Ex Utero Culture of Mouse Embryos from Pregastrulation to Advanced Organogenesis
Summary
An enhanced platform for whole-embryo culture allows continuous and robust ex utero development of postimplantation mouse embryos for up to six days, from pregastrulation stages until advanced organogenesis. In this protocol, we detail the standard procedure for successful embryo culture using static plates and rotating bottle systems.
Abstract
Postimplantation mammalian embryo culture methods have been generally inefficient and limited to brief periods after dissection out of the uterus. Platforms have been recently developed for highly robust and prolonged ex utero culture of mouse embryos from egg-cylinder stages until advanced organogenesis. These platforms enable appropriate and faithful development of pregastrulating embryos (E5.5) until the hind limb formation stage (E11). Late gastrulating embryos (E7.5) are grown in rotating bottles in these settings, while extended culture from pregastrulation stages (E5.5 or E6.5) requires a combination of static and rotating bottle cultures. In addition, sensitive regulation of O2 and CO2 concentration, gas pressure, glucose levels, and the use of a specific ex utero culture medium are critical for proper embryo development. Here, a detailed step-by-step protocol for extended ex utero mouse embryo culture is provided. The ability to grow normal mouse embryos ex utero from gastrulation to organogenesis represents a valuable tool for characterizing the effect of different experimental perturbations during embryonic development.
Introduction
Intrauterine development of the mammalian embryo has limited the study of the early stages of postimplantation development1,2. The inaccessibility of the developing embryo hampers the understanding of key developmental processes occurring after the embryo implants into the uterus, such as the establishment of the animal body plan, specification of the germ layers, or the formation of tissues and organs. Moreover, the very small size of the early postimplanted embryo makes it difficult to observe by intravital imaging in utero before E103. The inability to observe and manipulate living embryos at these stages has restricted the study of early postimplantation embryogenesis to snapshots during development.
Protocols for in vitro culture of preimplantation mammalian embryos are well established, reliable, and regularly utilized4. Nevertheless, attempts to establish ex utero culture systems capable of supporting proper mammalian postimplantation embryo growth had limited success5. A variety of culture techniques have been proposed for over a century, mainly by culturing the embryos in conventional static plates6,7,8 or rotating bottles (roller cultures)5,9,10. These platforms proved helpful in expanding the knowledge on mammalian development after implantation11,12, despite being highly inefficient for normal embryo survival and limited to short periods. The embryos began to display developmental retardation and morphological anomalies as early as 24-48 h after culture initiation.
This study provides a detailed description for setting up the ex utero embryo culture system that allows continuous development from pregastrulation to advanced organogenesis stages over up to six days of postimplantation development13. This paper describes the improved roller culture protocol that supports the growth of E7.5 embryos (neural plate and headfold-stage) until the hind limb formation stage (~E11) and the extended culture from E5.5/E6.5 by combining culture on static plates and roller culture platforms.
Protocol
All animal experiments were performed according to the Animal Protection Guidelines of Weizmann Institute of Science and approved by relevant Weizmann Institute IACUC (#01390120-1, 01330120-2, 33520117-2). Healthy pregnant women were asked to give their informed consent to collect blood from their umbilical cord, as approved by the Rambam Medical Center Helsinki Committee (#RMB-0452-15). Healthy adults were asked for their informed consent to have blood collected according to the guidelines of the Weizmann Institute of Science Helsinki Committee (#1566-3).
1. Media preparation
- Prepare the dissection medium using 500 mL of Dulbecco's Modified Eagle Medium (DMEM) without phenol red and L-glutamine, supplemented with 10% fetal bovine serum, 5 mL of penicillin/streptomycin, and filter-sterilize using a 0.22 µm filter.
NOTE: Keep the dissection medium at 4 °C for up to 1 month. - Prepare ex utero embryo culture medium (EUCM) freshly every culture day inside a biological hood using 25% of embryonic medium plus 50% rat serum and 25% human umbilical cord blood serum (HCS) or human adult blood serum (HBS).
- To prepare embryonic medium, add 5 mL of glutamine, 5 mL of HEPES, and 5 mL of penicillin/streptomycin in 500 mL of DMEM without phenol red and L-glutamine. Prepare 10-15 mL aliquots and store them at 4 °C for up to two months.
NOTE: Double the antibiotic concentration if experiencing any contamination. - Heat-inactivate the rat serum at 56 °C for 30-45 min and filter through a 0.22 µm polyvinylidene difluoride filter. Use the serum for culture on the same day of inactivation. Thaw HCS or HBS and use it immediately for experiments.
NOTE: Commercial rat serum provides consistent results (a minimum of 4 lots were tested without evident variation). Alternatively, high-quality rat serum can be obtained in-house as described previously8,14. If HCS is not available, replace it with in-house-collected HBS. Rat serum, HCS, and HBS can be refrozen once and kept at -20 °C for use on a different day. Thaw and combine the refrozen serum with a larger volume of freshly thawed serum and do not refreeze it.
2. Collection of human umbilical cord blood serum and human adult blood serum
- To isolate HCS, collect umbilical cord blood from healthy pregnant women on the day of the scheduled caesarian delivery.
- Double-clamp the umbilical cord 5-7 cm from the umbilicus and transect between the clamps immediately after delivery of the infant.
- Manually draw blood using a large-bore 14 G needle and a 50 mL syringe directly from the umbilical vein while the placenta remains in situ to avoid any traces of hemolysis.
NOTE: Collect blood after the infant is removed from the field of surgery, and umbilical blood has been drawn for clinical tests.
- Dispense the collected blood to 5 mL procoagulant sterile test tubes and immediately transfer them to 4 °C for 15 min to allow complete coagulation. Centrifuge the coagulated test tubes at 2,500 × g for 10 min at 4 °C.
- Discard tubes showing signs of hemolysis (pink-red serum). Collect the serum (yellow-color) using a 5 mL pipette and filter it through a 0.22 µm filter. Heat-inactivate the serum immediately in a 56 °C water bath for 45 min.
- Prepare 1-1.5 mL aliquots of the inactivated serum and store them at -80 °C for up to six months. If needed, ship at -70 °C using dry ice.
- For adult human blood serum collection, draw blood from healthy adults and immediately isolate serum following the same protocol described for umbilical cord blood serum. Store the HBS as heat-inactivated and filtered aliquots at -80 °C for up to six months.
NOTE: Human umbilical cord serum and adult human serum prepared freshly in-house gave superior results to commercially available serum. Collect HCS from healthy women, over the age of 18 and under 40 years scheduled for cesarian section delivery. Exclude women who gave vaginal birth and women with any chronic illness or active medical conditions, including gestational diabetes or hypertension.
3. Ex utero roller culture of embryos from E7.5 to E11
- Setting up the roller culture incubator and gas regulator
- Culture E7.5 or more advanced embryos using the roller culture incubator system. Turn on the rotator and the heating unit at 37 °C for at least 1 h before the embryo dissections. Add autoclaved water to the gas inlet bottle and the outlet test tube.
NOTE: Place a thermometer adjacent to the rotating drum and frequently check the stability of the temperature inside the incubator. Open the incubator as quickly as possible as prolonged opening time will increase the temperature and affect embryo development. When necessary, add more autoclaved water to the gas inlet bottle and the outlet test tube to keep them to a minimum of half capacity during culture. Protect the embryos inside the incubator from light by covering the incubator with a cloth. - Turn on the gas regulator module by pressing the main switch and subsequently turning on the Oxygen/Nitrogen and CO2 controllers (Figure 1A). Set the oxygen and CO2 values to 5% using the respective controllers. Open the gas regulator and set the gas pressure 세스 ~6.5-7 pounds per square inch (psi) by moving the voltage switch in the pressure transmitter (Figure 1B). Confirm the gas pressure value using a digital pressure gauge.
- Monitor the gas flow by checking the rate of bubbles created inside the outlet water-filled test tube allocated inside the precision incubator. Set the proper bubble rate by closing/opening the gas flow valve on the lid of the water bottle. Ensure that the gas flow allows the formation of bubbles at a rate of 2-4 bubbles per second or set the bubble flow at the first point where bubbling comes out to the water-filled outlet tube.
- Fill the culture bottles with 2 mL of culture medium and plug them into the hollowed rotating drum for preequilibration for 1 h. Use the hollowed silicon bungs to seal the bottles to the drum. Keep sealed the empty spaces in the rotating drum using the solid bungs.
NOTE: The absence of bubbles in the outlet tube (no gas coming through the system) or an exceptionally high gas flow may affect embryo development. In the case of a lack of bubble flow to the outlet test tube, check that all silicon bungs are properly positioned in the rotating drum and sealing the system, and check that the water bottle is closed properly and all the tubing is connected correctly. A lack of bubbling coming into the water bottle may indicate a malfunction in the gas regulator.
- Culture E7.5 or more advanced embryos using the roller culture incubator system. Turn on the rotator and the heating unit at 37 °C for at least 1 h before the embryo dissections. Add autoclaved water to the gas inlet bottle and the outlet test tube.
- Dissection of mouse embryos from pregnant mice
- Preequilibrate the dissection medium inside an incubator at 37 °C and 5% CO2 for a minimum of 1 h. Leave the lid slightly open to allow gas exchange.
- Sacrifice the pregnant female by cervical dislocation, clean the abdomen of the female with 70% ethanol, and cut the skin and abdominal wall with scissors. Locate one end of the uterus and cut at the intersection between the ovary and the uterus. Next, cut along the uterus until the other end and transfer it to a 100 mm Petri dish filled with room temperature Dulbecco's phosphate-buffered saline (DPBS).
NOTE: Use of non-hormone-primed, naturally mated females is recommended. - Quickly wash the conceptuses in DPBS and cut in pairs to facilitate embryo handling. Move all the pairs of conceptuses to preequilibrated dissection medium in a 60 mm Petri dish and cut into individual conceptuses.
- Remove the uterine wall of the conceptuses by tearing the uterine tissue using a pair of gross forceps. Use fine microsurgical forceps to cut the tip of the pear-shaped decidua. Insert the forceps next to the embryo parallel to its long axis, and open the forceps to split the decidua into halves.
- Finally, leave the intact ectoplacental cone attached to the egg cylinder by grasping the embryo from the decidua and peeling the parietal yolk sac off the embryo using fine forceps.
NOTE: To avoid affecting the embryo, perform dissections on a microscope equipped with a heating plate at 37 °C within a maximum of 30-40 min. Dissect one litter of embryos at a time. - Immediately after dissection, transfer the embryos to a new plate filled with equilibrated dissection medium using a glass Pasteur pipette to prevent the embryos from sticking to the tissue debris.
- Select those embryos in the neural plate/early head fold stage showing no damage in the epiblast and transfer 5-6 embryos per bottle to the preequilibrated glass culture bottles.
NOTE: To prepare the glass Pasteur pipette, cut the opening of the pipette to an adequate size to fit the embryos using a glass cutter, and keep it in ethanol to avoid contamination. Wash the glass pipette with PBS before transferring embryos. When transferring the embryos to the culture bottle, make sure to carry over as little volume of the dissection medium as possible to avoid diluting the EUCM. - Place the bottles in the rotating culture system at 37 °C, in an atmosphere of 5% O2 and 5% CO2.
- Each day of culture, remove the bottles one by one from the incubator to evaluate embryo development under a stereomicroscope. Make sure to close the empty hole in the drum with a solid bung when removing a bottle. Cut the tip of a sterile plastic Pasteur pipette to fit the embryo size and move the embryos to a Petri dish to facilitate observation. Make sure that the embryos are always covered by medium.
NOTE: Minimize the time for which the embryos are outside the incubator to avoid detrimental effects in the embryos due to a decrease in body temperature. Handle the embryos carefully to avoid rupture of the yolk sac blood vessels, affecting embryo survival. - At culture day 1 (equivalent to E8.5), transfer groups of 3 embryos to a new bottle with 2 mL of fresh and preequilibrated EUCM containing extra 3 mg/mL of D-glucose and a gas mixture of 13% O2, 5% CO2.
- After 48 h (equivalent to E9.5), transfer groups of two embryos to a new bottle with fresh preheated EUCM plus 3.5 mg/mL of glucose in a gas atmosphere of 18% O2 and 5% CO2.
- At 72 h of culture (equivalent to E10.5), move each embryo to an individual bottle containing 1.5-2 mL of fresh medium supplemented with 4 mg/mL of glucose and a gas supply of 21% O2 and 5% CO2.
NOTE: Embryos reach their maximum growth about midnight of culture day 4. - Use fine forceps to remove the yolk sac and amnion, and detach the umbilical cord for proper observation of embryo morphology. Turn off the gas regulation module and precision incubator.
NOTE: Preequilibrate the culture medium for 1 hour by incubation inside a glass bottle in the roller culture with an adequate gas atmosphere according to the embryo stage. Clean the culture bottles and all incubator glassware after every use by performing three washes with running distilled water followed by an overnight wash submerged in 70% ethanol. Rewash three times with running distilled water, let the bottles dry overnight, and sterilize by autoclave. Likewise, autoclave the silicon plugs regularly. Thoroughly clean all dissection tools with 70% ethanol and dry-sterilize them.
4. Extended embryo culture from E5.5/E6.5 to E11
- Culture pregastrulation (E5.5) and early gastrulation (E6.5) embryos until the early somite stage (E8.5) in static plates.
- Preequilibrate the dissection medium for 1 h in an incubator with 5% CO2 at 37 °C.
NOTE: To allow gas exchange, do not close the lid of the tube completely. - Prepare the culture plates by adding 250 µL of freshly prepared EUCM to each well. Place the plates inside a CO2 incubator at 37 °C for preequilibration.
NOTE: Although 8-well plates are well-suited for embryo growth, culture can be done in any other plate by adjusting the volume of the medium according to the size of the plate. - Dissect egg cylinder embryos out of the uterus following the technique described for E7.5. Remove the parietal yolk sac of the embryo and leave the intact ectoplacental cone attached to the egg cylinder.
- Transfer individual embryos into each well of the 8-well plate using a micropipette and place the plate inside the incubator with 5% CO2 at 37 °C.
- Image the embryos under a stereomicroscope and select for culture only those with a well-formed amniotic cavity, with no evident damage and without the Reichert's membrane.
NOTE: Use 20 µL pipette tips to transfer E5.5 embryos and 200 µL tips to transfer E6.5 embryos. In the case of cultures starting at E6.5, HCS can be replaced by freshly collected HBS. - Remove half of the medium and add 250 µL of freshly prepared prewarmed EUCM after 24 h of culture, ensuring that the embryos are always immersed in the culture medium. In the case of cultures starting at E5.5, after two days of culture (equivalent to E7.5), remove 200 µL and add 250 µL of new EUCM.
NOTE: During static culture, embryos might attach to the plate (mainly when using plastic plates), which will severely compromise embryo development. Attachment of the embryo to the plate is more frequent on the first day of culturing E5.5 embryos. To prevent attachment, carefully push the embryos away from the plate surface using fine, sterile forceps. Verify that only the ectoplacental cone remains attached to the surface of the plate. - Transfer the embryos into the roller culture at the early somite stage (4-7 somites; after three days of culture for embryos explanted at E5.5 and two days for cultures started at E6.5), following the same indications described previously for E8.5 stage embryos.
NOTE: Transferring the embryos to the roller culture at somite stages different than indicated above results in failure of further development. In contrast with E7.5 ex utero cultures, it is possible to maintain the embryos in a constant atmosphere of 21% oxygen and 5% CO2, providing a slightly higher efficiency of embryo development than atmospheres with dynamic oxygen concentration.
Representative Results
The roller culture conditions described for E7.5 embryos (late-gastrulation stage) support constant and normal embryo growth with an average efficiency close to 75% after 4 culture days (Figure 2 and Table 1). The efficiency of embryo development may vary across diverse mouse genetic backgrounds but is consistently robust (Figure 2C). Supplementation with HBS instead of HCS yields an efficiency of ~68% after 4 days of ex utero culture, depending on the genetic background of the mice (Figure 2D and Table 2). The embryos developed ex utero recapitulate proper development until approximately the 44-somite stage. Afterward, the embryos present embryonic abnormalities due to the absence of the allantoic placenta, resulting in insufficient oxygenation and nutrient supply given the increased body size at this stage.
Development of E6.5 embryos (early-streak) in static plates is correctly recapitulated with an efficiency of >90% until the early somite-stage E8.5, using EUCM with both HCS and HBS (Figure 3, Table 3, and Table 4) (see8,13 for a detailed description of embryo staging between E5.5 to E8.5). Ex utero culture from gastrulation to advanced organogenesis by combining cultures on static plates followed by the roller culture in a constant 21% oxygen atmosphere gives an estimated efficiency of proper development of 55% and 26% to the 44-somite stage, using HCS and HBS, respectively (Figure 3A, Table 3, and Table 4). There is a delay of 1-2 somite pairs in these embryos compared to embryos developed in utero. The greatest drop in efficiency occurs at the transition from E8.5 to E9.5 due to failure of axial turning and closure of the neural tube.
Cultures starting from E5.5 pregastrulating embryos show efficiency of proper development to the early-somite stage (E8.5) of approximately 46%, and nearly 17% of the embryos will complete proper development after six days of culture after being transferred to the roller culture (Figure 4 and Table 5). Extended ex utero culture prolongs the developmental delay in the embryos, with embryos explanted at E5.5 showing a delay of 2-4 pairs of somites compared to in vivo embryos. Nevertheless, morphogenesis and tissue development proceed properly until approximately the 42-somite stage.
The most common defects seen in the embryos for cultures initiated from E7.5, E6.5, and E5.5 are exemplified in Figure 5A–C. At the time of dissection, embryos with even minor damage to the epiblast or the extraembryonic region, as well as embryos retaining the Reichert's membrane, should be discarded. Likewise, early embryos will not grow properly (see Figure 5B for dead embryos) or display severe developmental delays (see Figure 5B for a delayed embryo). Attachment of the embryonic epiblast to the surface of the plate will affect development depending on the position and grade of attachment. Attachment of a part of the epiblast or the whole embryo will cause the failure of further development (see Figure 5B for an attached embryo).
The main abnormalities observed in the percentage of defective embryos at E8.5 (early somite stage) are the development of the posterior region outside the yolk sac or defects in the growth of the neural folds (Figure 5A,B). In the case of cultures started at E5.5, a frequently observed developmental defect is the presence of a small, underdeveloped epiblast (Figure 5C). At the time equivalent to E9.5, defects in the closure of the neural folds, failure of axial turning, or a deficiency in brain growth represent the most commonly observed abnormalities (Figure 5). The most frequently observed developmental defects at E10.5/E11 are anomalies in the head region, disruption of normal blood circulation in the yolk sac, and pericardial effusion (Figure 5). Rupture of one main blood vessel and blood outflow may cause subsequent death of the embryo. Notably, proper growth of the embryo itself might be reached even in the absence of evident yolk sac circulation. Embryos kept in culture beyond the stage equivalent to E11 exhibit body shrinkage and death after few hours due to a lack of proper tissue oxygenation.
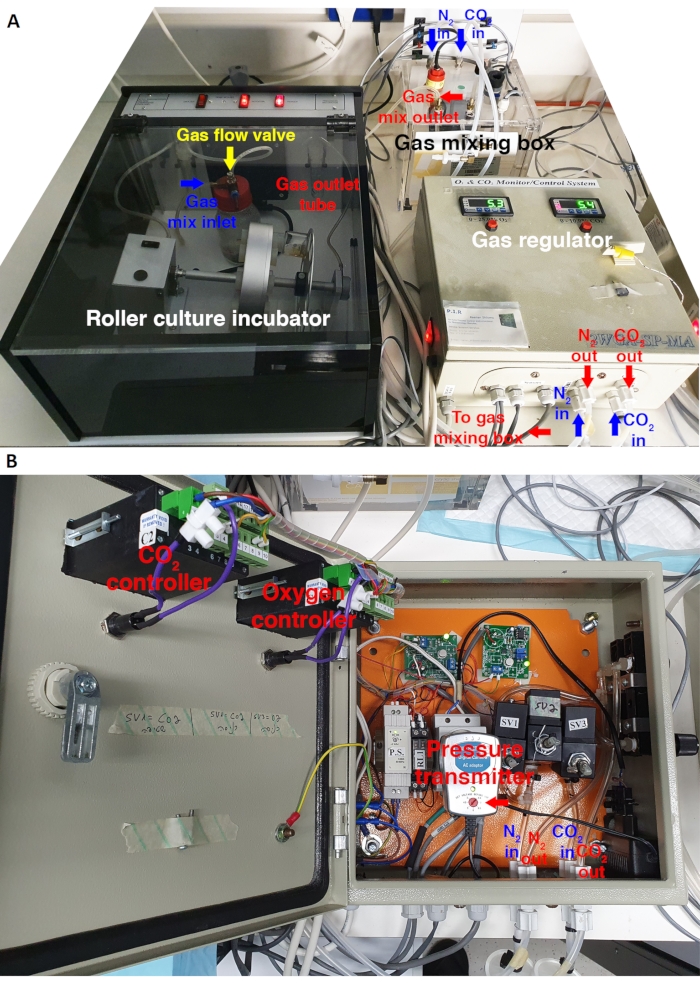
Figure 1: Gas and pressure regulation system adapted to a roller culture incubator. (A) Top view of the gas regulation module connected to the roller culture incubator. N2 and CO2 enter the gas regulator to allow precise control of the oxygen/CO2 concentrations and gas pressure. The gases are directed towards the mixing box, in which they are mixed by a centrifugal blower and injected into the incubator by a pump that generates positive pressure. The gas flows through the inlet into a water bottle and later to the sealed bottles. (B) Internal configuration of the electronic module for gas and pressure regulation. The voltage value set on the pressure transmitter regulates the pressure generated by the pump inside the gas mixing box (5-6 V to attain pressure of 6-7 psi in this specific model). Please click here to view a larger version of this figure.
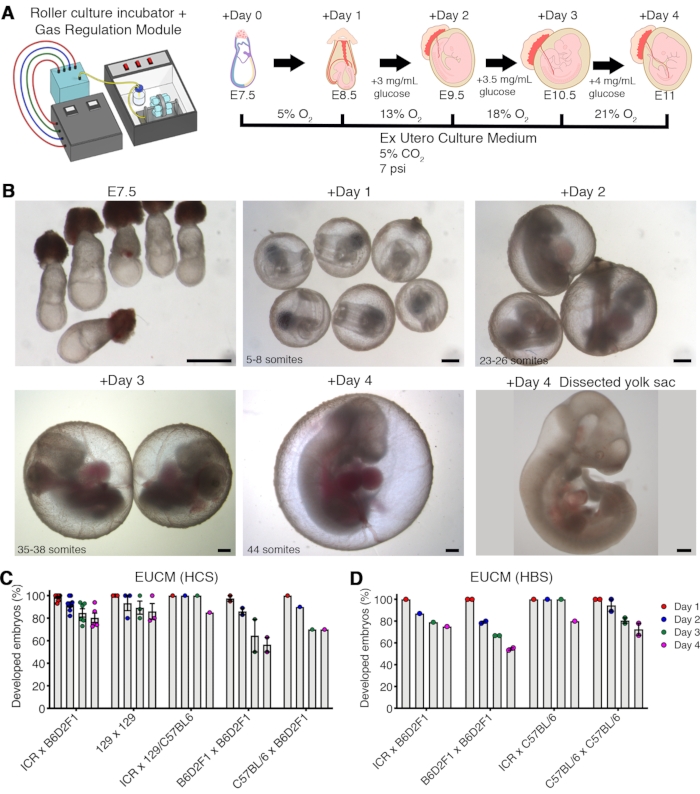
Figure 2: Ex utero culture platform supports growth of E7.5 embryos until advanced organogenesis. (A) Diagram depicting the E7.5 ex utero embryo culture protocol. (B) Representative bright-field images of groups of cultured embryos developing ex utero over 4 days, from late gastrulation (E7.5) to the 44-somite stage (E11). The typical variation in somite number assessed every 24 h is indicated. Scale bars = 500 µm. (C, D) Percentage of normally developed embryos at 1-4 days of culture starting from E7.5 divided by mouse parental strains and serum supplementation (C, human umbilical cord blood serum; D, human adult blood serum). Panel A has been modified from 13. Abbreviations: EUCM = ex utero embryo culture medium; HCS = human umbilical cord blood serum; HBS = human adult blood serum. Please click here to view a larger version of this figure.
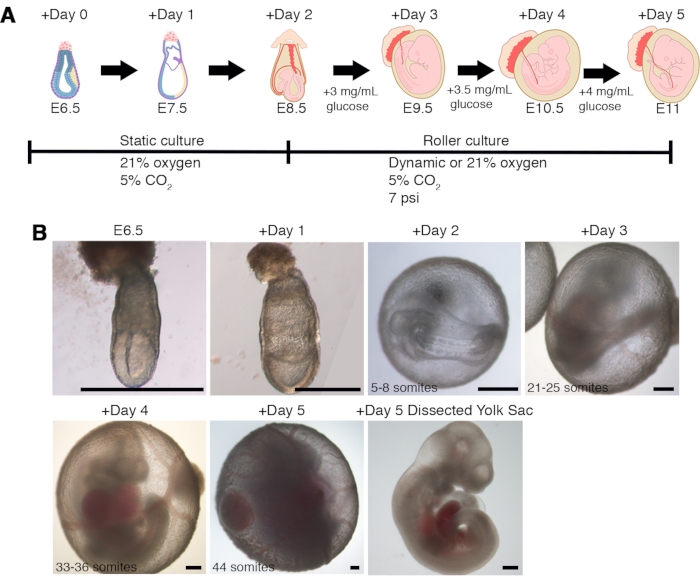
Figure 3. Extended ex utero culture protocol for growing E6.5 early-gastrulating mouse embryos until late organogenesis. (A) Schematic illustration of the extended ex utero culture protocol combining static plates and rotating bottles systems. (B) Bright-field images of embryos cultured ex utero for five days from E6.5 to the 44-somite stage. The typical variation in somite number assessed every 24 h is indicated. Scale bars = 500 µm. Please click here to view a larger version of this figure.
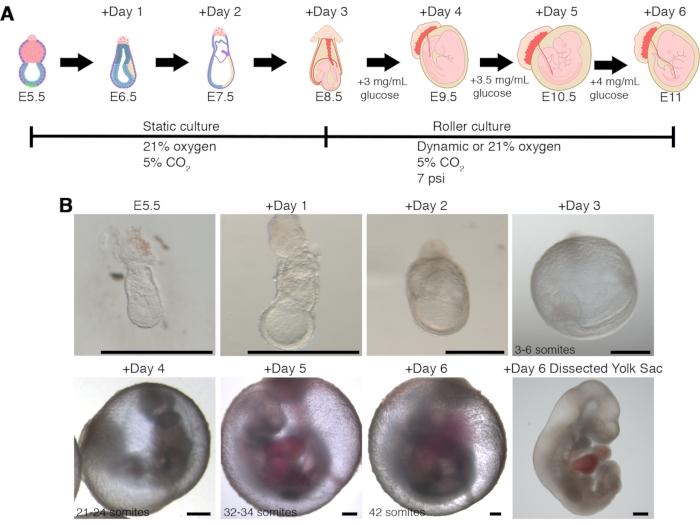
Figure 4: Continuous ex utero culture of pregastrulation mouse embryos from E5.5 until late organogenesis stages. (A) Schematic depiction of the ex utero culture the protocol for E5.5 embryos.(B) Representative bright-field images of embryos cultured ex utero for six days from E5.5 until the 42-somite stage. The typical variation in somite number assessed every 24 h is indicated. Scale bars = 500 µm. Please click here to view a larger version of this figure.
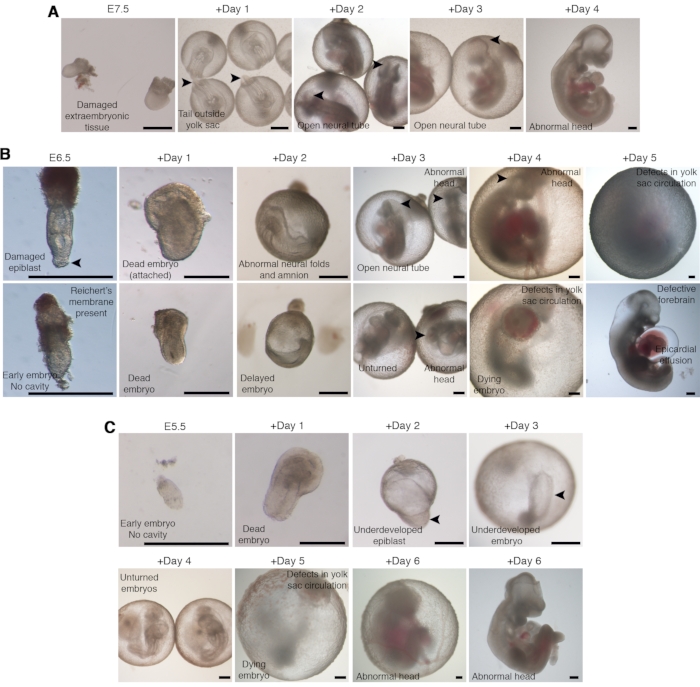
Figure 5: Representative developmental defects observed in embryos cultured ex utero. (A–C) Bright-field microscopy images of abnormal mouse embryos grown ex utero starting from E7.5 (A), E6.5 (B), or E5.5 (C). A general description of the defect is provided on each image. Scale bars = 500 µm. Please click here to view a larger version of this figure.
Table 1: Efficiency of proper development of embryos isolated at E7.5 days post coitum. The embryos were cultured ex utero for 4 days in EUCM (25% Human Umbilical Cord Blood Serum). [-] indicates cultures not continued due to experimental requirements. Please click here to download this Table.
Table 2: Efficiency of proper development of embryos isolated at E7.5, cultured ex utero for 4 days, replacing Human Umbilical Cord Blood Serum with freshly isolated Human Adult Blood Serum. [-] indicates cultures not continued due to experimental requirements. Please click here to download this Table.
Table 3: Efficiency of proper development of embryos (B6D2F1/ICR) isolated at E6.5 and cultured ex utero for 5 days using EUCM (25% Human Umbilical Cord Blood Serum). The ex utero culture was done in static culture for two days (21% O2) followed by three days in rotating bottles at 21% O2. [-] indicates cultures not continued due to experimental requirements. Abbreviation: NA = not acquired. Please click here to download this Table.
Table 4: Efficiency of proper development of embryos (B6D2F1/ICR) isolated at E6.5 and cultured ex utero for 5 days using EUCM (replacing Human Umbilical Cord Blood Serum with freshly isolated Human Adult Blood Serum). The embryos were developed in static culture for two days (21% O2) followed by three days in rotating bottles at 21% O2. [-] indicates cultures not continued due to experimental requirements. Please click here to download this Table.
Table 5: Efficiency of proper development of embryos (B6D2F1/ICR) isolated at E5.5 and cultured ex utero for 6 days using EUCM (25% Human Umbilical Cord Blood Serum). The embryos were developed in static culture for three days (21% O2) followed by three days in rotating bottles at 21% O2. [-] indicates cultures not continued due to experimental requirements. Please click here to download this Table.
Discussion
The culture protocol presented herein can sustain proper and continuous mouse embryo development ex utero for up to six days, from E5.5 to E11. Previously, embryos at these developmental stages could develop normally in culture only for short periods (up to 48 h)15. The coupling of the gas regulation module to the roller culture incubator for precise control of oxygen concentration and hyperbaric gas pressure is critical for the proper mouse embryo culture described herein. Increasing the gas pressure to 7 psi enhances oxygen diffusion, allowing embryo development up to E11 in an atmosphere of up to 21% O2/5% CO2, in contrast to the previously employed conditions of 95% O216, which may be harmful to the embryo in the long term. Further, oxygen concentration is known to play a critical role in embryonic development, as early postimplantation embryogenesis takes place under hypoxic conditions17. Accordingly, the successful culture of late-gastrulating embryos requires an initial 5% O2 atmosphere, with a dynamic increase in oxygen concentration as the embryo grows. Remarkably, culturing pre/early-gastrulating embryos in static culture at 5% O2 drastically decreased the efficiency of proper embryo development compared to 21% O2, and they could not develop further to E9.5. The latter might be explained by the slower rate of nutrient and oxygen diffusion through the embryonic tissues in the static culture compared to the culture in rotating bottles1,10.
Furthermore, the high content of rat and human umbilical cord blood serum provides more consistent results for growing early postimplanted embryos than media supplemented with only rat serum13,18. Importantly, the serum used for embryo culture should be prepared from freshly extracted blood. Although high-quality rat serum for whole-embryo culture is commercially available, human serum should be isolated in-house. Supplementation with human umbilical cord blood serum can be replaced by serum isolated from human adult blood, which is widely accessible and still provides consistent and efficient embryo growth.
Successful and efficient embryo development ex utero is also highly dependent on accurate embryo isolation. First, the dissection procedure should be performed in dissection medium warmed at 37 °C, and the dissected embryos should be transferred into the culture bottles/plates within 30 min. Second, precise embryo isolation from the decidua and removal of the Reichert’s membrane without damaging the epiblast is key for obtaining high efficiency of embryo development. Third, only embryos at the adequate stage should be selected for culture, as early/delayed embryos will not grow properly.
Handling the embryos during transfer is another essential point during the ex utero culture, mainly after the development of the embryonic yolk sac. Embryos should be transferred carefully because damage to major yolk sac blood vessels could affect proper development. Generally, the longer the period of embryo culture, the lower the efficiency of normal embryo development, i.e., embryos explanted at E7.5 will develop with higher efficiency than those explanted at E6.5 or E5.5. Moreover, the presence of antibiotics in the medium is fundamental to prevent contamination if a dissection microscope allocated inside a biological hood is not available.
It cannot be ruled out that other platforms, pressure levels, or conditions might enable similar or enhanced outcomes to the results obtained with the present protocol. Further optimization of the conditions described in this study is needed to reach an efficiency of embryo development equal to that observed by intrauterine development. Moreover, future development of a defined serum-free medium could help define the specific metabolic and chemical requirements during mammalian embryo development and reduce batch-to-batch serum variability. The need for constant nutrient and gas mixing after E8.5 using the rotating bottles culture in the current settings limits the long-term imaging capabilities during organogenesis stages. Future development of microfluidics devices enabling gas and nutrient mixture in static culture coupled to microscopy setups could help overcome this challenge.
Embryos cultured ex utero can be experimentally manipulated and kept in culture up to advanced organogenesis stages outside the uterus. We previously demonstrated the ability to introduce diverse perturbations in developing embryos, such as genetic manipulation by electroporation or lentiviral infection, live imaging, cell grafting, and teratogenic studies13. Ultimately, this platform may help uncover cell fate specification and organ formation mechanisms in mammals by allowing real-time experimentation in living mouse embryos for up to six days of early postimplantation development.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by Pascal and Ilana Mantoux; European Research Council (ERC-CoG-2016 726497-Cellnaivety); Flight Attendant Medical Research Council (FAMRI); Israel Cancer Research Fund (ICRF) professorship, BSF, Helen and Martin Kimmel Institute for Stem Cell Research, Helen and Martin Kimmel Award for Innovative Investigation; Israel Science Foundation (ISF), Minerva, the Sherman Institute for Medicinal Chemistry, Nella and Leon Benoziyo Center for Neurological Diseases, David and Fela Shapell Family Center for Genetic Disorders Research, Kekst Family Institute for Medical Genetics, Dr. Beth Rom-Rymer Stem Cell Research Fund, Edmond de Rothschild Foundations, Zantker Charitable Foundation, Estate of Zvia Zeroni.
Materials
| 0.22 µm pore size filter (250 mL) | JetBiofil | FCA-206-250 | |
| 0.22 µm pore size syringe PVDF filter | Millipore | SLGV033RS | |
| 8-well µ-plates glass bottom/ibiTreat | iBidi | 80827/80826 | |
| Bottle with adaptor cap for gas inlet | Arad Technologies | ||
| Bungs (Hole) | B.T.C. Engineering, Cullum Starr Precision Engineering | BTC 06 | Used to seal the bottles to the drum |
| Bungs (Solid) | B.T.C. Engineering, Cullum Starr Precision Engineering | BTC 07 | Used to seal the rotating drum |
| Culture bottles | B.T.C. Engineering, Cullum Starr Precision Engineering | BTC 03/BTC 04 | Either Glass Bottles (Small) BTC 03 or Glass Bottles (Large) BTC 04 |
| D(+)-glucose Monohydrate | J.T. Baker | ||
| Diamond knife | Fine Science Tools | 10100-30/45 | |
| Digital Pressure Gauge | Shanghai Benxu Electronics Technology co. Ltd | BX-DPG80 | |
| DMEM | GIBCO | 11880 | |
| Dulbecco's Phosphate Buffered Saline | Biological industries | 02-020-1A | |
| Fetal Bovine Serum | Biological industries | 04-013-1A | |
| Gas regulation module | Arad Technologies | HannaLab1 | |
| Glutamax | GIBCO | 35050061 | glutamine |
| Graefe forceps | Fine Science Tools | 11052-10 | |
| HEPES | GIBCO | 15630056 | |
| Microsurgical forceps (Dumont #5, #55) | Fine Science Tools | 11255-20 | |
| Pasteur pipettes (glass) | Hilgenberg | 3150102 | |
| Pasteur pipettes (plastic) | Alexred | SO P12201 | |
| Penicillin/Streptomycin | Biological industries | 03-031-1B | |
| Petri Dishes (60 mm and 100 mm) | Falcon | 351007/351029 | |
| Precision incubator system | B.T.C. Engineering, Cullum Starr Precision Engineering | BTC01 | BTC01 model with gas bubbler kit |
| Pro-coagulant sterile test tubes (5 mL) | Greiner Bio-One | #456005 | |
| Rat whole embryo culture serum | ENVIGO Bioproducts | B-4520 | |
| Stereoscopic microscope equipped with heating plate | Nikon | SMZ18 | |
| Sterile syringes (5, 10 ml) for sera filtration | Pic Solution | ||
| Surgical scissors | Fine Science Tools | 14094-11 |
References
- New, D. A. Whole-embryo culture and the study of mammalian embryos during organogenesis. Biological Reviews of the Cambridge Philosophical Society. 53 (1), 81-122 (1978).
- Tam, P. P., Behringer, R. R. Mouse gastrulation: the formation of a mammalian body plan. Mechanisms of Development. 68 (1-2), 3-25 (1997).
- Huang, Q., et al. Intravital imaging of mouse embryos. Science. 368 (6487), 181-186 (2020).
- White, M. D., et al. Long-lived binding of Sox2 to DNA predicts cell fate in the four-cell mouse embryo. Cell. 165 (1), 75-87 (2016).
- Tam, P. P. Postimplantation mouse development: whole embryo culture and micro-manipulation. International Journal of Developmental Biology. 42 (7), 895-902 (1998).
- Nicholas, J. S., Rudnick, D. The development of rat embryos in tissue culture. Proceedings of the National Academy of Sciences of the United States of America. 20 (12), 656-658 (1934).
- New, D. A., Stein, K. F. Cultivation of mouse embryos in vitro. Nature. 199, 297-299 (1963).
- Rivera-Pérez, J. A., Jones, V., Tam, P. P. L. Culture of whole mouse embryos at early postimplantation to organogenesis stages: developmental staging and methods. Methods in Enzymology. 476, 185-203 (2010).
- New, D. A. T., Coppola, P. T., Terry, S. Culture of explanted rat embryos in rotating tubes. Journal of Reproduction and Fertility. 35 (1), 135-138 (1973).
- Cockroft, D. L. A comparative and historical review of culture methods for vertebrates. International Journal of Developmental Biology. 41 (12), 127-137 (1997).
- Parameswaran, M., Tam, P. P. L. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Developmental Genetics. 17 (1), 16-28 (1995).
- Beddington, R. S. Induction of a second neural axis by the mouse node. Development. 120 (3), 613-620 (1994).
- Aguilera-Castrejon, A., et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature. 593 (7857), 119-124 (2021).
- Takahashi, M., Makino, S., Kikkawa, T., Osumi, N. Preparation of rat serum suitable for mammalian whole embryo culture. Journal of Visualized Experiments: JoVE. (90), e51969 (2014).
- Behringer, R., Gertsenstein, M., Nagy, K. V., Nagy, A. Isolation, culture and manipulation of postimplantation embryos. Manipulating the Mouse Embryo: a Laboratory Manual. , 149-193 (2014).
- Takahashi, M., Nomura, T., Osumi, N. Transferring genes into cultured mammalian embryos by electroporation. Development, Growth and Differentiation. 50 (6), 485-497 (2008).
- Mathieu, J., Ruohola-Baker, H. Metabolic remodeling during the loss and acquisition of pluripotency. Development. 144 (4), 541-551 (2017).
- Sturm, K., Tam, P. P. L. Isolation and culture of whole postimplantation embryos and germ layer derivatives. Methods in Enzymology. 225, 164-190 (1993).

