Screening Peptides that Activate MRGPRX2 using Engineered HEK Cells
Summary
Techniques for generating a library of short peptides that can activate mast cells via the MRGPRX2 receptor are described. Associated techniques are easy, inexpensive, and can be extended to other cell receptors.
Abstract
Identifying ligands specific to therapeutically significant cell receptors is crucial for many applications, including the design and development of new therapeutics. Mas related G-protein receptor-X2 (MRGPRX2) is an important receptor that regulates mast cell activation and, thus, directs the general immune response. Numerous ligands for MRGPRX2 have been identified and include endogenous peptides like PAMPs, defensins, LL-37 and other protein fragments (i.e., degraded albumin). Further identification of MRGPRX2 specific ligands requires the screening of a large number of peptides (i.e., peptide library); however, mast cells are difficult and expensive to maintain in vitro and, therefore, not economical to use for screening large numbers of molecules. The present paper demonstrates a method to design, develop, and screen a library of small peptide molecules using MRGPRX2 expressing HEK cells. This cell line is relatively easy and inexpensive to maintain and can be used for in vitro high-throughput analysis. A calcium sensitive Fura-2 fluorescent dye to mark intracellular calcium flux upon activation was used to monitor the activation. The ratio of fluorescence intensity of Fura-2 at 510 nm against excitation wavelengths of 340 and 380 nm was used to calculate calcium concentration. The peptide library used to verify this system was based on the endogenous proadrenomedullin N-terminal 12 (PAMP-12) secretagogue, which is known to bind MRGPRX2 with high specificity and affinity. Subsequent peptides were generated through amino acid truncation and alanine scanning techniques applied to PAMP-12. The method described here is simple and inexpensive yet robust for screening a large library of compounds to identify binding domains and other important parameters that play an important role in receptor activation.
Introduction
Mast cells are an integral part of the immune system and play a crucial role in both innate and adaptive immune responses. Mast cells are primarily activated either by the binding of an antigen to the immunoglobulin E (IgE) – FcεRI receptor complex, or by the recently discovered mas related G-protein receptor-X2 (MRGPRX2)1. MRGPRX2 activation has been linked to several immune and inflammatory diseases, and hence, it is important to understand the binding mechanism of the receptor to its ligands2. To do so, a library of small peptide molecules was developed and screened against MRGPRX2 receptors that were overexpressed in HEK cells. In the study, the peptide library was constructed using the simple and versatile techniques of alanine scanning and amino acid truncation. Alanine scanning involves replacing specific amino acids with an alanine residue. Alanine being small and neutral, strips the peptide of the specific properties conferred by the replaced residue and consecutively highlights the significance of the respective physiochemical properties of the amino acid in receptor interactions. On the contrary, in amino acid truncation, peptide sequences are designed such that it lacks one or more amino acid residues from the N-terminal, C terminal, or both. This set of peptides was used to identify the amino acid sequences crucial to MRGPRX2 binding.
Experience with human mast cells lines (LAD-2) has shown that these cells are difficult to culture and maintain in vitro: a doubling time of two weeks, expensive medium supplements, and direct attention required during passaging3. These attributes make the cells unsuitable for large scale screening of potential ligands. Herein, stably transfected HEK cells expressing MRGPRX2 receptor (HEK-X2) were used to screen the peptide library1. HEK-293 cells are widely used and studied for the heterologous expression of surface receptors due to their high transfection efficiency, faster doubling rate, and the need for non-expensive medium supplements to be cultured in laboratory4. The protocol to transfect HEK-293 cell line has been demonstrated and is well established5. HEK-293 cells stably expressing MRGPRX2 receptor (passage 13-19) were activated with the peptides generated through N-truncation, C-truncation, N+C-truncation, and alanine scanning1. Wild type HEK cells (HEK-WT) (passage 16-21) were used as control. Intracellular calcium release upon activation was monitored to study the MRGPRX2 based activation.
Cell activation by MRGPRX2 is followed by a cytosolic calcium mobilization. This regulated intracellular calcium release in mast cells is regulated by the store operated calcium entry (SOCE), coordinated by the stromal interaction molecule 1 (STIM1); and is central to the immune response cascade6,7. Various methods have been used to detect intracellular calcium concentration, including patch-clamps and fluorescent dyes8. Of all the techniques available, fluorometric calcium dyes in conjugation with various detection techniques are being widely used9. Two types of fluorometric dyes that have gained interests are namely, single wavelength dyes like Fluo-4, and dual wavelength ratiometric dyes like Indo -1 and Fura-2. The advantage that dual wavelength ratiometric dyes bring over single wavelength dyes is that the ratiometric dyes correct for experimental errors like dye loading, photo bleaching, and focusing10,11.
Fura-2 acetoxymethyl ester (Fura-2 AM) is a cell permeating, green-fluorescent dye whose excitation shifts to a lower wavelength upon calcium-binding. Experimentally, Fura-2 is excited at 340 and 380 nm, while the emission is recorded at 510 nm. Upon calcium binding, the fluorescent intensity at 340 nm increases while that of 380 nm decreases, as shown in Figure 1. Data is represented as a ratio of fluorescence intensity after excitation at 340 nm (F340) to that of intensity after excitation at 380 nm (F380) i.e., F340/F380. The F340/F380 ratio is proportional to intracellular calcium, the value of which can be calculated by the Grynkiewicz equation12. Since the fluorescence signal is obtained from the excitation of the dye at two wavelengths (340 nm and 380 nm), the ratio of the fluorescence signals corrects for experimental factors like dye loading, dye leakage, photobleaching, and cell densities.
Protocol
1. Design and development of peptide library
- To identify the ligands of the mast cell MRGPRX2 receptor based on a known ligand i.e., PAMP-1213, follow the steps below.
- Generate N-truncated peptide library by truncating the N-terminal amino acid residues of the ligand, in succession, by solid-phase peptide synthesis (SPPS).
- Generate C-truncated peptide library by truncating the C-terminal amino acid residues of the known ligand, in succession, by SPPS.
- Based on the results of 1.1.1 and 1.1.2, generate an N+C-truncated peptide library using SPPS by truncating the desired residues from the N and C-terminal, respectively.
- Use solid-phase peptide synthesis to synthesize the peptides13.
- Modify the N-terminal to acetyl (Ac) group and C-terminal to amide group.
- Characterize the peptide for its purity using high-pressure liquid chromatography (HPLC) and for the mass using a mass spectrophotometer.
- To study the significance of specific amino acids within the parent PAMP-12 molecule, follow the steps below.
- Generate an alanine scanning peptide library by replacing the respective amino acid residues in the peptide molecule with alanine, one at a time using SPPS. Modify the N-terminal to acetyl (Ac) group and C-terminal to amide group.
- Characterize the peptide for its purity using high-pressure liquid chromatography (HPLC) and for the mass using a mass spectrophotometer.
NOTE: Ensure that the synthesized peptides are of high purity. Characterize the peptides using mass spectroscopy and HPLC.
2. In vitro cell culture
- Culture HEK-X2 and HEK-WT cells by following the steps below.
- Prepare culture medium by supplementing high glucose DMEM with 10% fetal bovine serum (FBS), 2 mM L-Glutamine, 100 U/mL of penicillin and 100 µg/mL of streptomycin.
- Passage the cells in tissue culture (TC) treated T-75 culture flasks and grow in an incubator at 37 °C containing 5% CO2, till they are 75-80% confluent.
- Once 75% confluent, wash the cells and add 2-3 mL of trypsin for 2 – 3 min. Incubate in a 37 °C, 5% CO2 incubator to detach the cells.
- Once the cells have detached, collect the cells in trypsin. Add 6-9 mL of fresh medium.
- Centrifuge the cells at 1620 x g for 3-5 min.
- After centrifugation, discard the supernatant to collect the pellet. Resuspend the cells in a fresh culture medium. Dilute the cells as per the desired concentration.
NOTE: HEK cells are fast-growing cells and hence optimize the cell medium supplements. HEK cells are adherent cells; passage them in TC-treated culture flasks to support adhesion.
- Prepare a 96 well assay plate for the experiment.
- Add 200 µL of the cell suspension in each well, with a concentration of 200,000 cells/mL, to seed 40,000 cells/well.
- Grow the cells for 24 h in a 37 °C, 5% CO2 incubator.
NOTE: Optimize the cell density per well based on the plate size and the cell strain type. Conduct the experiment in triplicates in a black TC-treated 96 well plate with flat transparent bottom.
3. Fura-2 AM calcium assay
- Prepare dye by following the steps below.
- Use Fura-2 AM dye for the experiment.
- Prepare HEPES-Tyrode's buffer (HTB) buffer containing 25 mM HEPES buffer, 120 mM NaCl, 5 mM KCl, 1 mg/mL glucose, 1 mg/mL bovine serum albumin (BSA) and freshly added 1.8 mM CaCl2 in autoclave sterilized water.
- Add 50 µL of DMSO in 50 µg Fura-2 AM vial to prepare 1 mM stock solution of Fura-2 AM dye. Add 1 µL of 1 mM Fura-2 AM dye per mL of fresh medium to prepare the dye loading medium having 1 µM dye concentration.
- Remove the 96 well plate from the incubator and discard the medium. Replace the medium with a fresh dye loading medium. Add 200 µL of dye loading medium in each well. Incubate the cells for 30-40 min in a 37 °C, 5% CO2 incubator.
- After 40 min of incubation, remove the medium. Wash the cells with the HTB buffer. Add 100 µL of HTB buffer for fluorescence reading. Take the plate for fluorescence reading.
NOTE: Optimize the concentration of dye in the dye loading medium. Dye leakage and photobleaching are possible concerns associated with the dye. Add CaCl2 fresh into the HTB buffer to avoid precipitation.
4. Cell activation and fluorescent reading
NOTE: Fluorescence plate reader with an automated pipetting system allows for the automatic transfer of compounds from a compound source to the assay plate without taking the plate out of the plate reader.
- While the cells are being incubated, set the Plate Reader.
- Set the Temperature to 37 °C.
- In Settings, select Flex.
- Set the Read Mode 세스 Fluorescence and Bottom read.
- In Wavelengths, set the number of Wavelengths to 2. Set the Excitation to 340 nm and 380 nm. Set the Emission to 510 nm.
- Leave the Sensitivity 세스 Default.
- In Timing, set the Interval to 3.9 s. Set Run Time to 94 s to get 25 reads.
- Next, select the Assay Plate Type.
- Next, select the Wells to Read.
- In Compound Transfer, set Transfers to 1 and Initial Volume to 100 µL. Set the Pipette Height to 100 µL, Volume to 50 µL, and Time Point to 36 s, to add the compound at the 10th reading.
- Next, select the Compound Source plate type.
- Leave Triturate 세스 Not Used.
- Select the tips in the Pipette Tips Layout.
- For Compound and Tip Columns, ensure that the compounds to be transferred are in column 1 of the compound plate. Set the Tip Column to 1 and Compound Column to 1.
- Leave the Autocalibrate as ON.
- Click OK.
- When the temperature has reached to 37 °C, load the plates into the Plate Reader.
- Press the Reading Chamber to put the Assay Plate into the fluorescence Plate Reader.
- Press the Source to put the Compound Plate. Prepare the Compound Plate by adding 200 µL of respective peptides, ionomycin and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA)-Triton X-100 solutions.
- Press the Tip Rack to put the Tip Box. Use a black tip to avoid tip autofluorescence.
- Once the plates are loaded, review the settings of the software and press Read.
5. Data analysis
- Determine the calcium concentration from the fluorescence ratio by the Grynkiewicz equation –

where, Kd is the dissociation constant of Fura-2 AM, R is the emission ratio after excitation at 340 nm and 380 nm (F340/F380) for respective peptides, Rmax is the maximum fluorescence ratio observed by the addition of 50 µL of 30 µM ionomycin, Rmin is the minimum fluorescence observed by the addition of 50 µL of 100 mM EGTA/2.5%Triton X-100, and F380min and F380max are the absolute fluorescence intensity of Fura-2 AM in calcium free and bound state, respectively.
NOTE: The machine dispenses the liquid with some force. Do not set the peptide dispensing height too close to the bottom of the plate; it may detach the cells. Use black pipette tips to avoid autofluorescence. Read the maximum fluorescence and the minimum fluorescence for each plate for each experiment.
Representative Results
Table 1 contains the peptide sequences generated through terminal amino acid truncation and alanine scanning. As shown in Table 1, peptide sequence RKKWNKWALSR lacks N-terminal phenylalanine (F) with respect to its parent PAMP-12 and hence is a representative peptide in N-truncated library. Similarly, in FRKKWNKWALS, PAMP-12 C-terminal serine has been removed, representing a C-truncated peptide library derived from PAMP-12. In N+C-truncated peptide library, amino acid from both N and C-terminal are removed. Truncation of 4 amino acid from N-terminal and 1 residue from C-terminal of PAMP-12 results in WNKWALS. Peptide library derived from alanine scanning has one amino acid replaced with alanine, as with ARKKWNKWALSR, where N-terminal phenylalanine is replaced with alanine. Fura-2 AM dye was used to study the activation potential of peptides against MRGPRX2 transfected HEK cells1. The data was recorded on a fluorescence plate reader. If the peptide ligand activated the cell, the fluorescence pumped at 340 nm (F340) increases, while the same decreases for 380 nm wavelength (F380) (Figure 1a). For a blank (or for that matter non activating peptide or HEK-WT control) however, the relative increase and decrease would be respectively low, as shown in the Figure 2a. Calcium concentration is, however, represented by F340/F380 ratio as shown in Figure 1b,2b. The ratio F340/F380 can be further substituted in the Grynkiewicz equation to get the calcium concentration using in situ calibrations (Figure 3). The peptides listed in Table 1 were characterized by mass spectroscopy (Figure 4a) and HPLC (Figure 4b) and found to be of high purity.
| Peptide Technique | Representative Peptide |
| Parent peptide | Ac-FRKKWNKWALSR-Amide |
| N-Truncation | Ac-RKKWNKWALSR-Amide |
| C-Truncation | Ac-FRKKWNKWALS-Amide |
| N+C-Truncation | Ac-WNKWALS-Amide |
| Alanine Scanning | Ac-ARKKWNKWALSR-Amide |
Table 1: Representative peptide sequences generated after N, C and N+C – truncation, and alanine scanning. The N-terminal of the peptides were acetyl modified while the C-Terminal contained an amide group.
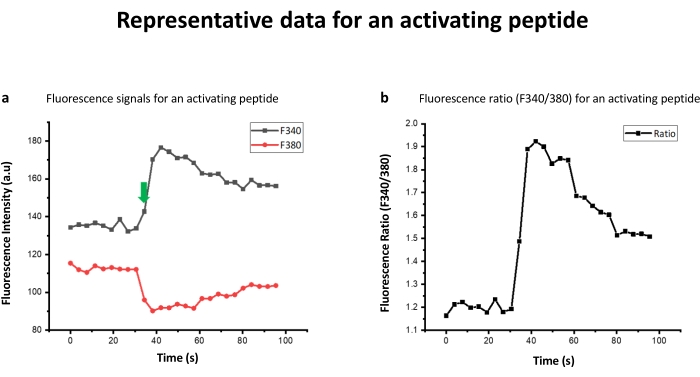
Figure 1: Representative data for an activating peptide. (a) The fluorescence signals for an activating peptide. Represented data corresponds to PAMP-12 (FRKKWNKWALSR). Peptide was added after generating a baseline for 10 reading cycles (36 s), as shown by the arrow. (b) The ratio of the fluorescence emission after excitation at 340 nm (F340) to that of fluorescence emission after excitation at 380 nm (F380) (F340/F380). Please click here to view a larger version of this figure.
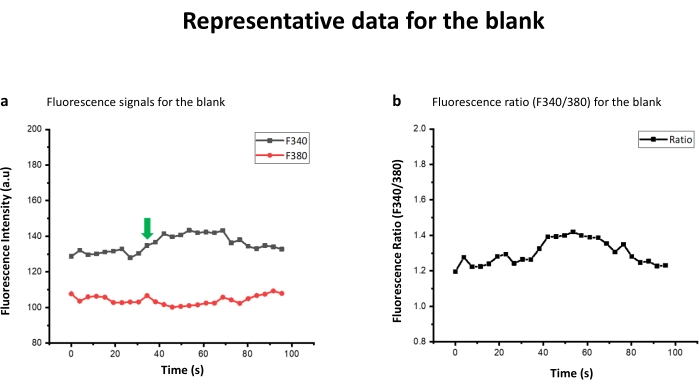
Figure 2: Representative data for the blank. (a) The fluorescence signals for the Blank. HTB was added after generating a baseline for 10 reading cycles (36 s), as shown by the arrow. (b) The ratio of the fluorescence emission after excitation at 340 nm (F340) to that of fluorescence emission after excitation at 380 nm (F380) (F340/F380). Please click here to view a larger version of this figure.
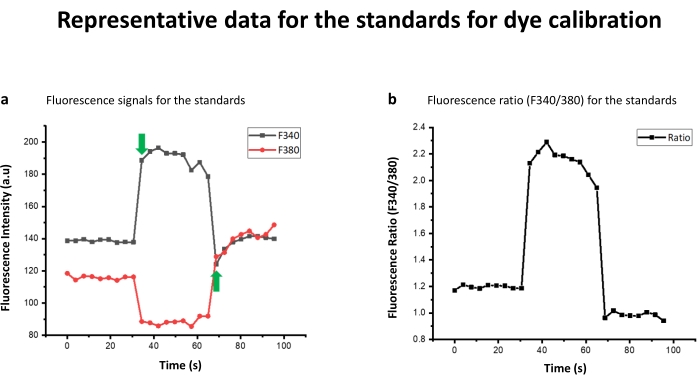
Figure 3: Representative data for the standards for dye calibration. (a) Ionomycin was added at 10th readings, as shown by the arrow, to get maximum fluorescence in Ca+2 bound state. EGTA-Triton X-100 was added after 20 readings, as shown by the arrow, to get a minimum signal. (b) The ratio of the fluorescence emission after excitation at 340 nm (F340) to that of fluorescence emission after excitation at 380 nm (F380) (F340/F380). These values are further put in the Grynkiewicz equation to get the intracellular calcium concentration. Please click here to view a larger version of this figure.
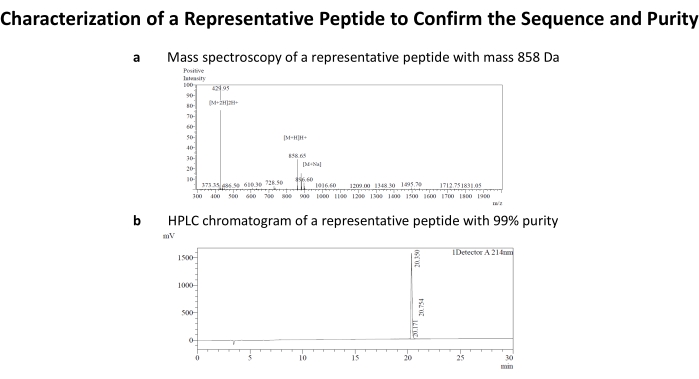
Figure 4: Characterization of a representative peptide to confirm the sequence and purity. (a) The theoretical mass of the representative peptide sequence WNKWAL was 857.90 Da, which was shown by the m/z ratio in mass spectroscopy. (b) Peptide's purity of 99% as confirmed by HPLC. This peptide belongs to N+C-truncated peptide library. Please click here to view a larger version of this figure.
Discussion
Calcium signaling is central to mast cell degranulation and has been widely used in the study of receptor-ligand interactions, ligand identification, and drug discovery14. MRGPRX2 is a recently discovered mast cell receptor that has been found to play a key role in many inflammatory diseases like itch, asthma, and atopic dermatitis, among others2. Furthermore, several approved drugs have been shown to elicit an inflammatory response through the MRGPRX2 receptor15. It is, therefore, imperative to study the ligand-receptor interaction, identify new ligands, and understand the activation mechanism. This study shows the use of common peptide techniques in designing a peptide library (Table 1) and in studying the MRGPRX2 based mast cell activation. The underlying calcium mobilization was employed as the indicator of mast cell activation.
Fura-2 is a calcium sensitive dye used to measure intracellular calcium concentration. The acetoxymethyl ester variant (Fura-2 AM) increases the permeability of the cell membrane yielding an easy method for quantifying the cytoplasmic calcium releases. The dye has frequently been used with various characterization techniques like flow cytometer, microscopy, and fluorimeters9,16,17. Though widely used, there are challenges associated with the dye, which need to be addressed before these can be efficiently employed. Intracellular calcium binding relies on the de-esterification of the dye, which is largely dependent on the dye loading conditions, cell system, and cell culture types. Partial de-esterification results in inadequate fluorescence signals. Furthermore, incomplete de-esterification also results in the localization of dye into the cell organelles resulting in inaccurate data. Leakage of de-esterified dye from the cell is another issue faced with Fura-210,11.
Apart from the dye loading, the use of the detection technique also plays an important role. The inability of flow cytometers to operate with UV lasers makes them unfavorable for the dye9. Similarly, fluorescent imaging techniques require advanced training in microscope operation. The transient nature of calcium release upon ligand activation requires a rapid response in the change in wavelengths and thus a faster shutter speed of the microscope. In addition, the use of specialized cell imaging chambers, use of coverslips and constant image focusing makes it a cumbersome technique for large-scale screening16.
A significant amount of time was invested in the method optimization. Cuvette based fluorometer, wherein cells after detaching from the culture flask, were incubated with the dye-loaded medium in the dark and then were washed and resuspended in the HTB buffer for the study. Cells suspended in HTB buffer were taken in a cuvette and were read using a photomultiplier based fluorometer. The detection systems could only hold few (2-4) cuvettes at a time and thus was not suitable for the large-scale screening of peptides. Further, HEK cells being adherent cells, the cell suspension reading showed great variations within individual repeats within an experiment. Consequently, fluorescent imaging technique was used, which again proved to be tedious and slow. The requirement for an advanced microscope with fast wavelength changing capability, microscope-specific cell chambers, and excellent imaging skills impeded the study. Additionally, in situ peptide simulation was time extensive and resulted in the loss of important data. Inefficient cell processing technique resulted in cell flotations during readings which made it difficult to focus and gave inconsistent results.
A fluorescence plate reader system with an automated pipetting system that can dispense compounds during experiments was used for the study. The fact that many samples are read in quick succession in a 96 well plate is an added benefit of this technique. The results showed that the readings were more consistent when cells were attached as compared to suspension. Cell counts of 40,000 cells/well in a TC-treated 96 well plate grown for a day gave the best results. A dye incubation time of 30-40 min at 37 °C incubator was optimum. However, when experiments were done with experimental repeats, variations in the corresponding wells of different columns were observed. To overcome this, a positive (PAMP-12) and a negative control (Blank) for each column of the plate was used. This gave more consistent and reproducible data. The method described here is an easy and versatile method, which can be efficiently employed for large-scale calcium-based screening. However, there are several factors which determines the quality of data and thus the method needs to be optimized for a given cell-instrument system.
Disclosures
The authors have nothing to disclose.
Acknowledgements
SR and LDU would like to acknowledge Alberta Innovates Strategic Research Project, NRC, and NSERC-Discovery grant for this project.
Materials
| Bovine Serum Albumin | Sigma Aldrich | 5470 | |
| Calcium Chloride | Sigma Aldrich | 793939 | |
| Corning 96 Well | Sigma Aldrich | CLS3603 | |
| Black Polystyrene Microplate | Sigma Aldrich | CLS3603 | |
| DMEM | Thermo Fischer | 11995065 | High Glucose |
| DMSO | Thermo Fischer | D12345 | Sterile, biological grade |
| EGTA | Sigma Aldrich | E3889 | |
| Fetal Bovine Serum | Thermo Fischer | 12483-020 | |
| Flexstation 3 | Molecular devices | FV06060 | |
| Fura-2 AM | Thermo Fischer | F1221 | |
| Glucose | Sigma Aldrich | D8270 | |
| HEPES buffer | Thermo Fischer | 15630-080 | |
| Ionomycin | Sigma Aldrich | I9657 | |
| L Glutamine | Thermo Fischer | 25030-081 | |
| Pen Strep | Thermo Fischer | 15140122 | |
| Peptides | RS Syntehsis | Custom | ≥95% pure; N terminal – acetyl group C terminal – amide group |
| Potassium Chloride | Sigma Aldrich | 12636 | |
| Sodium Chloride | Sigma Aldrich | S9888 | |
| TritonX-100 | DOW Chemical | 166704 |
References
- McNeil, B. D., et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 519 (7542), 237-241 (2015).
- Subramanian, H., Gupta, K., Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. Journal of Allergy and Clinical Immunology. 138 (3), 700-710 (2016).
- Rådinger, M., Jensen, B. M., Kuehn, H. S., Kirshenbaum, A., Gilfillan, A. M. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Current protocols in immunology. , (2010).
- Thomas, P., Smart, T. G. HEK293 cell line: A vehicle for the expression of recombinant proteins. Journal of Pharmacological and Toxicological Methods. 51 (3), 187-200 (2005).
- Subedi, G. P., Johnson, R. W., Moniz, H. A., Moremen, K. W., Barb, A. High yield expression of recombinant human proteins with the transient transfection of HEK293 cells in suspension. Journal of Visualized Experiments. (106), e53568 (2015).
- Occhiuto, C. J., et al. Store-operated calcium entry via STIM1 contributes to MRGPRX2 induced mast cell functions. Frontiers in Immunology. 10, 3143 (2020).
- Baba, Y., et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nature Immunology. 9 (1), 81-88 (2008).
- Hoth, M., Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 355 (6358), 353-356 (1992).
- Assinger, A., Volf, I., Schmid, D. A novel, rapid method to quantify intraplatelet calcium dynamics by ratiometric flow cytometry. PLoS One. 10 (4), 0122527 (2015).
- Roe, M. W., Lemasters, J. J., Herman, B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 11 (2-3), 63-73 (1990).
- Malgaroli, A., Milani, D., Meldolesi, J., Pozzan, T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. The Journal of Cell Biology. 105 (5), 2145-2155 (1987).
- Grynkiewicz, G., Poenie, M., Tsienb, R. Y. A New Generation of Ca2 + Indicators with Greatly Improved Fluorescence Properties. Journal of Biological Chemistry. 260 (6), 3440-3450 (1985).
- Lu, L., Parmar, M. B., Kulka, M., Kwan, P., Unsworth, L. D. Self-assembling peptide nanoscaffold that activates human mast cells. ACS Applied Materials and Interfaces. 10 (7), 6107-6117 (2018).
- Lansu, K., et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nature Chemical Biology. 13 (5), 529-536 (2017).
- Navinés-Ferrer, A., et al. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Scientific Reports. 8 (1), 11628 (2018).
- Johnson, M. Calcium imaging of store-operated calcium (Ca 2+) entry (SOCE) in HEK293 cells using Fura-2. Calcium Signalling. , 163-172 (2019).
- Tinning, P. W., Franssen, A. J. P. M., Hridi, S. U., Bushell, T. J., McConnell, G. A 340/380 nm light-emitting diode illuminator for Fura-2 AM ratiometric Ca2+ imaging of live cells with better than 5 nM precision. Journal of Microscopy. 269 (3), 212-220 (2018).

