Pneumatically Driven Microfluidic Platform for Micro-Particle Concentration
Summary
The present protocol describes a pneumatic microfluidic platform that can be used for efficient microparticle concentration.
Abstract
The present article introduces a method for fabricating and operating a pneumatic valve to control particle concentration using a microfluidic platform. This platform has a three-dimensional (3D) network with curved fluid channels and three pneumatic valves, which create networks, channels, and spaces through duplex replication with polydimethylsiloxane (PDMS). The device operates based on the transient response of a fluid flow rate controlled by a pneumatic valve in the following order: (1) sample loading, (2) sample blocking, (3) sample concentration, and (4) sample release. The particles are blocked by thin diaphragm layer deformation of the sieve valve (Vs) plate and accumulate in the curved microfluidic channel. The working fluid is discharged by the actuation of two on/off valves. As a result of the operation, all particles of various magnifications were successfully intercepted and disengaged. When this technology is applied, the operating pressure, the time required for concentration, and the concentration rate may vary depending on the device dimensions and particle size magnification.
Introduction
Due to the importance of biological analysis, microfluidic and biomedical microelectromechanical systems (BioMEMS) technologies1,2 are used to develop and study devices for the purification and collection of micromaterials2,3,4. Particle capture is categorized as active or passive. Active traps have been used for external dielectric5, magnetophoretic6, auditory7, visual8, or thermal9 forces acting on independent particles, enabling precise control of their movements. However, an interaction between the particle and external force is required; thus, the throughput is low. In microfluidic systems, controlling the flow rate is very important because the external forces are transmitted to the target particles.
In general, passive microfluidic devices have micropillars in microchannels10,11. Particles are filtered through interaction with a flowing fluid, and these devices are easy to design and inexpensive to manufacture. However, they cause particle clogging in micro-pillars, so more complex devices have been developed to prevent particle clogging12. Microfluidic devices with complex structures are generally suitable for managing a limited number of particles13,14,15,16,17,18.
This article describes a method to fabricate and operate a pneumatically driven microfluidic platform for large particle concentrations that overcomes the shortcomings18 as mentioned above. This platform can block and concentrate particles by deformation and actuation of the thin diaphragm layer of the sieve valve (Vs) plate that accumulates in curved microfluidic channels. Particles accumulate in curved microfluidic channels, and the concentrated particles can separate by discharging the working fluid via the actuation of two PDMS seals on/off valves18. This method makes it possible to process a limited number of particles or concentrate a large number of small particles. Operating conditions such as the magnitude of flow rate and compressed air pressure can prevent unwanted cell damage and increase cell trapping efficiency.
Protocol
1. Designing the microfluidic platform for particle concentration
- Design the pneumatic microfluidic platform consisting of one pneumatic valve for fluid flow in the 3D flow network and three pneumatic valves for sieve (Vs), fluid (Vf), and particle (Vp) valve operation (Figure 1).
NOTE: Vs blocks concentrate particles from the liquid, and Vf and Vp allow fluid and particle release after concentration. Three pneumatic ports provide compressed air from the fluid/pneumatic supply layer (normally open) and the pneumatic valve light outlet to actuate the valve. The microfluidic channel network is designed with a CAD program18,19. - Design the channel to be a pneumatic supply layer and a 3D channel network layer (Figure 2).
NOTE: The fluid network is interconnected with the curved channels in the anterior part and the rectangular chamber in the posterior region. Vs block the inlet, and particles accumulate in the collection area of the curved fluid channel. Particle-free fluids (particle-free liquids) are exited through the Qf outlet and the concentrated particles through the Qp outlet (Figure 3). - According to the above conditions, prepare four types of SU-8 molds.
NOTE: The four molds include a mold that allows the valve to be controlled via pneumatics, two molds that create fluid channels, and a clean mold without shape (Figure 4 and Table 1). The four types of molds mentioned are fabricated using standard photolithography processes. This mold making consists of a SU-8 mold on a silicon wafer as per previously published reports18,19. Figure 5 depicts the device chip.
2. Fabrication of the microfluidic platform for particle concentration
NOTE: Figure 6 illustrates the fabrication of a microfluidic platform that concentrates particles.
- Replicate the PDMS layer using a prepared pneumatic valve channel SU-8 mold (step 1.3) for pneumatically controlling the valve.
- Pour 10 mL of liquid PDMS and 1 mL of curing agent (see Table of Materials) into a prepared pneumatic valve channel mold (step 1.3) and heat-activate at 90 °C for 30 min.
- After the PDMS structures are cured, separate the SU-8 mold of step 2.1.1.
- Punch three 1.5 mm pneumatic ports (Vs, Vf, and Vp) into the pneumatic valve channel manufactured according to step 2.1.2 using a 1.5 mm puncture (see Table of Materials).
- Pour 10 mL of liquid PDMS and 1 mL of curing agent into a prepared clean SU-8 mold prepared in step 1.3 and spin-coat at 1,500 rpm for 15 s using a spin coater (see Table of Materials). Then heat-activate at 90 °C for 30 min.
- After the PDMS structures are cured, separate the SU-8 mold of step 2.1.4.
NOTE: The valve diaphragm layer controls fluid flow according to the pneumatic pressure. - Treat atmospheric plasma (see Table of Materials) to the PDMS structures prepared in steps 2.1.3 and 2.1.5 for 20 s.
- Align directly plasma-treated PDMS structures from step 2.1.6 according to the channel structure by checking with a microscope.
- Bond the aligned PDMS structures prepared in step 2.1.7 by heating at 90 °C for 30 min.
- Punch a 1.5 mm diameter hole in the fluid channel inlet (Qfp) and fluid channel outlets (Qf and Qp) within the pneumatic channel part to which the thin diaphragm layer is bonded, using a 1.5 mm puncture.
- Replicate both sides of the PDMS layer using two SU-8 molds to make a microfluidic channel. Use a curved and rectangular microfluidic channel mold on the front and a microfluidic interconnection channel mold on the rear.
- Pour 10 mL of liquid PDMS and 1 mL of curing agent into the curved and rectangular microfluidic channel mold and spin-coat at 1,200 rpm for 15 s. Then create molds for the curved fluid chamber and fluid channels by thermal activation at 90 °C for 30 min (Figure 6A).
- Separate the PDMS layer on which the microfluidic channel is formed, then make a heat-activated mold covering the sealed vent wall by bonding to the glass wafer by treating atmospheric plasma for 20 s (Figure 6B).
- Pour 3 mL of liquid PDMS into the interconnection channel of the SU-8 mold (Figure 6C).
- Arrange the structure fabricated in step 2.2.2 with the interconnection channel mold in liquid PDMS on the microfluidic interconnect channel mold, and dry the superimposed structure at 130 °C for 30 min (Figure 6D).
NOTE: While curing the rear structure, the PDMS mold fabricated in step 2.2.2 is inflated by the thermal pressure of the air layer, and the deformed PDMS layer is thermally activated (Figure 6E)16. - After curing, remove the front SU-8 mold from the microfluidic channel network layer and carefully peel off the rear PDMS mold (Figure 6F).
NOTE: The 3D fluidic network layer allows the creation of an anterior curved fluid chamber and microfluidic channels. - Pour 10 mL of liquid PDMS and 1 mL of curing agent into a clean SU-8 mold. Then heat-activate at 90 °C for 30 min.
- After the PDMS structures are cured, separate the SU-8 mold.
NOTE: This step creates the additional sealing layer. - Treat the atmospheric plasma to PDMS structures prepared in steps 2.2.3 and 2.2.7 for 20 s.
- Align directly plasma-treated PDMS structures according to the channel structure by checking with a microscope.
- Bond the aligned PDMS structures by heating at 90 °C for 30 min.
- Align the PDMS structures prepared in steps 2.1 and 2.2 according to the channel structure and bond them by treating atmospheric plasma for 20 s.
3. Setting up the device
NOTE: Figure 7 shows fabricating a microfluidic platform that concentrates particles.
- Manually fill the microfluidic channel with bubble-free demineralized water using a 10 mL syringe.
- To control the P_Qfp and the three pneumatic valves (P_Vs, P_Vf, and P_Vp) that control the microbead flow, insert a precision pressure controller with four or more output channels (see Table of Materials) for the working fluid (Qfp) into the microfluidic platform.
NOTE: A precision pressure controller with four output channels can be replaced with multiple precision pressure controllers. In this experiment, the operating pressure of P_Qfp was 10 kPa, P_Vs was 15 kPa, and P_Vf and P_Vp were both 18 kPa (Figure 8 and Table 2). Figure 8 shows the working fluid flow rate over time as particles are concentrated by the microfluidic platform with P_Vs of 15 kPa, and Table 2 shows the actuation results according to the pneumatic valves. - Prepare carboxyl polystyrene test particles of various sizes in distilled water (see Table of Materials).
NOTE: The particle sizes used in this experiment were 24.9, 8.49, and 4.16 µm; particles of various sizes can be used depending on the pressure of P_Vs. - To control the flow rate of the working fluid, fill a glass bottle half full with water (working fluid) and connect the glass bottle cap to the controller output channel and microvalve.
NOTE: Connect one tube to the microvalve to receive compressed air from the controller and the other tube to inject water. - Observe platform operation through an inverted microscope for all platform operations and measure the operating flow rate over time at the outlet by a liquid flow meter (see Table of Materials).
4. Operation of the device
- Inject the particle/fluid mixture under pressure at the inlet (Qfp) with Vp (Figure 9A).
NOTE: The flow of particles and clean fluid from the outlet through the interconnected channels are controlled via Vp and Vf, respectively (Table 2). - Apply pressure to Vs at 15 kPa and Vp at 18 kPa to actuate the valve.
NOTE: At this time, the diaphragm is deformed, the particles of the fluid Qfp are blocked in the contact space between the curved fluid channel and the curved fluid cantilever, and the unwanted Qfp fluid is released through the open Qf (Figure 9B,C). - When the particles are concentrated, apply pressure only to Vf.
NOTE: At this time, when pressure is applied only to Vf, the clogged particles are released through Qp (Figure 9D).
Representative Results
Figure 8 shows the flow rate of the fluid rates for a four-stage platform operation, as mentioned in Table 2. The first stage is the loading state (a state). The platform was supplied with fluid with all valves open, and the working fluid (Qf) and particles (Qp) are almost identical as the microfluidic channel network exhibits structural symmetry. In the second stage (b state), compressed air was transported to Vs to block the particles, and as the Vs diaphragm deformed, the flow path narrowed, and the flow rate measured at the outlet port was reduced by hydraulic resistance. The flow rates of Qf and Qp were almost similar, and the difference was less than 2.67%. In the third stage (c state), compressed air was delivered to Vs and Vp for particle concentration, with Vs and Vp closed and Vf open. The measured Qp was close to zero, and the Qf was about 1.42 times that of the b state. In most cases, the flow rate doubles when both dissipation channels are in operation, but the platform has different types of hydraulic resistance in the main fluid channels and Vs, so the total flow of the working fluid is reduced. Finally (d state), compressed air was delivered only to Vf to collect the concentrated particles, and the flow rates of Qf and Qp were reversed. The flow was zero because Vf blocked Qf, and Qp was about 1.42 times the b state. The concentration ratio of the particles (Qp/(Qf+Qp) × 100) was 3.96-4.53. This shows that the sequential actuation programmed with the pneumatic valve works well due to flow changes.
Figure 9 shows the screen capturing concentrated particles. Figure 9A shows the flow state of the fluid with the three pneumatic valves not actuated, Figure 9B shows the method used to trap the particles, Figure 9C shows the sieve method, and Figure 9D shows the ejection of the concentrated beads. Particles were concentrated and accumulated in the collection area when Vs and Vp were closed, and all collected concentrated particles were released within 4 s when only Vf was closed. Therefore, the device successfully collects many particles suitable for particle collection and concentration.
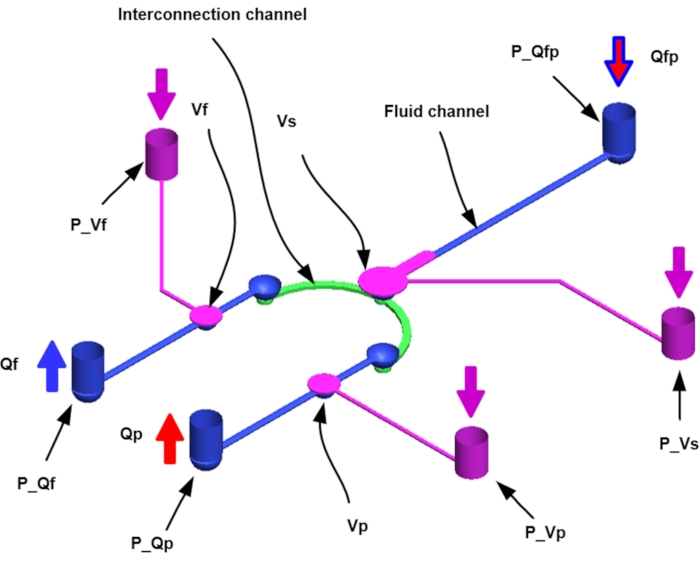
Figure 1: Schematic diagram of a pneumatic microfluidic platform for microparticle concentration (P, port; Q, flowrate; f, fluid; p, particle; V, valve; s, sieve). Please click here to view a larger version of this figure.
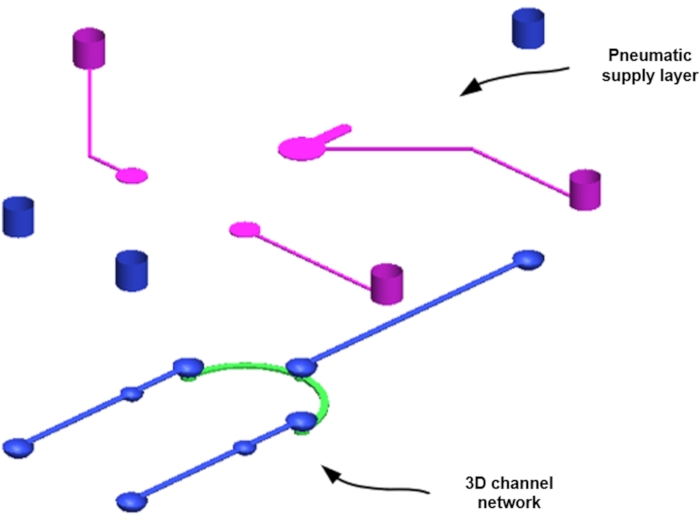
Figure 2: Assembly of the pneumatic microfluidic platform for microparticle concentration. Please click here to view a larger version of this figure.
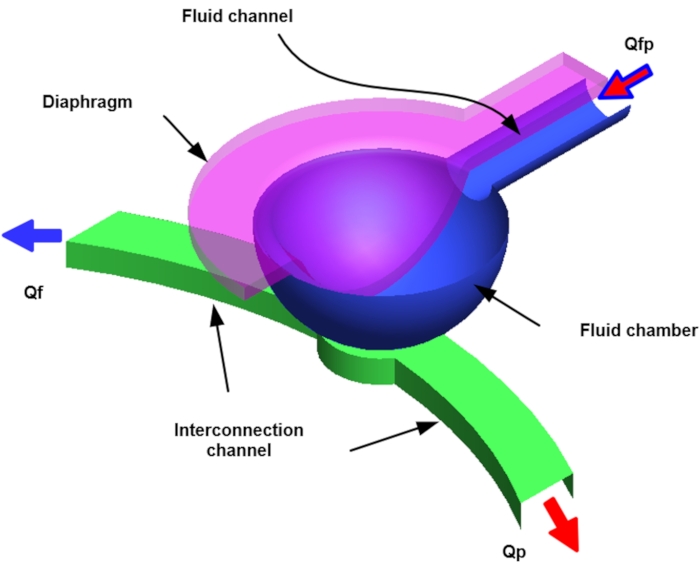
Figure 3: Schematic of Vs in the pneumatic microfluidic platform for microparticle concentration (P, port; Q, flowrate; f, fluid; p, particle; V, valve; s, sieve). Please click here to view a larger version of this figure.
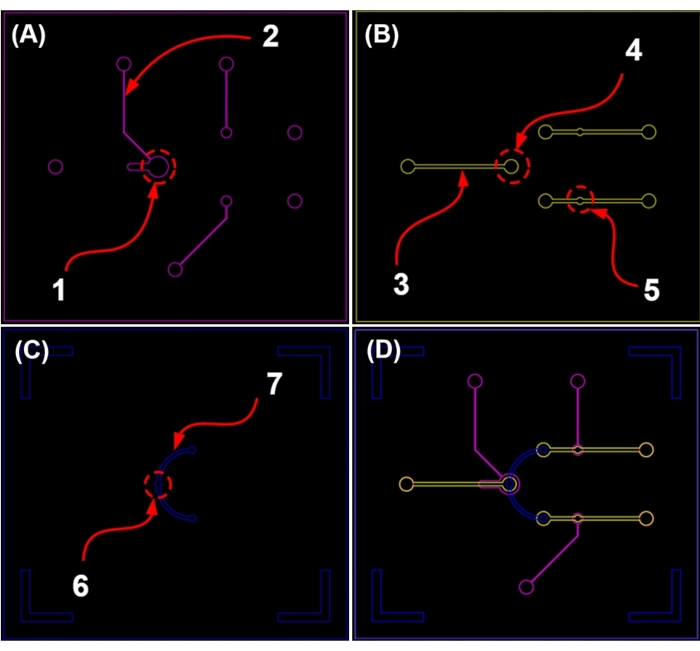
Figure 4: CAD image of the pneumatic microfluidic platform for microparticle concentration. (A) Pneumatic channel valve. (B) Main fluid channel. (C) Interconnection fluid channel. (D) Cross image of each channel (For the dimensions of 1 to 7, see Table 1). Please click here to view a larger version of this figure.
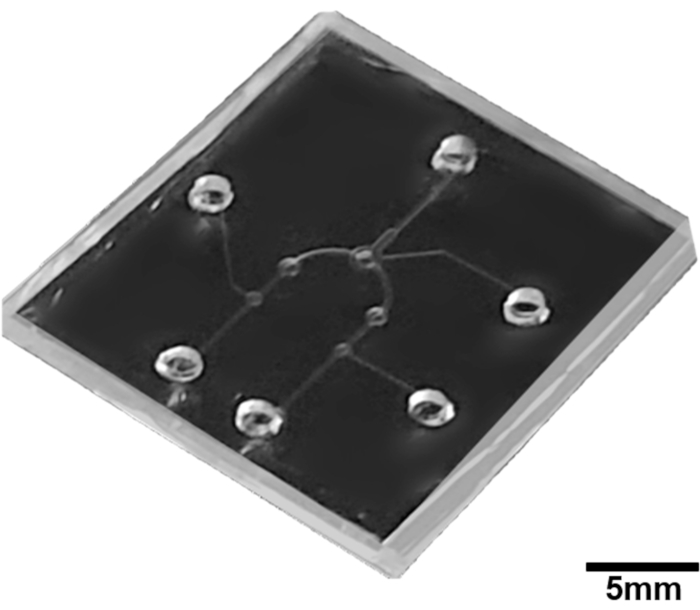
Figure 5: Fabrication image of the pneumatic microfluidic platform for microparticle concentration. Please click here to view a larger version of this figure.
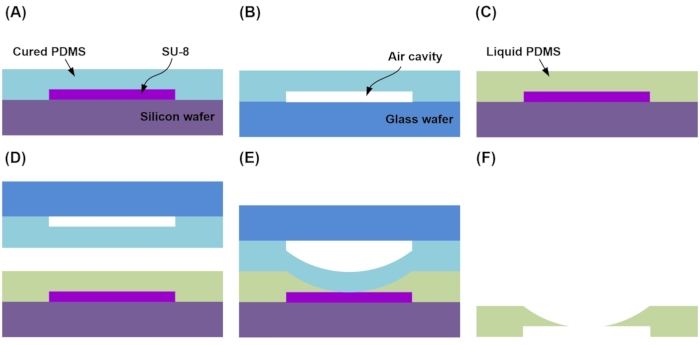
Figure 6: Schematic of the cross-section of the 3D fluidic channel network during fabrication. (A) Molds are created for the curved fluid chamber and fluid channel for replica molding. (B) Plasma bonding of the PDMS layer after curing to a glass wafer. (C) Liquid PDMS is poured into the SU-8 mold to create the interconnection channel. (D) The fluid chamber and fluid channel structure are arranged in liquid PDMS on the SU-8 mold. (E) The system is inflated by the thermal pressure of the air layer. (F) The inflated structure and SU-8 mold are removed. Please click here to view a larger version of this figure.
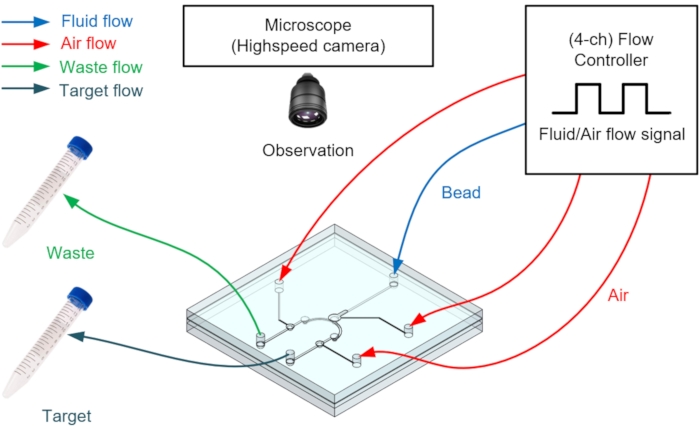
Figure 7: Schematic of the pneumatic microfluidic platform set up for micro-particle concentration. Please click here to view a larger version of this figure.
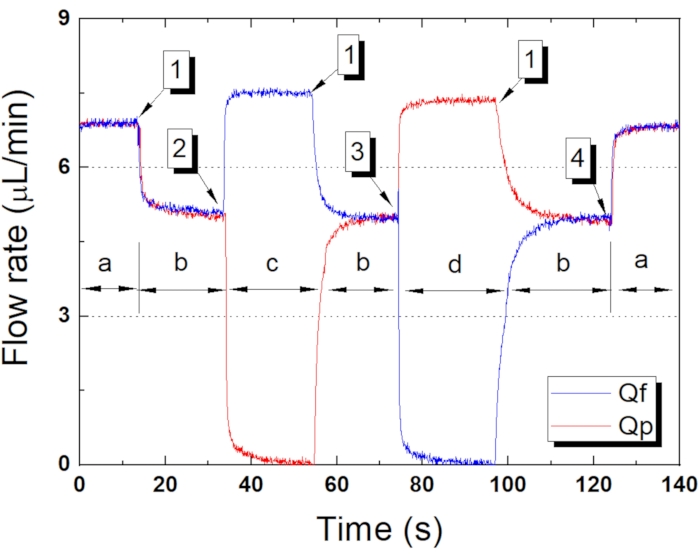
Figure 8: The flow rate of the fluid rates for a four-stage platform operation. The Qf and Qp working fluid flow rates following set Vf and Vp operating times (particle concentration times) in a pneumatic microfluidic platform with a Vs of 15 kPa. a-d show the pneumatic microfluidic platform operation state according to Table 2. (1) Sample loading, (2) Sample blocking, (3) Sample concentration, (4) Sample release. Please click here to view a larger version of this figure.
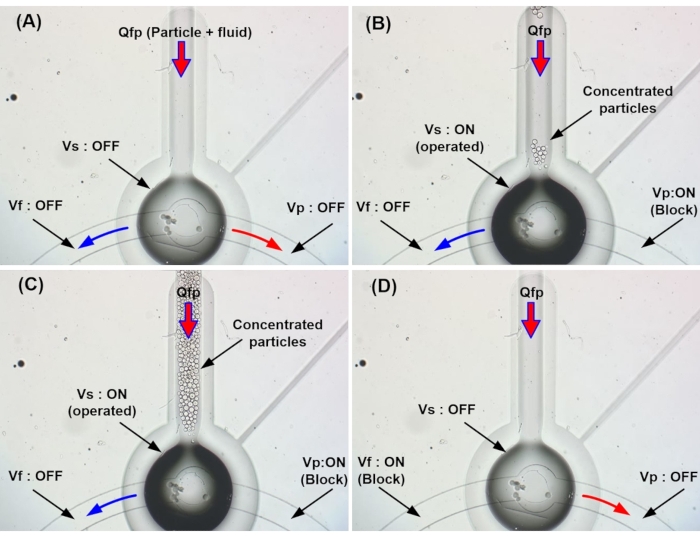
Figure 9: Operation of the microparticle concentrator. (A) Before the operation. (B) Microparticle sieving. (C) Microparticle sieve completion. (D) Release of concentrated particles. Please click here to view a larger version of this figure.
| Number | Structure | Width (W) or diameter (D), (μm) | |||
| 1 | Pneumatic chamber | 1200 (D) | |||
| 2 | Pneumatic channel | 50 (W) | |||
| 3 | Fluid channel | 200 (W) | |||
| 4 | Fluid chamber for Vs | 800 (D) | |||
| 5 | Fluid chamber for Vp (Vf) | 400 (D) | |||
| 6 | Interconnection chamber | 400 (D) | |||
| 7 | Interconnection channel | 200 (W) | |||
Table 1: Dimensions of the pneumatic microfluidic platform (1 to 7 in Figure 4).
| State | Pneumatic Microfluidic Platform Operation |
Pneumatic Valve Operation | |||
| Signal | Vs | Vf | Vp | ||
| a | Loading | 4 | OFF | OFF | OFF |
| b | Blocking | 1 | ON | OFF | OFF |
| c | Concentration | 2 | ON | OFF | ON |
| d | Release | 3 | OFF | ON | OFF |
Table 2: Pneumatic microfluidic platform operation by pneumatic valve operation, shown in Figure 8.
Discussion
This platform provides a simple way to purify and concentrate particles of various sizes. Particles are accumulated and released through pneumatic valve control, and no clogging is observed because there is no passive structure. Using this device, the concentration of particles of three sizes is presented. However, the operating pressure, the time required for concentration, and the rate may vary depending on the device dimensions, particle size magnification, and the pressure at Vs18,20,21.
When performing step 3.1, air bubbles may remain on the curved surface of the channel. When the air bubble remains, the environment in the channel changes, so it is necessary to check the channel very carefully through a microscope before operation.
Compared with previous studies, this platform has some advantages and disadvantages. In the dielectrophoretic method, fewer target particles are used22. An additional process was required to prepare particles to enhance the physical interaction between particles and external forces22,23. Complex design issues must be considered to increase the separation efficiency in magnetophoretic separation systems5,22. This platform showed higher separation efficiency than the ultrasonic method, which can separate samples at high flow rates24. However, because this platform does not have a passive structure, no clogging effect25,26,27 was observed when beads were trapped and accumulated, unlike the passive method.7,10 This platform can be used for water pretreatment when concentrating and extracting suspended bio-particles, as the operation is not affected by the properties of the physical particles18,21.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(Ministry of Science and ICT). (No. NRF-2021R1A2C1011380).
Materials
| 1.5 mm puncture | Self procduction | Self procduction | This puncture was made by requesting a mold maker based on the Miltex® Biopsy Punch with Plunger (15110-15) product. |
| 4 inch Silicon Wafer/SU-8 mold | 4science | 29-03573-01 | 4 inch (100) Ptype silicon wafer/SU-8 mold |
| Carboxyl Polystyrene Crosslinked Particle(24.9 μm) | Spherotech | CPX-200-10 | Concentrated bead sample1 |
| Flow meter | Sensirion | SLI-1000 | Flow measurement |
| High-speed camera | Photron | FASTCAM Mini | Observation of concentration |
| Hot plate | As one | HI-1000 | heating plate for curing of liquid PDMS |
| KOVAX-SYRINGE 10 mL/Syringe | Koreavaccine | 22G-10ML | Fill the microfluidic channel with bubble-free demineralized water. |
| Laboratory Conona treater/Atmospheric plasma | Electro-Technic | BD-20AC | Chip bonding/atmospheric plasma |
| Liquid polydimethylsiloxane, PDMS | Dow Corning Inc. | Sylgard 184 | Components of chip |
| Microscope | Olympus | IX-81 | Observation of concentration |
| PEEK Tubes | SAINT-GOBAIN PPL CORP. | AAD04103 | Inject or collect particles |
| Polystyrene Particle(4.16 μm) | Spherotech | PP-40-10 | Concentrated bead sample3 |
| Polystyrene Particle(8.49 μm) | Spherotech | PP-100-10 | Concentrated bead sample2 |
| Pressure controller/μflucon | AMED | μflucon | Control of air pressure |
| Spin coater | iNexus | ACE-200 | spread the liquid PDMS on SU-8 mold |
References
- Whitesides, G. M. The origins and the future of microfluidics. Nature. 442 (7107), 368-373 (2006).
- Desitter, I., et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Research. 31 (2), 427-441 (2011).
- Hayes, D. F., et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clinical Cancer Research. 12 (14), 4218-4224 (2016).
- Choi, S., Park, J. K. Microfluidic system for dielectrophoretic separation based on a trapezoidal electrode array. Lab on a Chip. 5 (10), 1161-1167 (2005).
- Jung, Y., et al. Six-stage cascade paramagnetic mode magnetophoretic separation system for human blood samples. Biomedical Microdevices. 12 (4), 637-645 (2010).
- Li, P., et al. Acoustic separation of circulating tumor cells. Proceedings of the National Academy of Sciences. 112 (16), 4970-4975 (2015).
- Lin, Y. H., Lee, G. B. Optically induced flow cytometry for continuous microparticle counting and sorting. Biosensors and Bioelectronics. 24 (4), 572-578 (2008).
- Gramotnev, D. K., et al. Thermal tweezers for surface manipulation with nanoscale resolution. Applied Physics Letters. 90 (5), 054108 (2007).
- Huang, L. R., et al. Continuous particle separation through deterministic lateral displacement. Science. 304 (5673), 987-990 (2004).
- Yin, D., et al. Multi-stage particle separation based on microstructure filtration and dielectrophoresis. Micromachines. 10 (2), 103 (2019).
- Yoon, Y., et al. Clogging-free microfluidics for continuous size-based separation of microparticles. Scientific Reports. 6 (1), 1-8 (2016).
- Alvankarian, J., Majlis, B. Y. Tunable microfluidic devices for hydrodynamic fractionation of cells and beads: a review. Sensors. 15 (11), 29685-29701 (2015).
- Irimia, D., Toner, M. Cell handling using microstructured membranes. Lab on a Chip. 6 (3), 345-352 (2006).
- Huang, S. B., et al. A tunable micro filter modulated by pneumatic pressure for cell separation. Sensors and Actuators B: Chemical. 142 (1), 389-399 (2009).
- Chang, Y. H., et al. A tunable microfluidic-based filter modulated by pneumatic pressure for separation of blood cells. Microfluidics and Nanofluidics. 12 (1-4), 85-94 (2012).
- Oh, C. K., et al. Fabrication of pneumatic valves with spherical dome-shape fluid chambers. Microfluidics and Nanofluidics. 19 (5), 1091-1099 (2015).
- Liu, W., et al. Dynamic trapping and high-throughput patterning of cells using pneumatic microstructures in an integrated microfluidic device. Lab on a Chip. 12 (9), 1702-1709 (2012).
- Jang, J. H., Jeong, O. C. Fabrication of a pneumatic microparticle concentrator. Micromachines. 11 (1), 40 (2020).
- McDonald, J. C., et al. Poly(dimethylsiloxane) as a material for fabricating microfluidic device. Accounts of Chemical Research. 35 (7), 491-499 (2002).
- Brivio, M., et al. A MALDI-chip integrated system with a monitoring window. Lab on a Chip. 5 (4), 378-381 (2005).
- Jeong, O. C., Konishi, S. The self-generated peristaltic motion of cascaded pneumatic actuators for micro pumps. Journal of Micromechanics and Microengineering. 18 (8), 085017 (2008).
- Taff, B. M., Voldman, J. A scalable addressable positive dielectrophoretic cell-sorting array. Analytical Chemistry. 77 (24), 7976-7983 (2005).
- Pamme, N., et al. On-chip free-flow magnetophoresis: Separation and detection of mixtures of magnetic particles in continuous flow. Journal of Magnetism and Magnetic Materials. 307 (2), 237-244 (2006).
- Harris, N. R., et al. Performance of a micro-engineered ultrasonic particle manipulator. Sensors and Actuators B: Chemical. 111, 481-486 (2005).
- Yoon, Y., et al. Clogging-free microfluidics for continuous size-based separation of microparticles. Scientific Reports. 6 (1), 1-18 (2016).
- Beattie, W., et al. Clog-free cell filtration using resettable cell traps. Lab on a Chip. 14 (15), 2657-2665 (2014).
- Cheng, Y., et al. A bubble- and clogging-free microfluidic particle separation platform with multi-filtration. Lab on a Chip. 16 (23), 4517-4526 (2016).

