Dural Stimulation and Periorbital von Frey Testing in Mice As a Preclinical Model of Headache
Summary
The most notable symptom of migraine is severe head pain, and it is hypothesized that this is mediated by sensory neurons innervating the meninges. Here, we present a method to locally apply substances to the dura in a minimally invasive manner while using facial hypersensitivity as an output.
Abstract
The cranial meninges, comprised of the dura mater, arachnoid, and pia mater, are thought to primarily serve structural functions for the nervous system. For example, they protect the brain from the skull and anchor/organize the vascular and neuronal supply of the cortex. However, the meninges are also implicated in nervous system disorders such as migraine, where the pain experienced during a migraine is attributed to local sterile inflammation and subsequent activation of local nociceptive afferents. Of the layers in the meninges, the dura mater is of particular interest in the pathophysiology of migraines. It is highly vascularized, harbors local nociceptive neurons, and is home to a diverse array of resident cells such as immune cells. Subtle changes in the local meningeal microenvironment may lead to activation and sensitization of dural perivascular nociceptors, thus leading to migraine pain. Studies have sought to address how dural afferents become activated/sensitized by using either in vivo electrophysiology, imaging techniques, or behavioral models, but these commonly require very invasive surgeries. This protocol presents a method for comparatively non-invasive application of compounds on the dura mater in mice and a suitable method for measuring headache-like tactile sensitivity using periorbital von Frey testing following dural stimulation. This method maintains the integrity of the dura and skull and reduces confounding effects from invasive techniques by injecting substances through a 0.65 mm modified cannula at the junction of unfused sagittal and lambdoid sutures. This preclinical model will allow researchers to investigate a wide range of dural stimuli and their role in the pathological progression of migraine, such as nociceptor activation, immune cell activation, vascular changes, and pain behaviors, all while maintaining injury-free conditions to the skull and meninges.
Introduction
Migraine pain remains a major public health issue worldwide. The World Health Organization ranks it as the sixth-most prevalent disease in the world, afflicting just under 15% of the Earth's population1 and causing a substantial socioeconomic burden on society2,3. Treatment options and their efficacy have been suboptimal and only provide symptomatic relief and do not significantly modify pathophysiological events that underly migraine occurrence4,5. The lack of treatment success is likely due to migraine being a multifactorial disorder whose pathology is poorly understood, leading to a limited number of therapeutic targets. Migraine is also challenging to fully capture in animal models, especially given that migraine diagnosis is made based on verbal communication with patients who describe their experience with migraine hallmarks such as aura, headache, photophobia, and allodynia. Notwithstanding, it is important to note that recent advances in migraine treatments are currently outperforming treatments for many neurological conditions that have been well validated by preclinical models. For instance, monoclonal antibodies and small molecules that target calcitonin gene-related peptide, or its receptor have been very successful in improving the quality of life of migraine sufferers and can potentially transform the clinical management of migraine. While there has been advancement in understanding this disorder, there continues to be much yet to be elucidated.
Based on preclinical animal models and human studies, it is widely accepted that migraine headaches are initiated by aberrant activation of nociceptive fibers within the meninges that signal through the trigeminal and upper cervical dorsal-root ganglia6,7,8,9,10. Despite this theory, many studies still use systemic administration of drugs to understand underlying contributing mechanisms in migraine. While systemic dosing of drugs has substantially strengthened our understanding, these findings do not directly assess whether local actions within the target tissue of interest play a role in migraine. Conversely, several studies have taken an approach to stimulate the dura; however, these experiments require cannula implantation via an invasive craniotomy and extended recovery times11,12. Because of these limitations, we developed a minimally invasive approach to locally stimulate the dura where the lack of a craniotomy eliminates post-surgical recovery and allows for immediate testing in awake animals12,13,14. These injections are performed under light isoflurane anesthesia and administered at the junction of the sagittal and lambdoid sutures in mice.
Several approaches have been developed to evaluate nociceptive behavioral responses in rodents15. Cutaneous allodynia has been reported in approximately 80% of migraine sufferers16,17 and represents a potential translational endpoint for use in rodents. In preclinical models, the application of von Frey filaments to the plantar region of the rodent paw has been used to assess pain behaviors in preclinical migraine models. The primary limitation of this approach is that it does not test the cephalic region. Facial grimace scoring has been used to capture pain behaviors in rodents by analyzing facial expressions after induction of pain stimuli18,19. However, its limitations include only capturing responses to acute stimuli and not chronic orofacial pain conditions. Facial grooming and decreased rearing are also considered outputs of behavioral responses in preclinical models of migraine20,21. Limitations of the former include difficulty in differentiating pain responses from normal routine grooming and other sensations such as itch. In the case of the latter, rearing behaviors typically decrease quickly after the introduction of rodents to novel environments. Although each of these behavioral endpoints is valuable in the understanding of various mechanisms that contribute to pain conditions, there is a critical need for preclinical models of pain disorders such as migraine to include endpoints that specifically capture cephalic hypersensitivity responses. Assessing tactile hypersensitivity of the periorbital skin following dural stimulation is a method that may provide better insight into mechanisms contributing to migraines where sensory symptoms are predominantly cephalic in nature. Here, we describe a method to administer substances onto the mouse dura as a preclinical model of migraine. Following dural application, we also present a detailed method for testing periorbital tactile hypersensitivity using calibrated von Frey filaments applied in the Dixon up-down method.
Protocol
All procedures were conducted with prior approval of the institutional Animal Care and Use Committee at the University of Texas at Dallas. ICR (CD-1) (30-35 g) and C57/BL6 (25-30 g) mice aged 6-8 weeks were used in this study.
1. Dural infuser
- Create the mouse infusers/injectors by modifying a commercially available internal cannula and infuser for unilateral injections with a non-metallic fused silica plastic cap that is adjustable and inserts into/extends below a 28 G guide cannula with inner diameter (I.D.) of 0.18 mm and an outer diameter (O.D.) of 0.35 mm (Figure 1A).
- Use a caliper or other measuring device to adjust the fused silica plastic cap on the infuser to a length of 0.6 mm; measured from the tip of the infuser to the edge of the silica plastic cap.
- Take caution not to bend or dull the infuser when adjusting the plastic cap.
- For other mouse strains that have not been previously used for dural injections, determine the optimal infuser length by setting the length to 0.6 mm and conducting pilot injections with ink or dye, adjusting the infuser length until it is observed that the dye is only in the dura mater and not on the brain or skull.
- Attach the long end (or the end that was not measured to be 0.6 mm) of the adjusted infuser to plastic tubing (pump tubing, 2-stop, I.D. 0.19 mm, length 406 mm).
- Cut the tubing to a minimum length of 8 in to ensure there is sufficient line to hold a volume of 5 µL.
- Make sure the tubing covers the metal part and the top of the plastic stopper located on the infuser. This will help prevent air bubbles from accumulating in the line.
- Attach the other end of the tubing to a 10 µL glass microsyringe (gas-tight; cemented needle; 21 G with a 10 mm projection), again, making sure to have a tight seal over the metal part of the syringe (Figure 1A).
- Once the line is connected, backfill the syringe with 5 µL of phosphate-buffered saline (PBS), synthetic interstitial fluid (SIF), or other vehicles of choice to prevent air bubbles from forming.
- If air bubbles are observed in the line, flood the line with the vehicle until the bubbles have dissipated.
NOTE: It may help to fill the syringe with the vehicle prior to attaching it to the line and then push the fluid through the line once connected.
- If air bubbles are observed in the line, flood the line with the vehicle until the bubbles have dissipated.
- After the line is backfilled with 5 µL of the vehicle and is working efficiently, load 5 µL of the drug/solution into Hamilton syringe (ink or dye may be used as an alternative to the drug/solution if learning or practicing this technique).
- Ensure that all vehicle solutions administered onto the dura are maintained at pH 7.4 and measured to an osmolality of 310. This reduces the potential activation of acid-sensing ion channels and other osmosensitive channels within the dura.
- The maximum volume tested in mice that did not cause leaking into the brain is roughly 10 µL. The behavioral effects after injections with this volume have not been tested. For this reason, administer only 5 µL of the solution onto the dura.
NOTE: These observations are based on the mouse strains/ages/weights of 6-8 week old CD1/ICR mice.
2. Dural injections
- Once the syringe is prepared and the drug is loaded, position a mouse flat onto its abdomen and anesthetize it under brief 3% isoflurane with an oxygen flow rate of 0.5-1 L/min via nosecone.
- After the mouse no longer displays a pinch reflex, adjust the anesthesia and sustain it at a 1.5% isoflurane.
- Once anesthetized, apply sterile opthalmic ointment to the eyes and shave the head of the animal, then disinfect the skin with povidone-iodine and ethanol. Following this, get in a position conducive to a successful injection.
- Use one hand to steady the head of the animal and hold the infuser with the other hand.
- Carefully probe and locate the junction of the sagittal and lambdoidal sutures on the skull of the mouse (Figure 1B, C).
- To locate this discreet junction through the skin, use the topographical features of the skull and gently probe the general location of the junction with the infuser.
- Verify the position of the junction by re-positioning the infuser along the skull and feeling for the exact location.
- Once the suture is located and the infuser is in place, very slowly and gently wiggle the infuser back and forth until it pierces through the skin and drops down into the junction all the way up to the plastic stopper.
NOTE: Ensure to insert the entire 0.6 mm tip of the infuser into the junction. - To verify the accuracy, use ink or dye as an injection solution and euthanize and decapitate the mouse.
- Remove the skull cap to visualize the dye within the dura mater (Figure 1C).
NOTE: Dye should not be observed on the brain or exterior of the skull. Likewise, mice in any experiment should be checked post-mortem to verify the accuracy of the injection as well as to ensure the integrity of the dura mater was undamaged.
- Remove the skull cap to visualize the dye within the dura mater (Figure 1C).
- Following the injection, remove the mouse from anesthesia, wait for it to regain consciousness and then return to its cage or place it into a testing chamber to begin the desired assays.
NOTE: Allow the mouse to recover from anesthesia for a minimum of 30 min prior to doing any behavioral experiments.
3. Periorbital von Frey
- Begin the study with a cohort of approximately 16-20 mice.
- One the day prior to habituation, handle each mouse for at least 5 min.
- Approximately 24 h after handling, habituate the mice to the testing room conditions and the von Frey testing apparatus (Figure 2A).
NOTE: The acrylic testing apparatus consists of individual compartments with lids that are approximately 3 in x 3.5 in x 5 in (W x H x D) and are supported by aluminum stands connected via 0.25 in 19 G square galvanized steel mesh wire.- Place the mice inside a horizontally placed 4-oz white paper cup that is odorless and does not contain polythene or paraffin wax.
NOTE: These types of cups are preferred because it reduces gastrointestinal upset in the mice if ingested (Figure 2B).
- Place the mice inside a horizontally placed 4-oz white paper cup that is odorless and does not contain polythene or paraffin wax.
- While the animals are in their respective chambers, place a pellet of the normal chow diet in the individual chamber of each mouse to calm the animals and avoids any unnecessary stress to the animals. Do this for 3 days prior to any von Frey behavior testing.
- Ensure that every time the mice are in the chamber that there is access to food.
- Number and assign each animal to the same space in the testing rack. Place the mouse in the same cup every day of the testing period to ensure that each animal becomes acclimated to its testing environment.
NOTE: Mice will gnaw on the cups and subsequently destroy the cups. If this should happen, replace the cup and label it with the corresponding mouse number.
- Following the initial 3 days of habituation, place the mice in their individual chambers.
- Allow the animals to acclimate to the testing room and chambers for at least 1 h prior to any von Frey testing to allow the mice to calm down and subsequently are easier to test.
- After acclimation to the room on the testing day, remove one mouse while still in its cup from its respective chamber.
- Maintain the cup in the horizontal position so that the mouse is on both forepaws and hind paws to help keep their weight evenly distributed.
NOTE: Unequal weight distribution may alter animal's responses and even prevent animals from responding.
- Maintain the cup in the horizontal position so that the mouse is on both forepaws and hind paws to help keep their weight evenly distributed.
- Place the cup with the mouse inside on the table below the testing rack on the absorbent pad.
- For periorbital von Frey testing, place the 0.07 g von Frey filament directly in the center of the face and between the eyes.
- Apply enough pressure on the filament to cause the von Frey hair to bend into a "C" shaped formation.
- Maintain contact with the region at least 3 s but no more than 5 s or until the mouse withdraws its head and swipes at the filament with its paw.
NOTE: If the filament slips or more than the tip of the filament touches the animal during testing, any responses should not be counted. These responses may be in response to the brush which are activated by different mechanoreceptors and therefore may not reflect accurate results. - Apply von Frey filaments according to the Dixon "up-down" method22,23.
- Initially, apply the von Frey filament which has a weight of 0.07 g. The lowest possible filament and the highest tested filament in this study are filaments with weights of 0.008 g and 0.6 g, respectively.
- Use the filaments of weight 0.008 g, 0.02 g, 0.04 g, 0.07 g, 0.16 g, 0.4 g, and 0.6 g to perform this assay.
- In this method, if an animal does not exhibit a response to the filament, apply the filament of the next higher gram weight.
- If the mouse does respond to a filament, consider that mouse responsive to that filament. If this is the case, apply the filament of the next lower gram weight.
- Repeat this pattern until the animal is tested 4 times after the initial response or the animal is determined to be unresponsive to any filaments tested in the assay.
NOTE: Refrain from applying any additional pressure stemming from the arm or wrist. A scale may be used to practice the application of the filament.
- Maintain contact with the region at least 3 s but no more than 5 s or until the mouse withdraws its head and swipes at the filament with its paw.
4. Testing for baseline withdrawal thresholds
- Prior to inclusion in an experiment, ensure that the mice reach a baseline withdrawal threshold between 0.5-0.6 g.
- A mouse reaches baseline if they fail to respond to any filament tested in the series mentioned in step 3.9.2.2 (0.07 g, 0.16 g, 0.4 g, and 0.6 g).
- Test mice daily when establishing baseline withdrawal thresholds.
- Testing allows animals to acclimate to the testing conditions and the pressure of the von Frey filaments.
- If the mice are still extremely hypersensitive after the third day of testing, try waiting 1 or 2 days before testing again.
NOTE: Too much time between testing days may result in the animal failing to adjust to the weight of the filament on their periorbital region, thus not reaching the targeted withdrawal threshold.
- Test mice for approximately 7 days before determining which animals do not meet the inclusion criteria for an experiment.
NOTE: Approximately 70% of mice will reach the targeted baseline level.- Prior to dural stimulation, analyze the baseline data to exclude any mouse that has not reached a baseline value of 0.5-0.6 grams or higher.
- After exclusion, randomly allocate each remaining mouse to a testing group. Achieve this by drawing out of a cup or writing a script on a spreadsheet to randomize numbers to a group.
5. Analysis of von Frey results
- Once the series of responses have been obtained, determine the delta, k value, the 50% threshold, and the withdrawal threshold in grams according to previously published methods24.
- Calculate the withdrawal threshold using this formula WT = 10(x*F+B), where WT= withdrawal threshold, F = paw withdrawal threshold calculated via the Chaplan method, and B = linear regression of log (bending force) = x*Filament number + B.
- Plot the data as either 50% withdrawal threshold or average withdrawal threshold in grams.
Representative Results
This injection method is used to administer stimuli onto the dura of mice so that subsequent behavioral testing may occur. The most common behavioral output measured with this model is cutaneous facial hypersensitivity assessed via von Frey12,13,14. Here we show how this model can be used to assess potential sex-specific contributions to migraine pathology (Figure 3).
This procedure has been used to examine the effects of dural prolactin (PRL) on mechanically evoked facial hypersensitivity14 (Figure 3). The results of this study demonstrated that female ICR mice show significantly reduced facial withdrawal thresholds in response to 5 µg of dural prolactin (Figure 3A). A ten-fold lower dose of 0.5 µg of prolactin (PRL) also showed responses similar to high dose of PRL (Figure 3B).
These injections have also been shown to produce spontaneous pain-related behaviors assessed via grimace. Dural 0.5 µg of PRL caused significant grimacing in female mice (Figure 3C), further demonstrating a clear role for dural PRL in female migraine-like behaviors. We performed grimace assays prior to all testing with von Frey filaments.
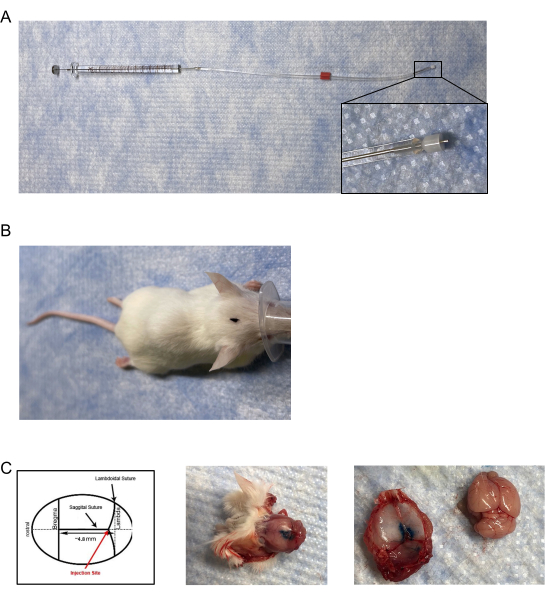
Figure 1: Dural infuser and injection placement. (A) The injectors/infusers consist of a modified cannula adjusted to the length of ~0.5 mm- 0.65 mm and attached to a needle cemented on a 10 µL gas-tight syringe via tygon tubing. (B) Aerial view of marked injection site location on the head of the mouse. (C) (Left panel) Diagram of the location of the dural injection. Placement of the injection is on the junction of the lambdoid and sagittal sutures at approximately 4.8 mm posterior to bregma. (Middle panel) Post-mortem aerial view of a mouse skull following dural injection of 5 µL of blue injection dye. (Right panel) Separation of the mouse skullcap from the brain. There was no observable leakage of blue injection dye on the brain. Please click here to view a larger version of this figure.
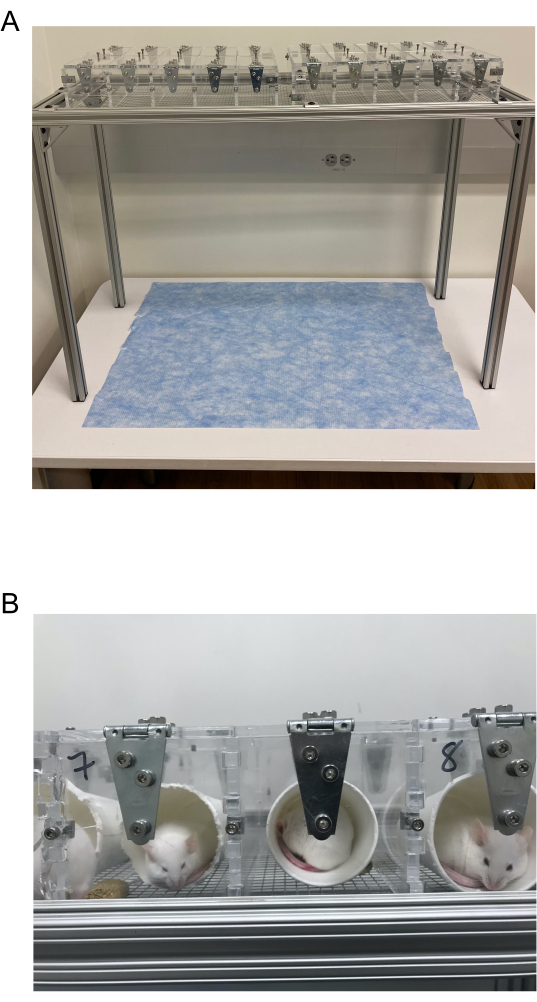
Figure 2: von Frey Testing chambers. (A) von Frey testing chamber composed of 3.5 in x 3.5 in individual acrylic chambers with lids placed on a wire mesh rack. These are connected via columns of 10 chambers organized in 2 rows. (B) Example of mice in their individual cups housed inside the von Frey testing chambers. Please click here to view a larger version of this figure.
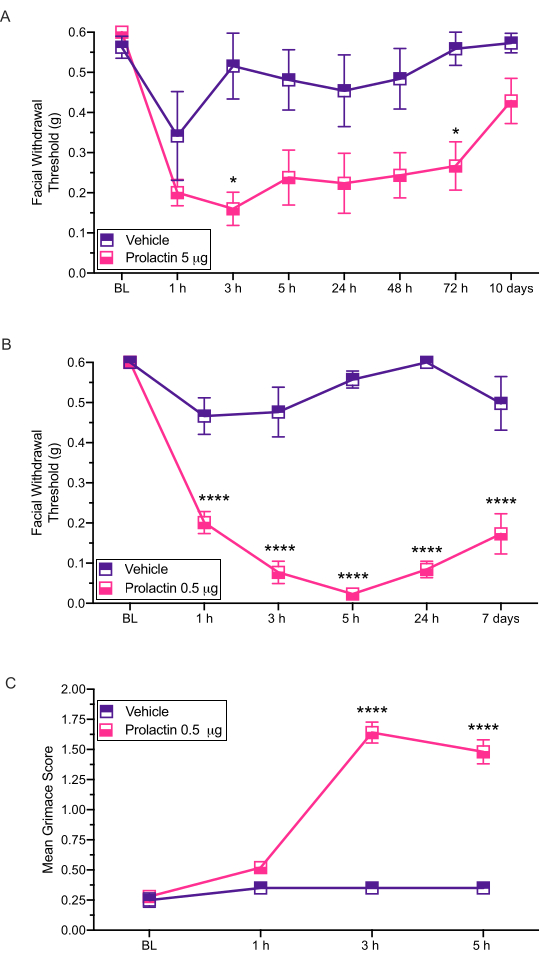
Figure 3: Dural application of prolactin induces behavioral responses in mice. Mechanical withdrawal thresholds were assessed following dural application of PRL (5 µg or 0.5 µg) in female mice. (A) Application of 5 µg of PRL (n = 7 PRL, n = 6 vehicle) induced facial hypersensitivity compared to vehicle. (B) Application of 0.5 µg of PRL (n = 5 PRL, n = 4 vehicle) induced long-lasting facial hypersensitivity. (C) Grimace was also assessed in the same mice treated with 0.5 µg of PRL at each time point. These mice exhibited significantly higher grimace scores compared to the mice treated with vehicle. Statistics: Two-way ANOVA followed by Bonferroni multiple comparison post-hoc analysis. Data are represented as means ± SEM. *p < 0.05, ****p < 0.0001. Please click here to view a larger version of this figure.
Discussion
Maladaptive changes in the local nociceptive system in the dura are considered a key contributor to the headache phase of migraine attacks despite a lack of tissue injury25,26. Here the study presents a method whereby minimally invasive stimulation of the dura can induce facial tactile hypersensitivity. Elucidating the mechanisms and events involved in dural nociceptor activation without causing damage to the cranium and tissues may more accurately reflect migraine mechanisms in a preclinical model.
Craniotomy and cannula implantation have long been used to assess functions and mechanisms that contribute to migraine pain11,12. However, it has been reported that a craniotomy can induce activation of dural mast cells and increase pial vascular permeability in rodents27. Given that mast cell activation in the dura is highly implicated in migraine7,8,28,29, this technique has major caveats that may skew the interpretation. Administering substances through the junction of the sagittal and lambdoidal sutures effectively diminishes the activation of nociceptors mediated by craniotomy-induced mast cell activation. Moreover, non-invasive dural stimulation does not require post-surgical recovery and administration of analgesics which may alter the interpretation of results. Local application of substances onto the dura allows researchers to focus on this specific target tissue, as opposed to systemic administration of drugs where the site of action is not easily determined12,13,14. While systemic administration of substances such as nitroglycerin and calcitonin gene-related peptide trigger experimental attacks in humans that are similar to migraines, they do not allow for assessment of the location of action in rodent models; more targeted tissue-specific models offer an alternate approach.
This technique described here involves injecting a drug or other solution directly onto the dura mater of the meninges through the junction where the sagittal and lambdoidal sutures of the skull meet. For best results, ICR (CD-1) or C57/BL6 mice aged 6-8 weeks should be used for these experiments. Younger mice may be used; however, the use of ICR (CD-1) mice that are older than 8 weeks are not recommended as their skull plate sutures are typically completely fused by this age, making it impossible to inject without damaging the skull. It is also critical to consider the weight/size of each mouse that will undergo this procedure. It is recommended that these injections are performed on animals that have a weight greater than 19 g as the skull is typically very thin at lower weights and may not withstand the pressure applied during the injection. Of importance, there are also likely factors that contribute to the age/weight at which skull plate fusion occurs (e.g., the composition of lab chow used in animal facilities). Therefore, experimenters may need to determine the age/weight range suitable under their own conditions. Different age ranges and animal weights may be required for other mouse strains or genotypes, depending on when the skull plates fuse in those animals, and may also require optimization of the injection itself.
When learning or practicing this technique, it is highly recommended that a level of comfort is obtained with locating the suture junction in euthanized mice. It may be best to first practice with the scalp excised or peeled back in these mice and slowly advance to locating the junction through the skin. Once establishing the precise location, inks and dyes can be injected into the dura to verify location accuracy and depth of the injection. This technique was developed and optimized using ICR (CD-1) mice (30-35 g) and C57/BL6 mice (25-30 g). An infuser length of 0.5-0.6 mm is sufficient to inject a mouse weighing within the range of 25-35 g. However, the length of the infuser may need to be calibrated if injecting mice that significantly differ from the mice used to optimize this technique. For example, a mouse smaller than 25 g would likely result in the use of an infuser that has a length less than 0.5 mm. Upon mastering this technique and when performed in age-appropriate mice, the success rate of this injection can be close to 100%; however, complications with the injection may stem from issues such as breaking the skull due to applying too much force to insert the infuser as well as abnormal bleeding caused by damaging meningeal blood vessels.
Alterations in tactile sensitivity are an important measurement when assessing pain behaviors in rodents. Here we demonstrate the use of periorbital von Frey testing to assess these behaviors in a preclinical migraine model. A major advantage of using this technique in migraine models is that we can assess hypersensitivity of the head, which has more relevance than other non-cranial locations such as paws. The critical step to ensure reproducible results is to make certain that the mice are fully baselined. This will require a well-trained experimenter that can apply von Frey filaments precisely. It is likely that it will take approximately 7 days for an animal to reach baseline. However, it is possible that not every animal will reach the targeted baseline. In our experience, after about 7 days of working with mice, only 60%-70% of animals will reach a baseline of 0.6 g in the periorbital region, but this is dependent on the cohort of animals. This timing should be considered prior to beginning an experiment to ensure sufficient numbers are used to account for dropout and that animals are the proper age post-baseline for using this non-invasive method to stimulate the dura. The steps for determining a baseline are outlined in protocol section 4.
A limitation to von Frey testing is that it can be difficult to distinguish between pain responses and routine grooming/itch. To help distinguish pain from grooming, it is important to notice the length of time this behavior occurs. Usually, a pain response is one swipe following the filament application, while grooming behaviors tend to be prolonged and can last for several seconds to minutes. If the grooming/itch behavior cannot be distinguished from a hypersensitive response, it is best not to record this as a response. Additionally, improper filament placement (e.g., filament slipping) can result in prolonged grooming of the animal, making it difficult to test properly. If this happens, the experimenter should wait until grooming has stopped and the mouse is calm enough to test. Continue from the same filament used prior to the beginning of the grooming behavior. If the mouse continues for very long bouts of time, place the mouse back in the testing chamber for approximately 5 min. Once the 5 min have passed, try testing the mouse again. If this behavior continues with no resolve, the mice have to be removed from the study. Of importance, it is not recommended to shave the fur on the face as it is unclear whether mouse skin retains the same sensitivity after hair is removed, and the process of hair removal (shaving, depilatory creams) may also influence skin sensitivity.
In most situations, it is ideal for administering substances onto the dura no more than 24 h after the mouse has reached baseline. It is recommended that mice are subjected to von Frey filament testing once per hour. If possible, testing every other hour gives enough time for the animals to calm down after testing. Additionally, experiments should be timed as not to interfere with their circadian patterns. Alterations to the circadian rhythm in mice may alter behavioral phenotypes and ultimately result in irreproducible results.
Periorbital von Frey testing can be used in combination with other behavioral assays to strengthen experimental conclusions. The grimace scale relies on spontaneous facial expressions in rodents rather than evoked responses18,19. This method has high accuracy and reliability when assessing and quantifying acute pain behaviors and has been used in many preclinical models of migraine12,30. When using both grimace and periorbital von Frey assays, the experimenter should consider scoring for grimace prior to application of von Frey filaments to the periorbital region of the mouse. This ensures that the grimacing behavior is spontaneous and not evoked by filament application. Hindpaw mechanical hypersensitivity can also be used in conjunction with periorbital von Frey testing. Contrary to grimace scoring, it is best to test facial hypersensitivity prior to assessing hind paw hypersensitivity. Hindpaw testing requires that the mouse is placed back in the chamber without the cup after periorbital von Frey testing is completed.
In conclusion, periorbital von Frey testing and non-invasive dural stimulation in mice add valuable options to the current range of preclinical models of migraine. When performed correctly, this technique presents a refined approach to generating a headache-like phenotype in rodents, as it does not require surgical implantation of a cannula. In rats, cannulas are prone to bacterial infection, can become clogged, may fall off, and require each animal to be single-housed, creating unnecessary stress on the animal. Furthermore, the dural stimulation protocol can easily be modified to use with several drug applications. Periorbital von Frey testing paradigms can also be modified to best fit the experimental specifications. Additionally, periorbital von Frey testing can be used in other orofacial pain disorders. These techniques are an important tool to help further understand the complex underlying mechanisms of migraine pain.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Institutes of Health (NS104200 and NS072204 to GD).
Materials
| 4 oz Hot Paper Cups | Choice Paper Company | 5004W | https://www.webstaurantstore.com/choice-4-oz-white-poly-paper-hot-cup-case/5004W.html |
| Absorbent Underpads | Fisherbrand | 14-206-65 | https://www.fishersci.com/shop/products/fisherbrand-absorbent-underpads-8/p-306048 |
| C313I/SPC Internal 28 G cannula | P1 Technologies (formerly Plastics One) | 8IC313ISPCXC | I.D. 18 mm, O.D. 35 mm |
| Gastight Model 1701 SN Syringes | Hamilton | 80008 | https://www.hamiltoncompany.com/laboratory-products/syringes/80008 |
| Ismatec Pump Tubing, 0.19 mm | Cole-Palmer | EW-96460-10 | https://www.coleparmer.com/i/ismatec-pump-tubing-2-stop-tygon-s3-e-lab-0-19-mm-id-12-pk/9646010 |
| Stand with chicken wire | Custom | The galvanized steel chicken wire dimensions are 0.25 in. x 19-gauge | |
| Testing Rack with individual Chambers | Custom | Each chamber should have a division between each mouse and lids to contain the mouse. The chambers should also be large enough to hold a 4 oz. paper cup. | |
| von Frey Filaments | Touch test/Stoelting | 58011 | https://www.stoeltingco.com/touch-test.html |
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 390 (10100), 1211-1259 (2017).
- Woldeamanuel, Y. W., Cowan, R. P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. Journal of the Neurological Sciences. 372, 307-315 (2017).
- Burch, R. C., Loder, S., Loder, E., Smitherman, T. A. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 55 (1), 21-34 (2015).
- Ashina, M. Migraine. New England Journal of Medicine. 383 (19), 1866-1876 (2020).
- Ashina, M., et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. 397 (10283), 1505-1518 (2021).
- Jacobs, B., Dussor, G. Neurovascular contributions to migraine: Moving beyond vasodilation. 신경과학. 338, 130-144 (2016).
- Koyuncu Irmak, D., Kilinc, E., Tore, F. Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine. Frontiers in Cellular Neuroscience. 13, 136 (2019).
- Levy, D. Migraine pain, meningeal inflammation, and mast cells. Current Pain and Headache Reports. 13 (3), 237-240 (2009).
- Levy, D., Labastida-Ramirez, A., MaassenVanDenBrink, A. Current understanding of meningeal and cerebral vascular function underlying migraine headache. Cephalalgia. 39 (13), 1606-1622 (2019).
- Phebus, L. A., Johnson, K. W. Dural inflammation model of migraine pain. Current Protocols in Neuroscience. , (2001).
- Fried, N. T., Maxwell, C. R., Elliott, M. B., Oshinsky, M. L. Region-specific disruption of the blood-brain barrier following repeated inflammatory dural stimulation in a rat model of chronic trigeminal allodynia. Cephalalgia. 38 (4), 674-689 (2018).
- Avona, A., et al. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. The Journal of Neuroscience. 39 (22), 4323-4331 (2019).
- Burgos-Vega, C. C., et al. Non-invasive dural stimulation in mice: A novel preclinical model of migraine. Cephalalgia. 39 (1), 123-134 (2019).
- Avona, A., et al. Meningeal CGRP-Prolactin interaction evokes female-specific migraine behavior. Annals of Neurology. 89 (6), 1129-1144 (2021).
- Deuis, J. R., Dvorakova, L. S., Vetter, I. Methods used to evaluate pain behaviors in rodents. Frontiers in Molecular Neuroscience. 10, 284 (2017).
- Lipton, R. B., et al. Cutaneous allodynia in the migraine population. Annals of Neurology. 63 (2), 148-158 (2008).
- Goadsby, P. J. Migraine, allodynia, sensitisation and all of that. European Neurology. 53, 10-16 (2005).
- Langford, D. J., et al. Coding of facial expressions of pain in the laboratory mouse. Nature Methods. 7 (6), 447-449 (2010).
- Mogil, J. S., Pang, D. S. J., Silva Dutra, G. G., Chambers, C. T. The development and use of facial grimace scales for pain measurement in animals. Neuroscience & Biobehavioral Reviews. 116, 480-493 (2020).
- Vuralli, D., Wattiez, A. S., Russo, A. F., Bolay, H. Behavioral and cognitive animal models in headache research. The Journal of Headache and Pain. 20 (1), 11 (2019).
- Mason, B. N., et al. Induction of migraine-like photophobic behavior in mice by both peripheral and central CGRP mechanisms. The journal of Neuroscience. 37 (1), 204-216 (2017).
- Dixon, W. J., Mood, A. M. A method for obtaining and analyzing sensitivity data. The Journal of the American Statistical Association. 43 (241), 109-126 (1948).
- Dixon, W. The up-and-down method for small samples. The Journal of the American Statistical Association. 60, (1965).
- Bonin, R. P., Bories, C., De Koninck, Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Molecular Pain. 10, 26 (2014).
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Seminars in Immunopathology. 40 (3), 301-314 (2018).
- Edvinsson, L., Haanes, K. A., Warfvinge, K. Does inflammation have a role in migraine. Nature Reviews Neurology. 15 (8), 483-490 (2019).
- Stokely, M. E., Orr, E. L. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. Journal of Neurotrauma. 25 (1), 52-61 (2008).
- Theoharides, T. C., Donelan, J., Kandere-Grzybowska, K., Konstantinidou, A. The role of mast cells in migraine pathophysiology. Brain Research Reviews. 49 (1), 65-76 (2005).
- Conti, P., et al. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. European Journal of Pharmacology. 844, 87-94 (2019).
- Rea, B. J., et al. Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain. 159 (11), 2306-2317 (2018).

