut away, gently remove the injected pancreas with forceps, and place in a 10 cm Petri dish filled with chilled ECS.
- Use scissors to remove the adipose, connective tissue, fibrotic tissue, and parts of the pancreas that are not injected with agarose.
NOTE: Parts of the tissue that should be removed will not have strongly established structures and will appear somewhat gelatinous. - After trimming the tissue, use scissors to cut it into smaller sections that are approximately 5 mm in diameter while leaving it submerged under ECS. Cut the tissue carefully, taking care to not push the agarose out of the tissue.
- Remove the pieces of tissue from the ECS, and place them on a two-ply wiper (see the Table of Materials). Gently roll them on the wiper using forceps to remove excess liquid.
- Using forceps, carefully place the pieces of tissue in a 35 mm Petri dish with no more than 4 pieces per plate. Place the flattest side of the tissue block facing downward. Gently press down on the tissue using forceps.
NOTE: Make sure there is space, at least a few millimeters, between the pieces of tissue, and that they are not touching the edge of the plate. - Slowly pour the 37 °C agarose into the dish, taking care not to pour it directly on to the tissue. Pour enough so that the tissue pieces are completely covered. Make sure there is a layer of agarose above the tissue pieces as this part will be glued to the specimen holder.
- Carefully transfer the dish with the pieces of tissue to a refrigerator to allow the agarose to set. Ensure the tissue pieces do not shift or start floating. If they do, quickly readjust them using forceps.
NOTE: Setting of the agarose should only take a few minutes. - Once the agarose has set, use a scalpel to cut around the tissue in straight lines to make agarose blocks as if making a grid between the pieces of tissue. Be sure to leave a few millimeters of agarose surrounding all sides of the tissue.
NOTE: There should not be any tissue protruding from the agarose. Each block should be a cube of approximately 5 mm x 5 mm x 5 mm volume. - Use the scalpel to flip out the empty sections of agarose that were cut around the edges of the plate. Remove the blocks with the tissue from the dish by lifting them carefully with forceps.
3. Mouse pancreatic slice generation
- Using forceps, arrange the blocks on the specimen holder; place them sideways, keeping in mind that they will be flipped onto the super glue. Arrange the blocks so that they do not extend further than the blade width. As the vibratome moves slowly, arrange the blocks so that the blade has to move forward the least possible distance.
NOTE: Two rows of three or four blocks each with a few millimeters between the rows works well. The blocks within the same row can touch each other, but when both rows touch, it can be difficult to retrieve slices as they come off the blocks. - Apply a line of super glue on the specimen holder, and use the end of the glue dispenser to spread the glue out into a thin layer. Flip the tissue blocks onto the glue so that the side closest to the tissue faces upward. Gently push down on the blocks, and let the glue dry for three minutes.
- Attach the blade holder and plate to the vibratome, typically with either a screw or magnet, depending on the vibratome model. Adjust the blade height and distance travelled so that the blade moves over the length of the blocks and just barely above them.
NOTE: Forceps can be helpful while adjusting the blade height. They can be placed on top of the tissue block to help place the blade as close to the top of the block as possible without touching the block. - Ensure that the glue has dried by gently nudging the blocks with forceps, and fill the vibratome tray with chilled ECS until the blade is covered. Set the vibratome to make 120 µm thickness slices at a speed of 0.175 mm/s, a frequency of 70 Hz, and an amplitude of 1 mm.
NOTE: The vibratome speed can be adjusted depending on the ease of cutting the tissue. - Start the vibratome, and watch for when slices start coming off of the tissue blocks. Use 10 cm Graefe forceps or a small No. 4 paintbrush to carefully remove the slices once they float off the block, and place them in 10 cm plates with KRBH containing 3 mM D-glucose and trypsin inhibitor. Pick up the slices by placing a paintbrush or forceps below them and gently lifting the slices. Do not incubate more than 15 slices together in a single plate.
NOTE: It is normal for the vibratome to have a few passes over the blocks where no slices are made, but these should be minimized for time. Have scissors ready in case the slices do not fully separate from the tissue blocks. If this happens, a corner or edge of the slice will be stuck to the block after the vibratome blade passes. Do not pull off the slice or block when removing the stuck slice. - Place the plates with the slices on a rocker at room temperature and at 25 rpm. Let the slices rest at room temperature for an hour. If they are going to be left for longer, place the slices in 15 mL of slice culture medium (see Supplemental Table 1) in an incubator. Incubate slices prepared for same-day studies at 37 °C, and culture slices that are cultured overnight at 24 °C, transferring them to 37 °C at least 1 h before experiments.
NOTE: Over the long term, the slices have better viability when cultured at 24 °C, although 37 °C is closer to their native physiological environment, probably due to the lower activity of the secreted protease enzymes at lower temperature. Mouse and human pancreatic tissue slices are both cultured at the same temperature and with a maximum of 15 slices per dish. However, the media recipes differ for human and mouse slices. Both formulations are listed in Supplemental Table 1. Additionally, the procedure is the same for generating mouse and human slices with the exception of the mouse pancreas requiring injection with agarose for stabilization. Human slices are acquired through the nPOD Pancreas Slice Program. Both mouse and human slices are 120 µm thick. A variety of experiments can be performed on the slices; choose staining panels that work best for planned experiments.
4. Slice preparation for staining procedures
- Culture the slices at 37 °C for at least 1 h ahead of the planned experiments. Warm KRBH containing 3 mM D-glucose in a 37 °C water bath. Transfer 2 mL of KRBH containing 3mM D-glucose in a 35 mm dish, and use a paintbrush to gently place the slice in the dish.
- If the slice is being transferred from medium, wash it twice with KRBH containing 3 mM D-glucose. Carefully aspirate the KRBH with 3 mM D-glucose using a transfer pipette or Pasteur pipette, taking care not to disturb the slice. Keep the slice in the plate with KRBH containing 3 mM D-glucose while the staining panels are prepared.
5. Dithizone staining
NOTE: Although dithizone can be used to stain the islets red, it will kill the slice as it has been found to be cytotoxic to islets17.
- Measure 12.5 mg of dithizone, add it to 1.25 mL of dimethylsulfoxide, and take this mixture up in a 50 mL syringe. Fill the syringe to a volume of 25 mL using Hanks Balanced Salt Solution, and attach a filter to the end of the syringe. Aliquot 2 mL of KRBH with 3 mM D-glucose, and add 2 drops of the filtered dithizone solution from the 50 mL syringe into a 35 mm dish.
- Using a paintbrush, carefully place a slice in a 35 mm petri dish. Image the slice with the islets indicated by red dithizone staining using a stereomicroscope.
6. Viability staining
NOTE: This section of the protocol describes how to assess slice viability using calcein-AM and blue-fluorescent SYTOX Blue (see the Table of Materials). Calcein-AM should be used at a concentration of 4 µM and SYTOX Blue at 1 µM.
- Aliquot 2 mL of KRBH containing 3 mM D-glucose, and add 2 µL of calcein-AM dye and SYTOX Blue to separate aliquots. Vortex the mixtures for 5 s.
- Add 200 µL of KRBH containing 3 mM D-glucose and calcein-AM dye to each well of an 8-well chambered coverglass.
NOTE: Alternative plates and/or imaging chambers other than an 8-well chambered coverglass can be used. - Using a paintbrush, carefully place a slice in each well of the plate, and transfer the plate with the slices to a 37 °C incubator for 20 min. Wash the slices twice with KRBH containing 3 mM D-glucose. Carefully aspirate the KRBH using a transfer or Pasteur pipette, taking care not to disturb the slice.
- Place the slice in a 35 mm coverglass-bottom Petri dish containing 2 mL of KRBH with 3 mM D-glucose and 2 µL of the SYTOX Blue at a concentration of 1 µL per 1 mL of solution. Cover the slice with a slice anchor, ensuring that the side with the harp faces downward. Take images of the slice.
NOTE: If the slice anchor keeps floating, wet it on both sides with KRBH containing 3 mM D-glucose to submerge it in the solution. It is critical to always maintain the slices in solutions containing protease inhibitor, even during dye loading. Viability stains used can be adapted for the specific experiment or microscope setup.
7. Slice Ca2+ indicator staining
NOTE: This section of the protocol describes how to stain slices for Ca2+ recordings using Oregon Green 488 BAPTA-1, AM and SYTOX Blue in mouse slices (see the Table of Materials). The Oregon Green 488 BAPTA-1, AM should be used at a concentration of 5.6 µM and the SYTOX Blue at 1 µM. In human slices, Fluo-4-AM should be used at a concentration of 6.4 µM.
- Aliquot 2 mL of KRBH containing 3 mM D-glucose, add 7 µL of the Oregon Green 488 BAPTA-1, AM and vortex the mixture for 5 s.
NOTE: For human tissue slices, use Fluo-4-AM instead of the Oregon Green 488 BAPTA-1, AM. The Fluo-4-AM is preferable because it is brighter when the intracellular Ca2+ concentration increases; however, it does not load well in mouse pancreatic tissue. The protocol is the same as described above for Fluo-4-AM with the exception that Fluo-4-AM only needs to be incubated for 30 min. - Add 200 µL of KRBH containing 3mM D-glucose and the dye to each well of an 8-well chambered coverglass. Using a paintbrush, carefully place a slice in each well of the chambered coverglass. Transfer the chambered coverglass with the slices to a 37 °C incubator for 45 min.
- Wash the slices twice with KRBH containing 3 mM D-glucose. Carefully aspirate the KRBH with 3 mM D-glucose using a transfer or Pasteur pipette, taking care to not disturb the slice.
- Place a slice in an imaging plate or chamber with KRBH containing 3 mM D-glucose and SYTOX Blue at a concentration of 1 µL per 1 mL, and cover with a slice anchor, ensuring that the harp faces downward. Take images of the slice.
NOTE: In this protocol, a 35 mm dish filled with 2 mL of KRBH containing 3 mM D-glucose and 2 µL of SYTOX Blue was used. If the slice anchor keeps floating, wet it on both sides with KRBH containing 3 mM D-glucose to submerge it in solution.
8. Mouse slice Ca2+ recordings
NOTES: The following section describes how to perform Ca2+ recordings on mouse pancreatic tissue slices using the Oregon Green 488 BAPTA-1, AM and SYTOX Blue. Imaging was performed on a confocal laser-scanning microscope (see the Table of Materials for details). The lasers used were 405 nm for the SYTOX Blue, 488 nm for the Oregon Green 488 BAPTA-1, AM, and 638 nm for reflectance. A HyD detector was used for the Oregon Green 488 BAPTA-1, AM. Photomultiplier tube (PMT) detectors were used for reflectance and the SYTOX Blue. The Ca2+ imaging protocol is the same for human pancreatic tissue slices except that Fluo-4-AM was used as the indicator. Laser power levels, gain, and pinhole size should be adjusted based on the sample and particular islet imaged. Typically, a pinhole of 1.5 airy units and a laser power of 1% are good starting points.
- At least 1 h before recording, switch on the microscope and equilibrate the stage-top or cage-style incubator to 37 °C. Place the 35 mm coverglass-bottom Petri dish containing the slice on the stage after removing the lid. Focus by setting the microscope to the 10x objective and brightfield mode. Locate islets using brightfield by looking for orange-brown ovals within the slice.
- Once a probable islet is located, switch the microscope to the confocal imaging mode. To confirm islets by reflectance, switch on the 638 nm laser, set the laser power between 1% and 2%, and switch off the 638 nm notch filter that would normally remove backscattered light. Set the detection limits on the PMT detector to a bandwidth of approximately 20 nm centered around 638 nm.
NOTE: Exercise caution as operating the microscope in reflectance mode could damage the detector. Due to the high granularity of the endocrine tissue, reflected light can now be used to locate islets. The islets will appear as groups of brightly backscattering granular cells on this reflectance channel. - To view the SYTOX Blue, switch on the 405 nm laser and PMT detector, and set the laser power between 1% and 2%. Center the islet of interest in the field of view using the X and Y knobs of the stage controller. Once an islet of interest is located and confirmed by backscatter, switch to the 20x objective, and zoom in so the islet takes up most of the frame.
- Take a z-stack of the islet with a z-step size of 1.5 µm. Find the best optical section of the islet where most of the cells are alive (negative for the SYTOX Blue) and well-loaded with the Oregon Green 488 BAPTA-1, AM or Fluo-4-AM.
NOTE: It is not unusual to see cells that are overloaded with a large amount of dye and that are very bright. These may be dying pancreatic cells in which Ca2+ storage in the endoplasmic reticulum may be released, resulting in high levels of loading; these are not ideal cells to record. Look for cells that have clearly loaded the dye, but are not oversaturating the detector so that an increase in brightness that occurs when cytosolic Ca2+ levels fluctuate is visible. Dye loading of islets within slices is variable; however, the dye typically loads well through ~10-15 µm of the islet. However, dye loading can be difficult to visualize if the cells are deep in the tissue. - To prevent dye fading during the recording, ensure that the laser power on the 488 nm channel does not exceed 2%. Increase the pinhole to 2 airy units to collect more signal with lower excitation power.
- Set the microscope to record in XYZT mode. Optimize the settings to reduce the frame rate to 2 s or less per frame.
NOTE: Settings adjustments that can be made to help decrease the frame rate include turning on bidirectional scanning, decreasing or turning off the line averaging, and increasing scanning speed. - Once the settings have been optimized, record several minutes of basal activity.
NOTE: Another good indicator of tissue viability is if the cells appear active and are visibly flashing during this recording of basal activity. - Add 100 µL of 20x concentrated glucose and KCl in KRBH to the plate using a 200 µL micropipette at the given time points to achieve a final concentration of 16.7 mM glucose or 30 mM KCl.
NOTES: Add the solutions carefully, taking care not to disturb the slice during the recording. Be sure not to bump the plate with the micropipette. It is typical to see the tissue contract in response to these stimulations. The Ca2+ flux recordings were processed and quantified in ImageJ18. Using ImageJ software, the staining intensity of the Oregon Green 488 BAPTA-1, AM was measured in the cells by manually selecting regions of interest (ROIs). The fluorescence intensity from these ROIs was calculated by dividing the fluorescence values at later timepoints by the initial fluorescence values of the cells (F/F0). A perfusion system can be used along with a specialized imaging chamber to administer the solutions to slices dynamically as opposed to adding them manually. Perfusion system and imaging chamber recommendations can be found in the Table of Materials.
9. Staining of mouse T cells in live pancreatic slices
NOTE: This section of the protocol describes how to stain immune cells within mouse slices. The mouse strain used is the NOD.Rag1-/-.AI4α/β as this model consistently develops disease with significant insulitis. The CD8+ T cells in this mouse all target an epitope of insulin, allowing the use of a phycoerythrin (PE)-labelled insulin-Db tetramer15. The CD8 antibody should be used at a concentration of 1:20 and the insulin tetramer at 1:50.
- Aliquot 100 µL of KRBH containing 3 mM D-glucose, add 2 µL of PE insulin tetramer and 5 µL of allophycocyanin (APC) CD8 antibody, and vortex the mixture for 5 s.
- Add 100 µL of KRBH containing 3 mM D-glucose and the tetramer and antibody to a well of an 8-well chambered coverglass. Using a paintbrush, carefully place a slice in the well of the chambered coverglass. Transfer the chambered coverglass with the slice to a 37 °C incubator for 30 min.
- Wash the slice twice with 2 mL KRBH containing 3 mM D-glucose. Carefully aspirate the KRBH with 3 mM D-glucose using a transfer or Pasteur pipette, taking care not to disturb the slice.
- Place the slice in a 35 mm coverglass-bottom Petri dish containing KRBH with 3 mM D-glucose and the SYTOX Blue at a concentration of 1 µL per 1 mL, and cover with a slice anchor, placing the side with the harp facing downward. Take images of the slice.
NOTE: If the slice anchor keeps floating, wet it on both sides with KRBH containing 3 mM D-glucose to submerge it in the solution. The diluted antibody and tetramer can be reused once. After staining two slices, a fresh antibody mixture should be made.
10. Recording of mouse immune cells
NOTE: The following section describes how to perform immune cell recordings on mouse pancreatic tissue slices using CD8 antibody, PE insulin tetramer, and SYTOX Blue. The imaging setup is as described in section 8. Recordings were made at 800 × 800 pixel resolution. The lasers used were 405 nm for the SYTOX Blue, 488 nm for the insulin tetramer, and 638 nm for CD8 antibody and reflectance. HyD detectors were used for CD8 antibody and PE insulin tetramer. PMT detectors were used for reflectance and the SYTOX Blue. The immune cell imaging protocol is the same for human pancreatic tissue slices except for the use of different antibodies and antigen-complexed HLA-multimers for human tissue. For both insulin tetramer staining in mouse tissue and HLA-multimer staining in human tissue, an immune cell co-stain should be used to verify the presence of the specific antigen-reactive T cells. Here, an anti-CD8 antibody was used. Antibodies, such as anti-CD3 or anti-CD4, can also be used depending on the target cell population.
- At least 1 h before recording, switch on the microscope, and equilibrate the stage-top incubator to 37 °C. Secure the 35 mm coverglass-bottom Petri dish containing the slice on the stage. Focus the microscope by setting the 10x objective in the brightfield mode. Locate the islets using the brightfield mode by looking for orange-brown colored ovals within the slice.
- Switch the microscope to confocal imaging by pressing the CS button on the microscope's touch screen controller. To view islets by reflectance, turn on the 638 laser and PMT detector, set the laser power between 1% and 2%, and turn off the notch filters.
NOTE: Due to the increased granularity of the endocrine tissue, reflected light can now be used to locate islets. The islets will appear as groups of bright granular cells on this channel. - To view the SYTOX Blue, CD8 antibody, and insulin tetramer, check that the laser power is between 1% and 2%. Use the following settings to view each of the three: for the SYTOX Blue, turn on the 405 nm laser and PMT detector; for the CD8 antibody, turn on the HyD detector; and to view the insulin tetramer, turn on the 488 nm laser and HyD detector.
- Center the islet of interest in the field of view using the X and Y knobs of the stage controller. Once an islet of interest is located, switch to the 20x objective, and zoom in so the islet takes up most of the frame. Take a z-stack of the islet with a z-step size of 1.5 µm. Find the best optical sections (a series of between 5 to 10 sections) of the islet where most of the cells are alive (negative for the SYTOX Blue) and any surrounding immune cells are in focus.
NOTE: Try to find frames where there are multiple CD8-positive and insulin tetramer-positive cells surrounding or infiltrating the islet. - Set the microscope to record in XYZT mode. Optimize the settings to record a Z-stack of the selected steps every 20 min over a period of several hours.
NOTE: If possible, it is best to do these recordings in an imaging chamber where temperature and CO2 levels can be controlled, particularly when recording for over four hours. In the case of overnight recording, excess antibody can be added to the media to compensate for T cell receptor cycling and dye fading. Additionally, different fluorophores can be used for the T cell antibodies. Based on experience, antibodies in the far-red range work best for T cells.
Representative Results
This protocol will yield live pancreatic tissue slices suitable for both functionality studies and immune cell recordings. Slice appearance in both brightfield and under reflected light are shown in Figure 1A,B. As discussed, islets can be found in slices using reflected light due to their increased granularity that occurs because of their insulin content (Figure 1C) and are clearly observed compared to the background tissue when reflected light is used. Viability should be assessed following slice generation, and islets should not be recorded if more than 20% of the islet is not viable. An islet with high viability is shown in Figure 1D, whereas an example of a poorly processed slice is shown in Supplemental Figure 1. Islets with low viability will have heavy SYTOX Blue staining, and the tissue will be covered with the stained nuclei of dead cells. Additionally, calcein-AM and Ca2+ indicators such as the Oregon Green 488 BAPTA-1, AM used here and Fluo-4-AM will not load well in dead cells. Islets should be selected for Ca2+ recordings if they are viable and if the indicator is loaded throughout the islet. Ca2+ indicator loading is indicative of cell viability as both Ca2+ indicators discussed in this protocol (the Oregon Green 488 BAPTA-1, AM and Fluo-4-AM) are loaded in cells through the same mechanism as the viability dye, calcein-AM.
For both the Ca2+ indicator dyes and calcein-AM, when the stains are loaded into cells, the acetoxymethyl ester is hydrolyzed within the cell, and the molecule becomes membrane impermeable19. Another positive indicator for viability is observable basal activity throughout the islet with cells flashing on and off. Basal activity should also be observable in the exocrine tissue to a lesser degree. Although mouse tissue tends to have less visible basal activity than human tissue, it is still present. An islet from a slice made from a NOD.Rag1-/- mouse pancreas is shown in Figure 2A. As mentioned above, the Oregon Green 488 BAPTA-1, AM used here has a lower fluorescence intensity increase upon binding Ca2+ (~14-fold) than Fluo-4 (~100-fold). However, the Oregon Green 488 BAPTA-1, AM has the advantage of a lower calcium dissociation constant (Kd = 170 nM) than Fluo-4 (Kd = 335 nM), resulting in the Oregon Green 488 BAPTA-1, AM being more sensitive to lower concentrations of cytosolic Ca2+. However, responses are still quantifiable, as shown by Figure 2B. Examples of an islet within a control human pancreatic tissue slice at rest and of one exhibiting a strong high glucose response are shown in Figure 2C and Supplemental Video 1. Fluo-4-AM dye is loaded well and is visible throughout the islet at low glucose concentrations. As discussed above, a typical occurrence is for a percentage of cells to load large amounts of dye and appear very bright. Moreover, the image parameters have been set for this recording so that most of the cells within the islet do not appear too bright at low glucose concentrations. This enables the detector to pick up on the increases in brightness that occur during changes in intracellular Ca2+ concentrations in response to high glucose levels. The quantification of the fluorescence of individual cells during this response is shown in Figure 2D, with the expected peak following the high glucose stimulation. ImageJ software was used to calculate the staining intensity of Fluo-4-AM and the Oregon Green 488 BAPTA-1, AM by manually selecting ROIs. The fold-increase in fluorescence intensity for each ROI was calculated by normalizing the fluorescence values at later timepoints using the initial fluorescence values of the cells (F/F0).
Dithizone stains the islets red and is visible under a brightfield stereomicroscope. Intact islets and islets that are beginning to fall apart because of T1D onset can both be observed using this dye (Figure 3A,B). Islets can be found using reflected light (Figure 3C) and may begin to lose granularity due to immune cell infiltration and cell death (Figure 3D). Multiple CD3-positive cells can be seen infiltrating the islet in Figure 3D. Immune cell populations can be identified more specifically using CD8 antibody and insulin-tetramer staining. Imaging can then be applied to identify cells that co-stain for both markers (Figure 3E). The co-staining of the immune cells infiltrating the islet in Figure 3D indicates that the cells are effector T cells that are specifically targeting the insulin antigen. The CD8 co-stain is essential to distinguish that the areas that stain positive for tetramer are immune cells. The tetramer should not be used alone without an immune cell co-stain. A staining comparison of the mouse CD8 antibody and the isotype control Rat IgG2a, κ can be found in Supplemental Figure 2. An additional comparison of a control tetramer for lymphocytic choriomeningitis virus (LCMV) tetramer and the insulin tetramer can be found in Supplemental Figure 3. Some T cells remain stationary throughout the recording, many move slightly within a small area of the islet, and others are very mobile and can be seen moving throughout the islet and exocrine tissue. It is not unusual to see T cells exhibiting multiple mobility types within the same recording.
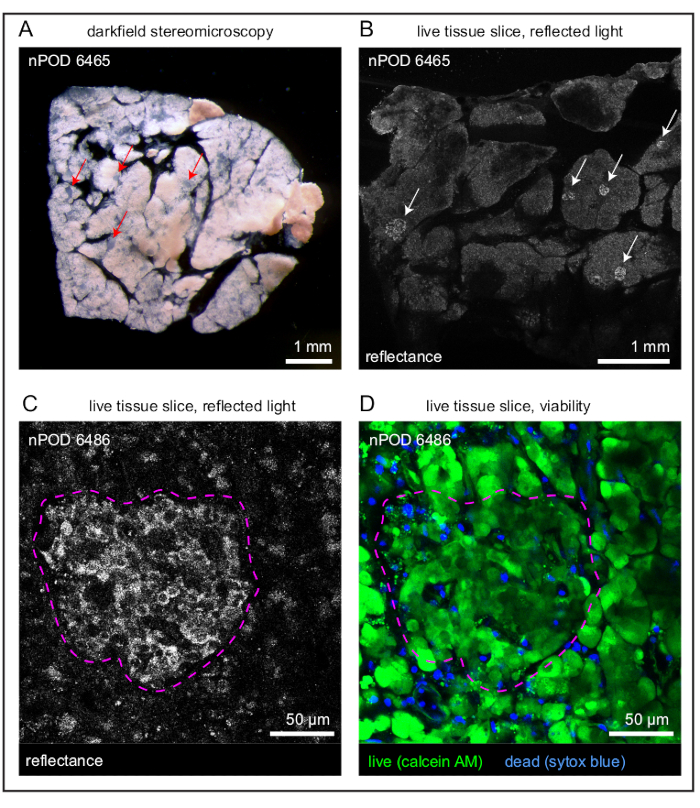
Figure 1: Overview of slices and individual islets. (A) Darkfield stereomicroscopy image of a live human pancreatic tissue slice with islets indicated by red arrows. (B) Reflected light image of a live human pancreatic tissue slice with islets indicated by white arrows. (C) Reflected light image of an islet (outlined in magenta) within a live human pancreatic tissue slice. (D) Viability staining of a high-viability islet (outlined in magenta) within a live human pancreatic tissue slice. Live cells are indicated in green and dead cells in blue. Scale bars (A, B) = 1 mm; scale bars (C, D) = 50 µm. Abbreviation: AM = acetoxymethyl ester. Please click here to view a larger version of this figure.
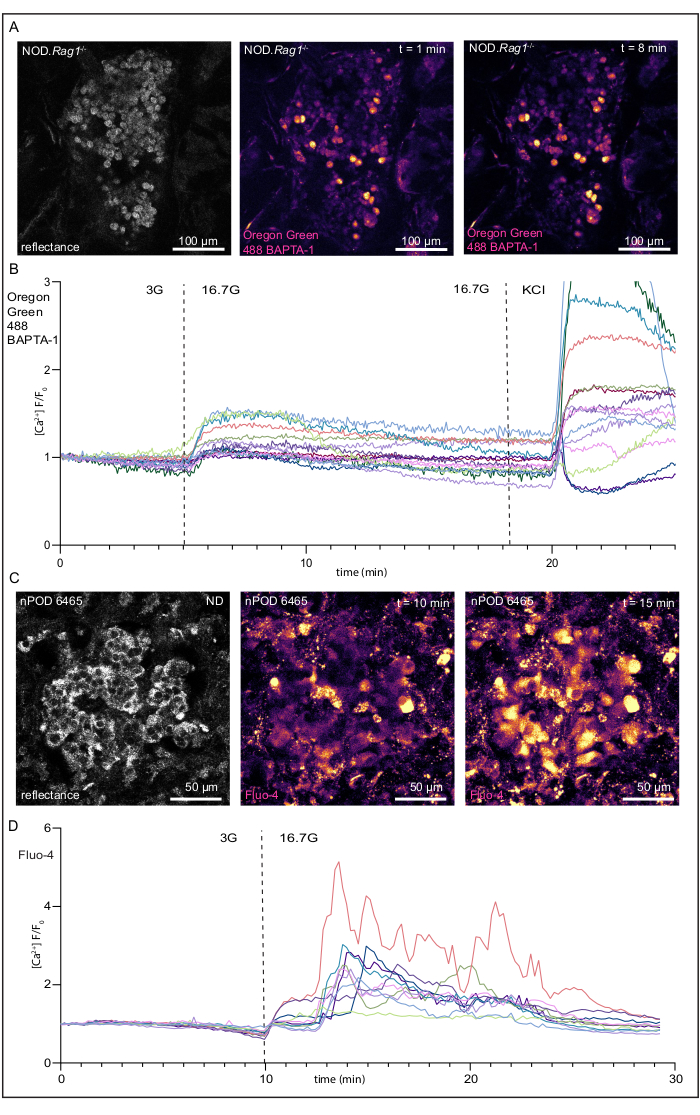
Figure 2: Recordings of changes in intracellular Ca2+ concentrations and responses to high glucose concentration of a live NOD.Rag1-/- mouse pancreatic tissue slice and human pancreatic tissue slice from a donor without diabetes. (A) Images of an islet within a live NOD.Rag1-/- mouse pancreatic slice loaded with a Oregon Green 488 BAPTA-1, AM (see the Table of Materials) undergoing glucose stimulation. From left to right, a reflected light image of the islet, the islet in low glucose, and the islet in high glucose. (B) Fluorescence traces of the Ca2+ response of an islet within a live NOD.Rag1-/- tissue slice with the expected response to high glucose concentration [KRBH with 16.7 mM D-glucose (16.7G)] and KCl [KRBH with 30 mM KCl and 3 mM D-glucose]. (C) Images of an islet within a live human pancreatic slice loaded with Fluo-4-AM undergoing glucose stimulation. From left to right, a reflected light image of the islet, the islet in low glucose, and the islet in high glucose. (D) Fluorescence traces of the Ca2+ response of an islet within a live human pancreas tissue slice with the expected response to KRBH with 16.7 mM D-glucose (16.7G). Scale bars (A) = 100 µm; scale bars (C) = 50 µm. Abbreviations: KRBH = Krebs-Ringer bicarbonate buffer; KCl = potassium chloride; NOD.Rag1-/- = non-obese diabetic-recombination activating gene-1-null; NOD.Rag1-/-.AI4α/β = T cell receptor transgenic (AI4) mouse strain. Please click here to view a larger version of this figure.
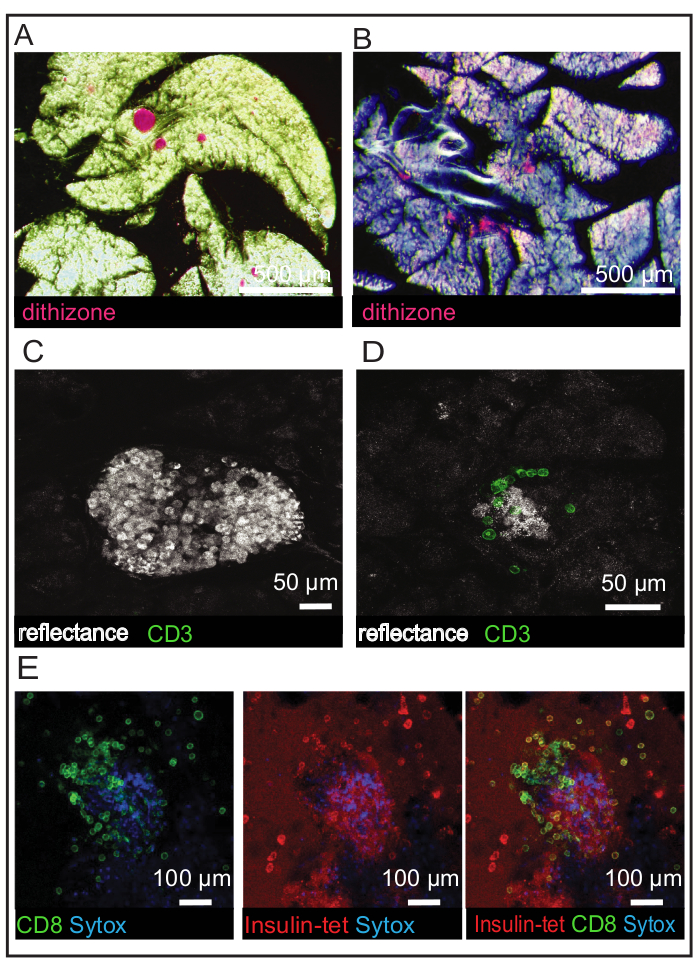
Figure 3: Identification of islets and immune cell populations in NOD.Rag1-/- and NOD.Rag1-/-.AI4α/β mouse slices. (A) Dithizone staining of islets in a NOD.Rag1-/- mouse slice with the islets indicated in red. (B) Dithizone staining of islets in a NOD.Rag1-/-.AI4α/β mouse slice with the islets indicated in red. Islets are losing their shape due to disease onset. (C) Reflected light image of an islet in a NOD.Rag1-/- mouse slice. (D) Reflected light image of an islet in a NOD.Rag1-/-.AI4α/β mouse slice with CD3 antibody staining (green). (E) Viability staining of dead cells (blue) and immune cell staining (CD8 in green and insulin tetramer in red) in a NOD.Rag1-/-.AI4α/β mouse slice. Scale bars (A) = 500 µm; scale bars (B) = 50 µm; scale bars (C) = 100 µm. Abbreviations: NOD.Rag1-/- = non-obese diabetic-recombination activating gene-1-null; NOD.Rag1-/-.AI4α/β = T cell receptor transgenic (AI4) mouse strain; CD = cluster of differentiation; insulin-tet = insulin tetramer. Please click here to view a larger version of this figure.
Supplemental Figure 1: NOD.Rag1-/- mouse pancreatic slice following improper preparation without trypsin inhibitor and an overnight incubation at 37 °C. (A) Darkfield stereomicroscopy image of a live NOD.Rag1-/- mouse pancreatic tissue slice; scale bar = 1 mm. (B) Reflected light image of a live mouse pancreatic tissue slice; scale bar = 50 µm. (C) Viability staining of low-viability tissue. Dead cells are indicated in blue; scale bar = 50 µm. Abbreviation: NOD.Rag1-/- = non-obese diabetic-recombination activating gene-1-null. Please click here to download this File.
Supplemental Figure 2: Rat IgG2a, κ isotype control antibody (left) and rat anti-mouse CD8 antibody (right) staining comparison in NOD.Rag1-/-.AI4α/β mouse slices. (A) Reflected light images of live NOD.Rag1-/-.AI4α/β mouse pancreatic tissue slices showing an islet (left) and blood vessel (right). (B) Antibody staining of live NOD.Rag1-/-.AI4α/β mouse pancreatic tissue slices. (C) Overlay of the reflected light and antibody channels. Scale bars for control antibody (left panels) = 20 µm; scale bars for CD8 antibody (right panels) = 50 µm. Abbreviations: NOD.Rag1-/- = non-obese diabetic-recombination activating gene-1-null; NOD.Rag1-/-.AI4α/β = T cell receptor transgenic (AI4) mouse strain; CD = cluster of differentiation; IgG = immunoglobulin G. Please click here to download this File.
Supplemental Figure 3: Lymphocytic choriomeningitis virus tetramer (left) and insulin tetramer (right) staining comparison in NOD.Rag1-/-.AI4α/β mouse slices. (A) Reflected light images of live NOD.Rag1-/-.AI4α/β mouse pancreas tissue slices showing a blood vessel in exocrine tissue (left) and islets (right). (B) Tetramer staining of a live NOD.Rag1-/-.AI4α/β mouse tissue slices. (C) Overlay of the reflected light and tetramer channels. Abbreviations: NOD.Rag1-/- = non-obese diabetic-recombination activating gene-1-null; NOD.Rag1-/-.AI4α/β = T cell receptor transgenic (AI4) mouse strain; LCMV = lymphocytic choriomeningitis virus; insulin-tet = insulin tetramer. Please click here to download this File.
Supplemental Video 1: Recording of cytosolic Ca2+ detected with Fluo-4 in response to high glucose stimulation in a human pancreatic tissue slice from a control donor without diabetes. Cells within the tissue can be observed to exhibit basal Fluo-4 activity in a low glucose solution (3.0 mM), followed by an increase in Fluo-4 fluorescence intensity in response to a stimulation with high glucose (16.7 mM). The video corresponds to the still images and traces shown in Figure 2C,D. Please click here to download this Video.
Supplemental Table 1: Please click here to download this Table.
Discussion
The objective of this protocol is to explicate the generation of pancreas slices and the procedures needed to employ the slices in functional and immunological studies. There are many benefits to using live pancreatic slices. However, there are several critical steps that are essential for the tissue to remain viable and useful during the described experiment protocols. It is imperative to work quickly. The length of time between injecting the pancreas and generating the slices on the vibratome should be minimized to maintain tissue viability. Viability is also improved by keeping the pancreas in cold ECS before slicing as opposed to room temperature ECS. Importantly, slices should never be in medium without protease inhibitor. When slices are incubated without the protease inhibitor, there are large decreases in viability.
When the slices were briefly left without inhibitor during dye loading, Ca2+ fluxes in response to high glucose and KCl could no longer be recorded despite basal activity still being visible in the slice. All solutions used with live slices including the KRBH with 3 mM D-glucose resting solution, the antibody incubation solution, and any culture media, must all contain protease inhibitor at a concentration of 0.1 mg per mL. The indicator panels used for slice imaging can be modified depending on the objective of the experiment and the availability of microscope lasers. There are numerous cell viability dyes in different colors that can be used instead of the SYTOX Blue used here (see the Table of Materials). For Ca2+ experiments, Fluo-4-AM works well in human tissue. Some researchers have success using the Oregon Green 488 BAPTA-1, AM used here (see the Table of Materials) for mouse slices, whereas others obtain good results with Fluo-4-AM20,21,22.
Additionally, mice engineered to express the genetically encoded Ca2+ indicator, GCaMP, in their islets could be used to circumvent the need to load the slices with a Ca2+ indicator dye. Although the Oregon Green 488 BAPTA-1, AM used here is not as bright as Fluo-4-AM, the Ca2+ responses are still observable and quantifiable. This is evidenced by the increase in fluorescent peaks shown in the NOD.Rag1-/- slice recordings following high glucose and KCl stimulation. Other substances, such as sulfonylureas and arginine, can be used as positive controls at the end of the Ca2+ protocol, but they have not yet been used with slices23,24,25. While there are many benefits to the live pancreatic tissue slice method, there are also some limitations. Although the slices can remain viable for several days, there are steep declines in viability and functionality if they are cultured for longer, unless special culture conditions are employed11,26. Additionally, as the slices contain live pancreatic exocrine tissue, acinar cells in the slices will continue to produce and release digestive enzymes that need to be inhibited using protease inhibitor. Therefore, when using this protocol for human or mouse studies, always maintain slices in solutions with protease inhibitor.
The live pancreatic tissue slice method avoids placing the pancreatic tissue under chemical stress by only exposing the tissue to mechanical force during slice generation as opposed to chemicals used during islet isolation procedures5. Furthermore, intact pancreatic tissue is maintained, allowing for a more holistic view of the pathologies and physiology that occur naturally within the organ5. Using the live pancreatic tissue slice method, immune cell activity can be observed in situ and real-time alongside tissue function. Additional in vitro imaging techniques, such as two-photon microscopy, have already been applied to tissue slices derived from thymus and could be applied to live pancreatic tissue slices27. Identification of immune cell populations that are present in the tissue along with their activities and impacts will allow for new knowledge to be gained on the pathogenesis of diseases such as T1D and T2D.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by NIH grants R01 DK123292, T32 DK108736, UC4 DK104194, UG3 DK122638, and P01 AI042288. This research was performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R), and The Leona M. & Harry B. Helmsley Charitable Trust (Grant #2018PG-T1D053). The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/. Thank you to Dr. Kevin Otto, University of Florida, for providing the vibratome used to generate mouse slices.
Materials
| #3 Style Scalpel Handle | Fisherbrand | 12-000-163 | |
| 1 M HEPES | Fisher Scientific | BP299-100 | HEPES Buffer, 1M Solution |
| 10 cm Untreated Culture Dish | Corning | 430591 | |
| 10 mL Luer-Lok Syringe | BD | 301029 | BD Syringe with Luer-Lok Tips |
| 27 G Needle | BD | BD 305109 | BD General Use and PrecisionGlide Hypodermic Needles |
| 35 mm coverglass-bottom Petri dish | Ibidi | 81156 | µ-Dish 35 mm, high |
| 50 mL syringe | BD | 309653 | |
| 8-well chambered coverglass | Ibidi | 80826 | µ-Slide 8 Well |
| APC anti-mouse CD8a antibody | Biolegend | 100712 | |
| BSA | Fisher Scientific | 199898 | |
| Calcium chloride | Sigma | C5670 | CaCl2 |
| Calcium chloride dihydrate | Sigma | C7902 | CaCl2 (dihydrate) |
| Compact Digital Rocker | Thermo Fisher Scientific | 88880020 | |
| Confocal laser-scanning microscope | Leica | SP8 | Pinhole = 1.5-2 airy units; acquired with 10x/0.40 numerical aperture HC PL APO CS2 dry and 20x/0.75 numerical aperture HC PL APO CS2 dry objectives at 512 × 512 pixel resolution |
| D-(+)-Glucose | Sigma | G7021 | C6H12O6 |
| ddiH2O | |||
| Dithizone | Sigma-Aldrich | D5130-10G | |
| DMSO | Invitrogen | D12345 | Dimethyl sulfoxide |
| Ethanol | Decon Laboratories | 2805 | |
| Falcon 35 mm tissue culture dish | Corning | 353001 | Falcon Easy-Grip Tissue Culture Dishes |
| FBS | Gibco | 10082147 | |
| Feather No. 10 Surgical Blade | Electron Microscopy Sciences | 7204410 | |
| fluo-4-AM | Invitrogen | F14201 | cell-permeable Ca2+ indicator |
| Gel Control Super Glue | Loctite | 45198 | |
| Graefe Forceps | Fine Science Tools | 11049-10 | |
| Hardened Fine Scissors | Fine Science Tools | 14090-09 | |
| HBSS | Gibco | 14025092 | Hanks Balanced Salt Solution |
| HEPES | Sigma | H4034 | C8H18N2O4S |
| Ice bucket | Fisherbrand | 03-395-150 | |
| Isoflurane | Patterson Veterinary | NDC 14043-704-05 | |
| Johns Hopkins Bulldog Clamp | Roboz Surgical Store | RS-7440 | Straight; 500-900 Grams Pressure; 1.5" Length |
| Kimwipes | Kimberly-Clark Professional | 34705 | Kimtech Science™ Kimwipes™ Delicate Task Wipers, 2-Ply |
| LIVE/DEAD Viability/Cytotoxicity Kit | Invitrogen | L3224 | This kit contains the calcein-AM live cell dye. |
| Low glucose DMEM | Corning | 10-014-CV | |
| Magnesium chloride hexahydrate | Sigma | M9272 | MgCl2 (hexahydrate) |
| Magnesium sulfate heptahydrate | Sigma | M2773 | MgSO4 (heptahydrate) |
| Magnetic Heated Platform | Warner Instruments | PM-1 | Platform for imaging chamber for dynamic stimulation recordings |
| Microwave | GE | JES1460DSWW | |
| Nalgene Syringe Filter | Thermo Fisher Scientific | 726-2520 | |
| No.4 Paintbrush | Michaels | 10269140 | |
| Open Diamond Bath Imaging Chamber | Warner Instruments | RC-26 | Imaging chamber for dynamic stimulation recordings |
| Oregon Green 488 BAPTA-1-AM | Invitrogen | O6807 | cell-permeable Ca2+ indicator |
| Overnight imaging chamber | Okolab | H201-LG | |
| PBS | Thermo Fisher Scientific | 20012050 | To make agarose for slice generation |
| PE-labeled insulin tetramer | Emory Tetramer Research Core | sequence YAIENYLEL | |
| Penicillin Streptomycin | Gibco | 15140122 | |
| Potassium chloride | Sigma | P5405 | KCl |
| Potassium phosphate monobasic | Sigma | P5655 | KH2PO4 |
| Razor Blades | Electron Microscopy Sciences | 71998 | For Vibratome; Double Edge Stainless Steel, uncoated |
| RPMI 1640 | Gibco | 11875093 | |
| SeaPlaque low melting-point agarose | Lonza | 50101 | To make agarose for slice generation |
| Slice anchor | Warner Instruments | 64-1421 | |
| Slice anchor (dynamic imaging) | Warner Instruments | 640253 | Slice anchor for dynamic imaging chamber |
| Sodium bicarbonate | Sigma | S5761 | NaHCO3 |
| Sodium chloride | Sigma | S5886 | NaCl |
| Sodium phosphate monohydrate | Sigma | S9638 | NaH2PO4 (monohydrate) |
| Soybean Trypsin Inhibitor | Sigma | T6522-1G | Trypsin inhibitor from Glycine max (soybean) |
| Stage Adapter | Warner Instruments | SA-20MW-AL | To fit imaging chamber for dynamic stimulation recordings on the microscope stage |
| Stage-top incubator | Okolab | H201 | |
| Stereoscope | Leica | IC90 E MSV266 | |
| SYTOX Blue Dead Cell Stain | Invitrogen | S34857 | blue-fluorescent nucleic acid stain |
| Transfer Pipet | Falcon | 357575 | Falcon™ Plastic Disposable Transfer Pipets |
| Valve Control System | Warner Instruments | VCS-8 | System for dynamic stimulation recordings |
| Vibratome VT1000 S | Leica | VT1000 S | |
| Water bath | Fisher Scientific | FSGPD02 | Fisherbrand Isotemp General Purpose Deluxe Water Bath GPD 02 |
References
- Uc, A., Fishman, D. S. Pancreatic disorders. Pediatric Clinics of North America. 64 (3), 685-706 (2017).
- Bluestone, J. A., Herold, K., Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 464 (7293), 1293-1300 (2010).
- Taylor, R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 36 (4), 1047-1055 (2013).
- Meier, R. P., et al. Islet of Langerhans isolation from pediatric and juvenile donor pancreases. Transplant International. 27 (9), 949-955 (2014).
- Marciniak, A., et al. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nature Protocols. 9 (12), 2809-2822 (2014).
- Panzer, J. K., Cohrs, C. M., Speier, S. Using pancreas tissue slices for the study of islet physiology. Methods in Molecular Biology. 2128, 301-312 (2020).
- Speier, S., Rupnik, M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Archive. 446 (5), 553-558 (2003).
- Panzer, J. K., et al. Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight. 5 (8), 134525 (2020).
- Dolai, S., et al. Pancreatitis-induced depletion of syntaxin 2 promotes autophagy and increases basolateral exocytosis. Gastroenterology. 154 (6), 1805-1821 (2018).
- Dolai, S., et al. Pancreas-specific SNAP23 depletion prevents pancreatitis by attenuating pathological basolateral exocytosis and formation of trypsin-activating autolysosomes. Autophagy. , 1-14 (2020).
- Qadir, M. M. F., et al. Long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nature Communications. 11 (1), 3265 (2020).
- Cohrs, C. M., et al. Dysfunction of persisting β cells is a key feature of early type 2 diabetes pathogenesis. Cell Reports. 31 (1), 107469 (2020).
- Liang, T., et al. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. Journal of Biological Chemistry. 292 (14), 5957-5969 (2017).
- Shultz, L. D., Ishikawa, F., Greiner, D. L. Humanized mice in translational biomedical research. Nat Reviews. Immunology. 7 (2), 118-130 (2007).
- Lamont, D., et al. Compensatory mechanisms allow undersized anchor-deficient class I MHC ligands to mediate pathogenic autoreactive T cell responses. Journal of Immunology. 193 (5), 2135-2146 (2014).
- Fish, R., Danneman, P. J., Brown, M., Karas, A. . Anesthesia and analgesia in laboratory animals. , (2011).
- Clark, S. A., Borland, K. M., Sherman, S. D., Rusack, T. C., Chick, W. L. Staining and in vitro toxicity of dithizone with canine, porcine, and bovine islets. Cell Transplantation. 3 (4), 299-306 (1994).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Monette, R., Small, D. L., Mealing, G., Morley, P. A fluorescence confocal assay to assess neuronal viability in brain slices. Brain Research Protocols. 2 (2), 99-108 (1998).
- Gál, E., et al. A novel in situ approach to studying pancreatic ducts in mice. Frontiers in Physiology. 10, 938 (2019).
- Stožer, A., Dolenšek, J., Rupnik, M. S. Glucose-stimulated calcium dynamics in islets of Langerhans in acute mouse pancreas tissue slices. PloS One. 8 (1), 54638 (2013).
- Stožer, A., et al. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLoS Computational Biology. 9 (2), 1002923 (2013).
- Früh, E., Elgert, C., Eggert, F., Scherneck, S., Rustenbeck, I. Glucagonotropic and glucagonostatic effects of KATP channel closure and potassium depolarization. Endocrinology. 162 (1), 136 (2021).
- Satin, L. S. New mechanisms for sulfonylurea control of insulin secretion. Endocrine. 4 (3), 191-198 (1996).
- Ren, J., et al. Slow oscillations of KATP conductance in mouse pancreatic islets provide support for electrical bursting driven by metabolic oscillations. American Journal of Physiology-Endocrinology and Metabolism. 305 (7), 805-817 (2013).
- Marciniak, A., Selck, C., Friedrich, B., Speier, S. Mouse pancreas tissue slice culture facilitates long-term studies of exocrine and endocrine cell physiology in situ. PLoS One. 8 (11), 78706 (2013).
- Dzhagalov, I. L., Melichar, H. J., Ross, J. O., Herzmark, P., Robey, E. A. Two-photon imaging of the immune system. Current Protocols in Cytometry. , (2012).

