Myocardial Infarction by Percutaneous Embolization Coil Deployment in a Swine Model
Summary
Myocardial infarction (MI) animal models that emulate the natural process of the disease in humans are crucial to understanding pathophysiological mechanisms and testing the safety and efficacy of new emergent therapies. Here, we describe an MI swine model created by deploying a percutaneous embolization coil.
Abstract
Myocardial infarction (MI) is the leading cause of mortality worldwide. Despite the use of evidence-based treatments, including coronary revascularization and cardiovascular drugs, a significant proportion of patients develop pathological left-ventricular remodeling and progressive heart failure following MI. Therefore, new therapeutic options, such as cellular and gene therapies, among others, have been developed to repair and regenerate injured myocardium. In this context, animal models of MI are crucial in exploring the safety and efficacy of these experimental therapies before clinical translation. Large animal models such as swine are preferred over smaller ones due to the high similarity of swine and human hearts in terms of coronary artery anatomy, cardiac kinetics, and the post-MI healing process. Here, we aimed to describe an MI model in pig by permanent coil deployment. Briefly, it comprises a percutaneous selective coronary artery cannulation through retrograde femoral access. Following coronary angiography, the coil is deployed at the target branch under fluoroscopic guidance. Finally, complete occlusion is confirmed by repeated coronary angiography. This approach is feasible, highly reproducible, and emulates the pathogenesis of human non-revascularized MI, avoiding the traditional open-chest surgery and the subsequent postoperative inflammation. Depending on the time of follow-up, the technique is suitable for acute, sub-acute, or chronic MI models.
Introduction
Myocardial infarction (MI) is the most prevalent cause of mortality, morbidity, and disability worldwide1. Despite current therapeutic advances, a significant proportion of patients develop adverse ventricular remodeling and progressive heart failure following MI, resulting in poor prognosis due to ventricular dysfunction and sudden death2,3,4. New therapeutic options to repair and/or regenerate injured myocardium are thus under scrutiny, and translational MI animal models are crucial in testing their safety and efficacy. Although several models have been used for cardiovascular research, including rats5,6, mice7,8, dogs9, and sheep10, pigs are one of the best choices for modeling cardiac ischemia studies because of their high similarity to humans in terms of heart size, coronary artery anatomy, cardiac kinetics, physiology, metabolism, and the post-MI healing process11,12,13,14,15.
In this context, many different open-surgical and percutaneous approaches are available to develop MI swine models. The open-chest approach involves a left lateral thoracotomy procedure and is useful in performing surgical coronary artery ligation16,17, myocardial cryo-injury, cauterization12, and coronary artery placement of a hydraulic occlude18 or an ameroid constrictor19, among others. Surgical coronary occlusion has been extensively used to test new therapeutic options such as cardiac tissue engineering and cell therapy, as it allows wide access and visual assessment of the heart; however, in contrast to human MI, it can result in surgical adhesions, adjacent scarring, and postoperative inflammation17. Myocardial cryo-injury and cauterization are easily reproducible techniques but do not reproduce the pathophysiological MI progression observed in humans12. On the other hand, several percutaneous techniques have been developed to produce temporary or permanent coronary blocking. These comprise transcoronary or intracoronary ethanol ablation20,21, occlusion by balloon angioplasty22, or delivery of thrombogenic materials such as agarose gel beads23, fibrinogen mixtures9, or coil embolization17,24. While balloon angioplasty is better suited for ischemia/reperfusion studies, coronary coil deployment is one of the best choices for modeling non-revascularized MI. This percutaneous approach is feasible, consistently reproducible, and avoids open-chest surgery. It allows precise control of the infarct location and results in pathophysiology similar to that of a human non-reperfused MI. Moreover, coil embolization is suitable for modeling acute, sub-acute, or chronic MI; chronic congestive heart failure; or valvular disease17.
The present protocol aims to describe how to develop an MI swine model by permanent coil deployment. Briefly, it comprises a percutaneous selective coronary artery cannulation through retrograde femoral access. Following coronary angiography, a coil is deployed at the target branch artery under fluoroscopic guidance. Finally, complete occlusion is confirmed by repeated coronary angiography.
Protocol
This study was approved by the Animal Experimentation Unit Ethical Committee of the Germans Trias i Pujol Health Research Institute (IGTP) and Government Authorities (Generalitat de Catalunya; Code: 10558 and 11208), and complies with all guidelines concerning the use of animals in research and teaching as defined by the Guide for the Care and Use of Laboratory Animals25.
1. Preprocedural preparation of animals
- Use crossbred Landrace X Large White pigs (30-35 kg) of either sex.
- Keep the animals in a fasting state for 12 h prior to the procedure.
2. Sedation, anesthesia, and analgesia
- Sedate the animal with an intramuscular (IM) injection of ketamine (3 mg/kg), midazolam (0.3 mg/kg), and dexmedetomidine (0.03 mg/kg). Wait for approximately 10-15 min.
- Once the pig is sedated, ventilate it with an oxygen (90-100%)-isoflurane (1-2%) mixture and a face mask to ensure optimal sedation.
- Place vet ointment on the pig's eyes to prevent dryness.
NOTE: Repeat every 20 min. - Place intravenously (IV) a 20 G catheter in a lateral ear vein. Administer propofol (1-2 mg/kg) to induce anesthesia.
- Once the pig has no swallowing reflex, intubate the animal using an endotracheal tube (size 6.5-7.0 for 30-35 kg).
NOTE: Adjust the size of the endotracheal tube according to the size of the pig. Intubation should be carried out rapidly to prevent a deeper anesthetic plane and prolonged apnea. - Administer IV buprenorphine (0.01 mg/kg) for intra-surgical analgesia. Use a fentanyl transdermal patch (100 µg/h) for post-operative analgesia.
NOTE: The fentanyl patch is applied to the inguinal skin, and it is active for 72 h to limit postoperative pain. Its pharmacological effect does not start immediately after delivery, thus apply it before starting the procedure. - Perform airway mask bag unit-ventilation (20 inflations/min) during the transport of the pig to the vascular interventional radiology (VIR) room.
- Connect the endotracheal tube to the anesthesia machine equipped with an airway sensor and capnography recording.
- Start mechanical positive pressure ventilation with FiO2 0.50, using a tidal volume of 10 mL/kg and a frequency of 16-20 breaths/min. Maintain the anesthesia with isoflurane (1-3%).
NOTE: To confirm the correct surgical anesthetic plane, the animal should not be respiring spontaneously nor have corneal or pupillary light reflexes.
3. Hemodynamic monitoring and preparation of the surgical area
- Place the animal on the operating table in the supine position and fix the limbs to the table with tape or bandage.
- Place electrocardiogram (ECG) probes subcutaneously in the animal's extremities for recording changes in ST-segment, T-waves, and heart rate during the experimental procedure.
- Place a pulse oximeter on the tongue or a corner of the lip of the animal and the non-invasive pressure cuff on the forelimb.
- Measure the rectal/esophageal temperature with a probe.
- Clean the right femoral area with surgical soap followed by alternating antiseptic povidone-iodine solution and alcohol 3 times under sterile conditions.
- Ensure that the surgeon performs surgical handwashing and wears a sterile gown and sterile gloves.
- Cover the animal with a sterile surgical drape.
- Prepare and flush with heparinized saline solution the needle, a 6F vascular sheath, a 0.035-inch J-tipped wire, a 6F JR4 90-cm guiding catheter, a 0.014-inch 200-cm guidewire, a 150-cm length/0.017-inch inner diameter microcatheter, and the contrast medium injection manifold kit.
4. Vascular access
- Puncture the right femoral artery via a percutaneous approach with ultrasound-guided puncture. Locate the bifurcation between the superficial femoral artery and the deep femoral artery.
- Position the transducer 2-3 cm proximal to the bifurcation, in the common femoral artery, and align the center of the transducer with the common femoral artery.
- Position the needle in the center of the transducer and puncture the artery at an angulation of approximately 45°. Subsequently, insert a 6F vascular sheath using the modified Seldinger technique26.
NOTE: In case of significant spasm or hematoma, crossover to the contralateral femoral artery. - Flush the catheters with heparinized saline solution. (5000 IU unfractionated heparin/1000 mL of 0.9% NaCL).
- Administer heparin through the sheath (300 IU/kg).
5. Coronary angiography
- Insert the J-tip wire into the JR4 guide catheter and advance the wire through the sheath into the ascending aorta, and then place the catheter up over the valvular surface.
- Remove the wire and connect the catheter to the injection manifold system. Purge the entire system.
- Under fluoroscopy, engage the catheter into the left main coronary artery and inject 10 mL of iodinated contrast medium to visualize the left coronary system (Figure 1A, C).
NOTE: It is important to ensure that the arterial pressure waveform is not damped before injecting to avoid the risk of coronary dissection. - Perform angiograms in two orthogonal views: left anterior oblique 40° and right anterior oblique 30° projections.
- Advance a 0.014-inch guidewire pre-assembled on the microcatheter to the middle left anterior descending (LAD) or distal left circumflex (LCX) coronary artery under fluoroscopic guidance.
6. Coil implantation
- Under fluoroscopic guidance, advance the microcatheter through the wire to the desired location where the coil implant should be deployed. In the case of LAD occlusion, place the coil distal to the first diagonal branch, and for LCX, place the coil distal to the first marginal branch.
NOTE: Proximal approaches (before the first diagonal or first marginal branches) have very low survival rates. - Remove the wire and select the coil.
NOTE: It is important to select the optimal coil size and length. A small or short coil may not position well in the vessel lumen and has a very high risk of distal migration due to contrast injections or spontaneous, resulting in smaller infarct size. A large or long coil may prolapse proximal to the vessel and produce a larger infarct than desired. The choice of the correct coil is especially important if non-detectable coils are used, as they cannot be removed. The optimal size is 1-2 mm larger than the lumen of the vessel to be embolized, and the length between 20-60 mm is usually adequate for 30-40-kg pigs. - Deliver the coil via microcatheter and slowly inject 5 mL of iodinated contrast medium under fluoroscopy to visualize the correct position of the coil.
- Remove the microcatheter inside the guide catheter and place the guide in a side branch to perform control injections and to ensure access to the artery in case a second coil needs to be implanted.
- Wait for the coil to thrombose and occlude the artery.
NOTE: When the artery is occluded, changes in the electrocardiogram can be observed. Another way to check complete arterial occlusion is to perform slow injections of iodinated contrast every 10 min (Figure 1B, D). If the artery does not occlude within 20-30 min, another coil implant may be required.
7. End of procedure
- Once the artery is occluded, administer a continuous IV infusion of lidocaine (50-100 µg/kg/min) for at least 1 h to prevent arrhythmic episodes.
- Perform an angiogram to ensure that there is no flow distal to the occlusion.
- Remove the wire, microcatheter, and guiding catheter.
- Remove the sheath and perform manual compression for 20 min.
8. Postoperative procedure and animal recovery
- Monitor the animal until it is fully recovered, using ECG, rectal temperature, pulse oximetry, and capnography.
NOTE: In case of ventricular arrhythmias, administer a bolus of lidocaine (1.5-3.5 mg/kg). - Administer an IM injection of tulathromycin (2.5 mg/kg) as prophylactic postoperative antibiotic therapy. For post-surgical analgesia, a transdermal fentanyl patch is administered before the surgical procedure (step 2.6).
- Turn off the isoflurane and maintain mechanical ventilation until the animal begins to breathe spontaneously.
- When the pig recovers the swallowing reflex, remove the endotracheal tube. NOTE: Check if the animal has a good SpO2 (more than 95%) before and after extubation.
- Transport the animal to an individual cage. Position the animal over a hot water blanket and cover it with a thermal drape to avoid post-surgical hypothermia.
NOTE: Do not return the pig to the company of other animals until it has fully recovered. - Monitor the animal until it has regained sufficient consciousness to maintain sternal recumbency.
9. Postoperative pain assessment and monitoring
- During the post-surgical follow-up, monitor the general condition of the animals, including the respiratory rate, food and water intake, activity and interaction with the other individuals, appearance and coloration of the skin, and the evolution of the surgical wound.
- Apply a daily supervision protocol according to the following scoring criteria: – Weight:
0: Normal
1: <10% weight loss
2: 10-20% weight loss
3:> 20% weight loss
– Body condition:
0: Good: non-prominent vertebrae, pelvic or spinal bones
2: Regular: evidence of spinal segmentation, palpable pelvic bones
3: Emaciation: extremely marked skeleton, little or no meat to cover
– Behavior:
0: Normal: Active and interactive in your environment
1: Slight decline in activity and less interactive
2: Abnormal: pronounced decline in activity, isolated
3: Abnormal: Immobile or hyperactivity, possible self-harm
– Physical appearance:
0: Normal: skin/hair shiny and eyes bright
1: Disappears embalming, skin/hair without shine
2: Poor skin/nasal secretions
3: Poor skin, abnormal or hunched posture
– Behavioral disorders:
0: None
1: Inability to move normally
2: Unable to reach food/drink, isolated from other animals
3: Intention to hide/corner, does not respond to stimuli (dying)
– Clinical signs:
0: None
1: Hypothermia, fever, mild respiratory failure
2: Infection of the surgical wound, moderate respiratory failure with muco-bloody secretions
3: Heart failure, severe respiratory failure (cyanosis, open mouth)
Score:
– 1-5: Supervise the animals once a day.
– 6-12: Provide supportive therapy if necessary.
– Any animal with a score of 3 in any of the above parameters or with a total score >12 will be euthanized.
NOTE: The animals should be monitored daily by the animal care staff and twice a week by the research and veterinary team. - Although no pain and distress are expected from the procedure, if any animal shows signs of pain, give analgesic therapy (tramadol, oral, 2-4 mg/kg, daily). If any animal does not respond to analgesic medication and shows signs of chronic pain (very low probability), euthanize the animal with an anesthetic overdose (sodium thiopental, IV, 200 mg/kg).
- If the surgical wound shows signs of infection (low probability) despite the antibiotic therapy administered, treat the wound daily and initiate a new antibiotic regimen (cefquinome sulphate, IM, 2 mg/kg, daily).
10. Euthanasia method
- Under previous sedation and anesthesia, as previously described, administer an IV sodium thiopental overdose (200 mg/kg).
- Confirm cardiorespiratory arrest and death by monitoring vital signs (electrocardiogram, blood pressure, capnography).
Representative Results
MI survival rates and location
Fifty-seven pigs underwent coronary coil implantation in the LCX marginal branch (n = 25; 12 females and 13 males) or in the LAD between the first and the second diagonal branches (n = 32; 16 females and 16 males) of the coronary artery and were followed up for 30 days. The survival rate of animals submitted to an MI at the LCX marginal branch was 80% (n = 20). Three pigs died as a result of fatal complications related to atrioventricular (AV) block and asystole before coil deployment, and 2 pigs died after ventricular fibrillation (VF) related to transmural MI after coil placement. The survival rate of animals submitted to MI at LAD was 72% (n = 23): 1 pig died due to an AV block and asystole after coil deployment and 8 animals after VF (5 after coil deployment, 2 at 12-48 h post-MI, and one 26 days post-MI). The survival rates differed between the LCX marginal branch (2-2.5 mm in diameter) and middle LAD (2.5-3 mm in diameter) MI, probably due to the larger infarct extension in the LAD model.
Magnetic resonance imaging (MRI) analysis was performed in all animals 30 days post-MI. Figure 2 illustrates late gadolinium-enhanced MRI images of the LCX marginal branch (Figure 2A,C) and distal LAD (Figure 2B,D) infarct models. As depicted, coil deployment in the LCX marginal coronary artery affects the LV lateral wall, while the interventricular septum is the most affected area in distal LAD placement. These results were also confirmed after heart sectioning (Figure 2E,F).
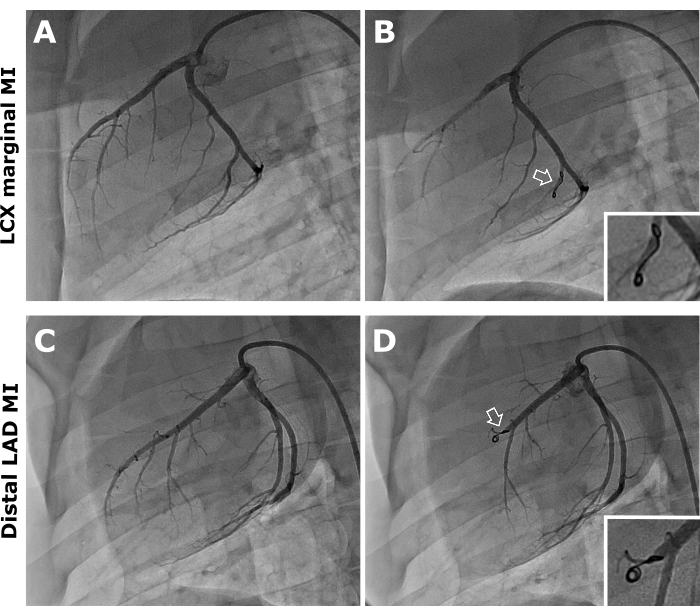
Figure 1: Coronary angiography, anteroposterior projection. Representative images of pre- (A,B) and post-coil (white arrows) deployment (C,D) in the LCX marginal branch and distal LAD coronary artery. Please click here to view a larger version of this figure.
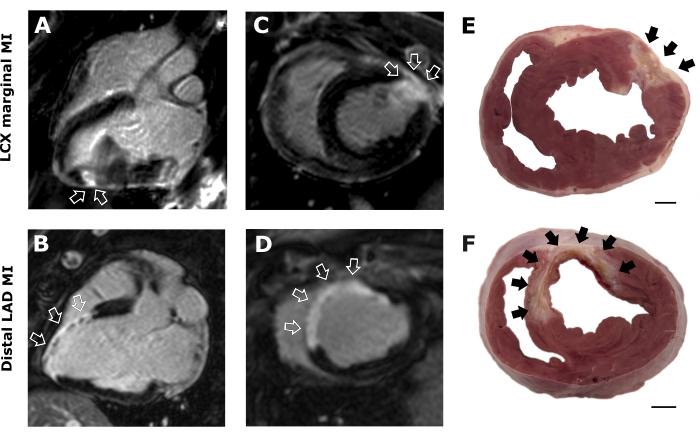
Figure 2: Magnetic resonance imaging and cardiac tissue sections. Representative T1 3-chamber (A,B) and short-axis (C,D) delayed enhancement images for LCX marginal and distal LAD infarction. Images reveal healthy (black) and infarcted (white) myocardium. Photographs of heart sections after LCX marginal (E) and distal LAD MI (F). Arrows indicate the location and extension of the infarcted area. Scale bar = 1 cm Please click here to view a larger version of this figure.
Discussion
A coil deployed in a coronary artery provides a reproducible and consistent pre-clinical non-reperfused MI model in swine that can be used to develop and test new cardiovascular therapeutic strategies.
In our hands, mortality at follow-up was 19% related to complications of MI, mostly within the first 24 h of the procedure. All these deaths are related to the natural history of the non-reperfused MI and were the primary outcomes of the study. One of the most critical steps in this protocol relies on the entry of the microcatheter into the coronary arteries. In some cases, microcatheter advancement caused a vagal reaction leading to severe hypotension, AV block, and finally asystole. Nevertheless, this can be avoided by administering an IV bolus of adrenaline (0.001 mg/kg) before advancing the microcatheter. Another complication is the occurrence of malignant arrhythmias that can lead to VF. These episodes usually occur 30 min after MI instauration. We recommend using a lidocaine continuous infusion rate (50-100 µg/kg/min) for at least 1h to reduce the risk of ventricular arrhythmias. As an alternative, a continuous infusion of amiodarone (50-80 µg/kg/min) can be administered. However, if ventricular arrhythmic events occur, we recommend delivering a bolus of lidocaine (1.5–3.5 mg/kg). In case of severe bradycardia, we recommend the administration of atropine bolus (0.01 mg/kg), noradrenaline perfusion (0.05-3 µg/kg/min) for mild or moderate hypotension, and adrenaline (0.03 mg/kg) for severe hypotension, electromechanical dissociation, AV block, or asystole. However, when a VF occurs, a 320J ventricular defibrillation has to be applied with a monophasic cardiac defibrillator and repeated until the animal recovers its cardiac rhythm. When several ventricular defibrillations are needed or asystole occurs, perform manual chest compressions (80-90 compressions/min), depressing the ribcage 4 inches, and connect the animal to the mechanical ventilator under 100% O2.
If the interventional procedure is extended for more than one hour, it is useful to monitor the anticoagulation level with the activated clotting time test to ensure that it is greater than 300 seconds. If it is shorter, an extra dose of heparin should be administered.
In case an occlusive thrombus does not form after coronary artery coil deployment, we recommend the placement of another coil. Another option could be to administrate protamine (1mg/100IU of UFH) to facilitate clot formation, although there is a risk of thrombus formation in the guiding catheter and subsequent embolization during control injection.
Many other occlusion models have been described to simulate MI based on cessation of coronary flow by arterial ligation, an ameroid constrictor, or balloon inflation. However, a deployed coil sets off the coagulation cascade with thrombus formation that occludes the coronary artery. This mechanism simulates as closely as possible the pathophysiology of human MI, compared with other non-invasive techniques like balloon occlusion. Despite the fact that non-reperfused MI results in more extensive scarring, less viable myocardium, and a greater reduction in terms of cardiac function than ischemia-reperfusion models27, it is more suitable for screening anti-inflammatory therapies, reverse cardiac remodeling, and gene or stem cell therapy for the treatment of cardiovascular disease28.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We express our gratitude to the Center of Comparative Medicine and Bioimaging of Catalonia (CMCiB) and staff for their contribution to the animal model execution. This work was supported by the Instituto de Salud Carlos III (PI18/01227, PI18/00256, INT20/00052), the Sociedad Española de Cardiología, and the Generalitat de Catalunya [2017-SGR-483]. This work was also funded by the Red de Terapia Celular – TerCel [RD16/0011/0006] and CIBER Cardiovascular [CB16/11/00403] projects, as part of the Plan Nacional de I+D+I, and cofunded by the ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER). Dr. Fadeuilhe was supported by a grant from the Spanish Society of Cardiology (Madrid, Spain).
Materials
| 6-F JR4 0-71"guiding catheter | Medtronic | LA6JR40 | 6F JR4 90 cm Guiding catheter |
| Adrenaline 1 mg/mL | B.Braun | National Code (NC). 602486 | Adrenaline |
| Atropine 1 mg/mL | B.Braun | NC. 635649 | Atropine |
| Betadine | Mylan | NC. 694109-1 | Povidone iodine solution |
| Bupaq 0.3 mg/mL | Richter Pharma AG | NC. 578816.6 | Buprenorphine |
| Dexdomitor 0.5 mg/mL | Orion Pharma | NC. 576303.3 | Dexmedetomidine |
| Draxxin | Zoetis | NC. 576313.2 | Tulathromycin |
| EMERALD Guidewire | Cordis | 502-585 | 0.035-inch J-tipped wire |
| External defibrillator | DigiCare | CS81XVET | Manual external defibrillator |
| Fendivia 100 µg/h | Takeda | NC. 658524.5 | Fentanyl transdermal patch |
| Guidewire Introducer Needle 18 G x 7 cm | Argon | GWI1802 | Introducer needle |
| Heparine 1% | ROVI | NC. 641647.1 | Heparin |
| Hi-Torque VersaTurn F | Abbott | 1013317J | 0.014-inch 200 cm Guidewire |
| IsoFlo | Zoetis | 50019100 | Isoflurane |
| Ketamidor | Richter Pharma AG, | NC. 580393.7 | Ketamine |
| Lidocaine 50 mg/mL | B.Braun | NC. 645572.2 | Lidocaine |
| MD8000vet | Meditech Equipment | MD8000vet | Multi-parameter monitor |
| Midazolam | Laboratorios Normon | NC. 624437.1 | Midazolam |
| Prelude.6F.11 cm (4.3").0.035" (0.89 mm).50 cm (19.7").Double Ended.Stainless Steel.6F.16 | Merit | PSI-6F-11-035 | 6F Vascular sheath |
| Propovet Multidosis 10 mg/mL | Zoetis | NC. 579742.7 | Propofol |
| RENEGADE STC-18 150/20/STRAIGHT/1RO | Boston Scientific | M001181370 | 150 cm length with 0.017-inch inner diameter Microcatheter |
| Ruschelit | Teleflex | 112482 | Endotracheal tube with balloon (#6.5) |
| SPUR II | Ambu | 325 012 000 | Airway mask bag unit-ventilation (AMBU) |
| Vasofix 20 G | B.Braun | 4269098 | 20 G Cannula |
| Visipaque 320 mg/mL USB 10 x 200 mL | General Electrics | 1177612 | Iodinated contrast medium |
| VortX-18 Diamond 3 mm/3.3 mm | Boston Scientific | M0013822030 | Coil |
| WATO EX-35 | Mindray | WATO EX-35Vet | Anesthesia machine |
References
- Khan, M., et al. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus. 12 (7), 9349 (2020).
- Bhatt, A. S., Ambrosy, A. P., Velazquez, E. J. Adverse remodeling and reverse remodeling after myocardial infarction. Current Cardiology Reports. 19 (8), 71 (2017).
- Verma, A., et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: The VALIANT (VALsartan In Acute myocardial iNfarcTion) echocardiographic study. JACC: Cardiovascular Imaging. 1 (5), 582-591 (2008).
- Konstam, M., Kramer, D., Patel, A., Maron, M., Udelson, J. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC. Cardiovascular Imaging. 4 (1), 98-108 (2011).
- Srikanth, G., Prakash, P., Tripathy, N., Dikshit, M., Nityanand, S. Establishment of a rat model of myocardial infarction with a high survival rate: A suitable model for evaluation of efficacy of stem cell therapy. Journal of Stem Cells and Regenerative Medicine. 5 (1), 30 (2009).
- Wu, Y., Yin, X., Wijaya, C., Huang, M. -. H., McConnell, B. K. Acute myocardial infarction in rats. Journal of Visualized Experiments: JoVE. (48), e2464 (2011).
- Takagawa, J., et al. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. Journal of Applied Physiology. 102 (6), 2104-2111 (2007).
- Yang, F., et al. Myocardial infarction and cardiac remodelling in mice. Experimental Physiology. 87 (5), 547-555 (2002).
- Suzuki, M., Asano, H., Tanaka, H., Usuda, S. Development and evaluation of a new canine myocardial infarction model using a closed-chest injection of thrombogenic material. Japanese Circulation Journal. 63 (11), 900-905 (1999).
- Rienzo, M., et al. A total closed chest sheep model of cardiogenic shock by percutaneous intracoronary ethanol injection. Scientific Reports. 10 (1), 12417 (2020).
- Spannbauer, A., et al. Large animal models of heart failure with reduced ejection fraction (HFrEF). Frontiers in Cardiovascular Medicine. 6, 117 (2019).
- Liu, J., Li, X. Novel porcine models of myocardial ischemia/infarction – Technical progress, modified electrocardiograms validating, and future application. Advances in Electrocardiograms – Clinical Applications. , 175-190 (2012).
- Hughes, G. C., Post, M., Simons, M., Annex, B. Translational physiology: porcine models of human coronary artery disease: implications for pre-clinical trials of therapeutic angiogenesis. Journal of Applied Physiology. 94 (5), 1689-1701 (2003).
- Tsang, H. G., et al. Large animal models of cardiovascular disease. Cell Biochemistry and Function. 34 (3), 113 (2016).
- Dixon, J. A., Spinale, F. G. Large animal models of heart failure. Circulation: Heart Failure. 2 (3), 262-271 (2009).
- Gálvez-Montón, C., et al. Transposition of a pericardial-derived vascular adipose flap for myocardial salvage after infarct. Cardiovascular Research. 91 (4), 659-667 (2011).
- Gálvez-Montón, C., et al. Comparison of two pre-clinical myocardial infarct models: coronary coil deployment versus surgical ligation. Journal of Translational Medicine. 12, 137 (2014).
- Domkowski, P. W., Hughes, G. C., Lowe, J. E. Ameroid constrictor versus hydraulic occluder: creation of hibernating myocardium. The Annals of Thoracic Surgery. 69 (6), 1984 (2000).
- Tuzun, E., et al. Correlation of ischemic area and coronary flow with ameroid size in a porcine model. Journal of Surgical Research. 164 (1), 38-42 (2010).
- Weismüller, P., Mayer, U., Richter, P., Heieck, F., Kochs, M., Hombach, V. Chemical ablation by subendocardial injection of ethanol via catheter – preliminary results in the pig heart. European Heart Journal. 12 (11), 1234-1239 (1991).
- Haines, D., Verow, A., Sinusas, A., Whayne, J., DiMarco, J. Intracoronary ethanol ablation in swine: characterization of myocardial injury in target and remote vascular beds. Journal of Cardiovascular Electrophysiology. 5 (1), 41-49 (1994).
- Li, K., Wagner, L., Moctezuma-Ramirez, A., Vela, D., Perin, E. A robust percutaneous myocardial infarction model in pigs and its effect on left ventricular function. Journal of Cardiovascular Translational Research. , (2021).
- Eldar, M., Ohad, D., Bor, A., Varda-Bloom, N., Swanson, D., Battler, A. A closed-chest pig model of sustained ventricular tachycardia. Pacing and Clinical Electrophysiology : PACE. 17 (10), 1603-1609 (1994).
- Dib, N., Diethrich, E. B., Campbell, A., Gahremanpour, A., McGarry, M., Opie, S. R. A percutaneous swine model of myocardial infarction. Journal of Pharmacological and Toxicological Methods. 53 (3), 256-263 (2006).
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. . Guide for the Care and Use of Laboratory Animals. 8th ed. , (2011).
- Carter, C., Girod, D., Hurwitz, R. Percutaneous cardiac catheterization of the neonate. Pediatrics. 55 (5), 662-665 (1975).
- Koudstaal, S., et al. Myocardial infarction and functional outcome assessment in pigs. Journal of Visualized Experiments JoVE. (86), e51269 (2014).
- Kumar, M., et al. Animal models of myocardial infarction: Mainstay in clinical translation. Regulatory Toxicology And Pharmacology RTP. 76, 221-230 (2016).

