Generation of Tissue Spheroids via a 3D Printed Stamp-Like Device
Summary
The present protocol describes a technique to produce tissue spheroids on a large scale cost-effectively using a 3D printed stamp-like device.
Abstract
Advances in 3D cell culture have developed more physiologically relevant in vitro models, such as tissue spheroids. Cells cultivated as spheroids have more realistic biological responses that resemble the in vivo environment. Due to their advantages, tissue spheroids represent an emerging trend toward superior, more reliable, and more predictive study models with a broad range of biotechnological applicability. However, reproducible platforms that can achieve large-scale production of tissue spheroids have become an unmet need in fully exploring and boosting their potential. Herein, the large-scale production of homogeneous tissue spheroids is reported using a low-cost and time-effective methodology. A 3D printed stamp-like device is developed to generate up to 4,716 spheroids per 6-well plate. The device is fabricated by the stereolithography method using a photocurable resin. The final device is composed of cylindrical micropins, with a height of 1.3 mm and a width of 650 µm. This approach allows the fast generation of homogeneous spheroids and co-cultured spheroids with uniform shape and size and >95% cell viability. Moreover, the stamp-like device is tunable for different sizes of well plates and Petri dishes. It is easily sterilized and can be reused for long periods. The efficient large-scale production of homogeneous tissue spheroids is essential to leverage their translation for multiple areas of industry, such as tissue engineering, drug development, disease modeling, and on-demand personalized medicine.
Introduction
Tissue spheroids are 3D micro-tissues formed by cell suspensions that undergo self-assembly without external forces1. These spheroids have been widely used in biofabrication protocols due to their resemblance with key features of the human physiological system2,3. Tissue spheroids provide more similar metabolism, cytoskeleton dynamics, cell viability, and metabolic and secretion activity than traditional monolayer cell culture1. Due to their fusion capability, they can also be used as building blocks (e.g., bioprinting protocols) to form complex tissue-engineered constructs with enhanced biological relevance4,5.
Due to their biological relevance, tissue spheroids have been used as a biotechnological tool for protocols ranging across tissue engineering, drug development, disease modeling, and nanotoxicological assessment, reducing time, space costs, and animal testing3,6,7,8. Nonetheless, to fully explore and leverage the potential of tissue spheroids, reliable and reproducible methods aiming at their large-scale production are highly necessary, and these remain an ongoing challenge.
Several methodologies produce spheroids, such as hanging drop, coated u-shaped bottom wells, microfluidics, and using a polymeric matrix9,10. Although these methodologies paved the way within the spheroid production market, they are still complex, time-consuming, labor-intensive, or expensive10.
The present protocol reports the large-scale production of homogeneous tissue spheroids using a low-cost and time-effective methodology. We have developed a 3D printed stamp-like device to generate up to 4,716 spheroids per 6-well plate. Moreover, the stamp-like device can be tailored to produce more spheroids per well, suitable for different cell culture plates. It is easily sterilizable and can be reused for long periods. The efficient large-scale production of homogeneous tissue spheroids is essential to translate their use to the clinics, contributing to multiple areas of industry such as tissue engineering, drug development, disease modeling, and on-demand personalized medicine.
Protocol
The L929 cell line, mouse fibroblasts, was used for the present study. The stamp-like 3D printed biodevice was obtained from a commercial source (see Table of Materials). Good cell culture practice and sterile techniques were followed throughout the protocol. The fabricated device was sterilized by wiping it with 70% alcohol and exposing it to UV light for 15 min. The cell culture media and solutions were warmed to 37 °C before contacting with the cells or tissue spheroids. A schematic representation of the protocol is shown in Figure 1.
1. Preparation of non-adherent molds from the stamp-like device
- Prepare 2% (w/v) agarose gel following the steps below.
- Dilute the agarose powder in phosphate-buffered saline (1x PBS) and homogenize the resulting suspension with circular movements.
NOTE: This solution can be placed in a glass bottle. In this step, the agarose solution will not be translucent. - Place the glass bottle into the microwave and set it for 30 s. Every 5 s, stop the microwave, remove the glass bottle, and manually homogenize the solution with circular movements. The heating process needs to be performed until the solution reaches a liquid-limpid state.
NOTE: A hot plate can also be used as a microwave alternative. After the heating process, the solution must be translucent/limpid. - Add 1 mL of the agarose solution to each well of a 6-well plate planned for the experiment.
- Wait for ~15 min or until the agarose solidifies.
NOTE: A cooling plate can be used to decrease the solidification time. - Add 1-2 mL of the agarose solution and gently insert the device above the liquid agarose.
NOTE: The placement of the device must be done carefully to prevent air bubbles in the agarose-device interface. - Wait for ~30 min or until the agarose solidifies.
NOTE: A cooling plate can be used to decrease the solidification time. - Gently remove the device from the agarose.
NOTE: The removal is critical. One should remove it carefully to maintain the agarose features intact; otherwise, the agarose can be disrupted. - Add 2 mL of DMEM media, wait for 10 min, discard the media, and replace it with fresh DMEM. Repeat this three times to wash the well properly.
- Add 2 mL of DMEM and place the 6-well plate in an incubator (at 37 °C in 5% CO2 and 80% humidity) until the cell seeding (Figure 2A-F, Supplementary Video 1).
- Dilute the agarose powder in phosphate-buffered saline (1x PBS) and homogenize the resulting suspension with circular movements.
2. Generation of tissue spheroids
NOTE: Different cell lineages have different adhesion properties. Hence, using this methodology, some types of cells may not form the tissue spheroids properly.
- Grow the cells following traditional monolayer culture (i.e., grow the cells in cell culture flasks using DMEM with low glucose supplemented with 10% fetal bovine serum (FBS), 100 µg/mL penicillin, and 100 µg/mL streptomycin) (see Table of Materials). Maintain the cells at 37 °C in a 5% CO2 incubator, and monitor until they reach 80% of confluence.
- After reaching the desirable confluence, wash the cells with 1x PBS.
NOTE: It is recommended to use 5 mL for 25 cm2 flasks , 10 mL for 75 cm2 flasks, and 15 mL for 150 cm2 flasks. - Add the dissociation enzyme and incubate the cells for 2-5 min at 37 °C in 5% CO2 and 80% humidity.
NOTE: The present study used 0.125% trypsin with 0.78 mM ethylenediamine tetraacetic acid (EDTA) as the dissociation enzyme (see Table of Materials). - Observe the detachment of the cells from the cell culture flasks and add a growth medium supplemented with FBS to neutralize the cell dissociation enzyme.
NOTE: For the present study, DMEM with low glucose (see Table of Materials) was used supplemented with 10% FBS. - Centrifuge the cell suspension at 400 x g for 5 min at room temperature. Then, count the cells manually.
- Take 50 x 105 cells per tube and add 5 mL of 1x PBS.
NOTE: The number of cells seeded influences the final tissue spheroid diameter. Hence, one can increase the cell number to generate tissue spheroids with larger diameters. - Centrifuge the cell suspension at 400 x g for 5 min at room temperature.
- Remove the supernatant using a pipette, add 1 mL of the cell culture medium, and homogenize the solution.
NOTE: In the present study, a complete culture medium of DMEM with low glucose, supplemented with 10% FBS, 100 µg/mL penicillin, and 100 µg/mL streptomycin, was used. - Remove 2 mL of medium from the 6-well plate (step 1.1.9) and add 1 mL of the cell suspension to the center of the agarose mold formed by the 3D-printed biodevice (step 1.1.7). Wait until the cells sediment in the micro resections (~20-30 min) and carefully add 1 mL of cell culture medium into the well.
NOTE: One needs to be extra careful in this step. It is recommended to gently add the medium, place the pipette tip close to the well wall, and dispense in small amounts. - Place the 6-well plate in the incubator (at 37 °C in 5% CO2 and 80% humidity) for the tissue spheroids to form (approximately 24-48 h, depending on the cell type) (Figure 3).
NOTE: Different cell types (e.g., cancer cells, primary cells) have different self-assembly kinetics11.
Representative Results
Generation of homogeneous micro resections using the 3D printed stamp-like device
The 3D printed stamp-like device was successfully manufactured by the stereolithography method12 using a photocurable resin (Figure 2A). The final device was composed of cylindrical micropins with a height of 1.3 mm and a width of 650 µm (Figure 2A). Its use as a master mold to produce non-adherent micro resections was achieved by maintaining the geometry (Figure 2B–F and Supplementary Video 1). The device was simple to use, easy to sterilize, and could be reused in the long term. Moreover, it is also tunable for different sizes of well plates (i.e., 6 wells, 12 wells, 24 wells, 96 wells) and Petri dishes (i.e., 30 mm, 50 mm, 90 mm, 150 mm). Here, we show data regarding the device for the 6-well plate. It generated 750 homogeneous micro resections per well or 4,716 per 6 well-plate (Figure 2C–D). Drawbacks such as disruption of the non-adherent mold and deformation of the micro resection geometry can occur if the device is withdrawn early (Figure 2G).
Large-scale production of tissue spheroids
The cells were seeded onto the non-adherent agarose molds, sedimented, and approximately 24 h later formed the tissue spheroids. The large-scale production of the spheroids was achieved by maintaining their shape, size (123 µm ± 3 µm), and viability (Figure 3A–D). This methodology supported the spheroids culture for months (data not shown).
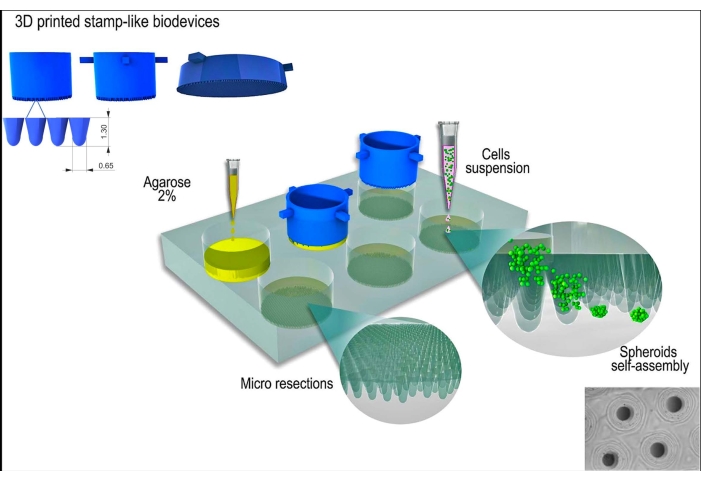
Figure 1: Schematic representation of tissue spheroid generation using a stamp-like 3D printed biodevice. The biodevice, composed of cylindrical micropins, molds a non-adherent hydrogel (e.g., agarose) to form an array of uniform micro resections. After the solidification of the agarose and the incubation time with the cell culture medium (to acclimatize the new non-adherent molds), the cell suspension is seeded on the molds, and the cells aggregate, self-assemble, and compact into a spherical shape. Please click here to view a larger version of this figure.
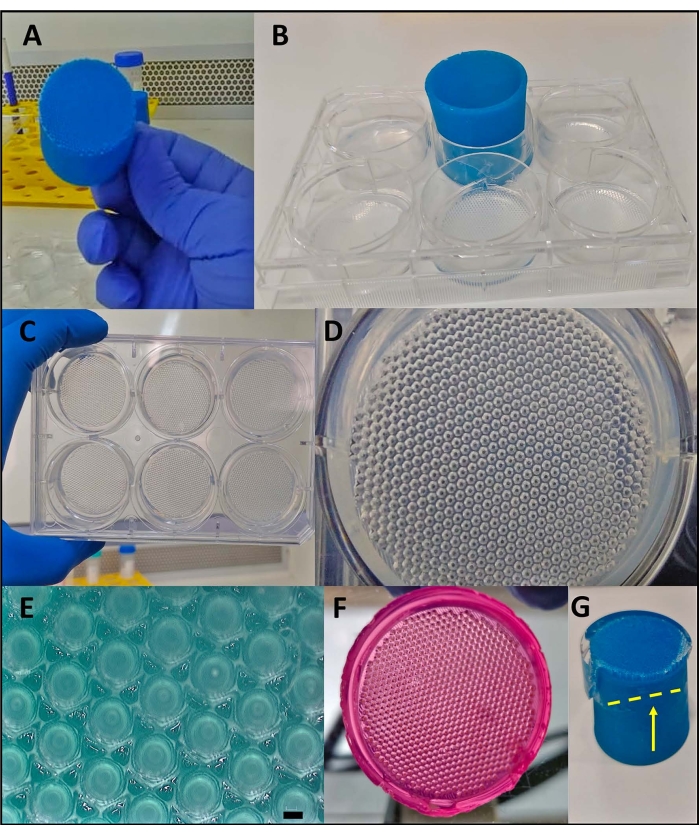
Figure 2: The 3D printed stamp-like device used as a master mold to form non-adherent micromolded agarose. (A) The 3D printed stamp-like device. (B) The insertion of the device into the liquid agarose to form the micro resections. (C–E) After the removal of the device, homogeneous resections are formed. (E) Scale bar = 200 µm. (F) Micromolded agarose after incubation with cell culture medium (pink color: cell medium). (G) Removing the device from the agarose when it is not completely solidified leads to its disruption (yellow line with arrow). Please click here to view a larger version of this figure.
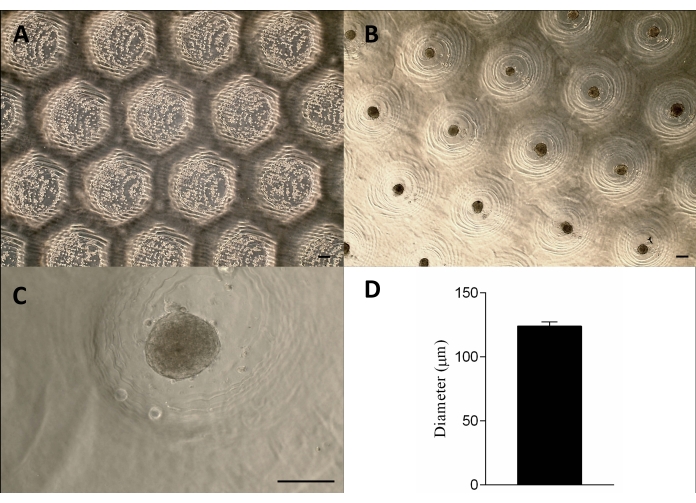
Figure 3: Cell seeding and spheroid formation. Phase-contrast microscopy (A) 0 h after the cells seeding in the micro resections (dots within the resections). (B–C) Spheroids formed approximately 24 h after the seeding. Scale bar = 100 µm. (D) Graphic representation of the spheroids' diameter, n = 4. The spheroids formed are homogeneous in shape and size. Please click here to view a larger version of this figure.
Supplementary Video 1: Micromolded agarose after removal of the stamp-like device. Please click here to download this Video.
Discussion
The present protocol describes a simple, fast, and inexpensive method for the large-scale production of tissue spheroids. A stamp-like 3D printed device was used as a master mold, which generated up to 4,716 spheroids per 6-well plate. It has been shown that cells cultivated as spheroids have more realistic biological responses that closely resemble the in vivo environment1. Due to their advantages, tissue spheroids represent an emerging trend toward superior, more reliable, and more predictive study models13,14. Hence, developing new methodologies to achieve large-scale production is pivotal to leveraging their application.
To successfully achieve the generation of tissue spheroids using this methodology, critical steps such as inserting the device into the liquid agarose need to be performed with caution to avoid air bubbles. The presence of air bubbles might impair the correct molding of the micro resections. Accordingly, removing the device before total solidification of the agarose may result in the rupture of the agarose mold. These steps are essential to produce agarose molds suitable for tissue spheroid formation. The shape and size variability for the generated batch of spheroids needs to be minimal. Therefore, one of the deterministic points is the slow addition of the culture medium after the cells are seeded in the agarose mold. This requires gentle dispensing of the liquid to not disturb the cells that have already entered the micro resections. Otherwise, some micro resections could have augmented concentrations of cells, which will lead to heterogeneity in size among the spheroids.
In addition to being simple, fast, and inexpensive, this protocol is also flexible (i.e., some modifications in it are possible to adjust to the experiment's particularities). Alternatively to agarose, one can use polydimethylsiloxane (PDMS) to form the molds. PDMS is an elastomer that is broadly used to fabricate microfluidic chips15. It has properties such as biocompatibility, optical transparency, being chemically inert, thermal stability, and gas permeability15. Different from agarose, molds made with PDMS are more resistant and can also be reused with preservation of the micro resection geometry.
Currently, the search for an alternative serum-free cell culture medium has increased worldwide16,17. Although FBS is a traditional supplement for cell culture, its use has ethical issues and possible batch variability. Nonetheless, considering the clinical application of spheroids, the risks of immune reactions and disease transmission impair the use of FBS as a medium supplement16. Thus, one can use a serum/xeno-free cell culture medium to form the spheroids following the present methodology. For instance, studies have used a special 3D culture medium composed of human albumin, ascorbic acid, insulin, transferrin, and selenium to form human mesenchymal/stromal stem cell spheroids4,8.
The successful formation of spheroids can be determined by their compact spheroidal shape. To date, some studies have reported the impact that cell adhesion mediators (e.g., E-cadherin, integrins), as well as cytoskeleton microtubules and actin filaments, have on cell self-assembly and further compaction into spheroids18,19. Therefore, cells that present disbalance in expressing these components might not be able to compact and form the spheroids. Hence, spheroid formation differs according to the cell line; thus, some types of cells might not be suitable options for the methodology described herein.
In the present protocol, a 3D printed stamp-like device, composed of cylindrical micropins, was used to mold a non-adherent hydrogel (e.g., agarose) to form an array of uniform microstructured resections. It is also possible to observe (i.e., using imaging) the cell's aggregation, self-assembly, and compaction in real time. Moreover, one has minimal labor to add/exchange medium and add drugs or other compounds. In addition, one can withdraw the spheroids for post-analysis, such as electron microscopy, cytometry, and histology, by merely applying a directed jet using a pipette loaded with medium or PBS. Several methodologies have been reported to generate spheroids, such as hanging drop, coated u-shaped bottom wells, microfluidics devices, and using a polymeric matrix. Nonetheless, they are usually complex, time-consuming, labor-intensive, expensive, or may generate heterogeneous spheroids 9,10. Although the large-scale production of spheroids was achieved using the hanging drop methodology20, it is still difficult to follow the spheroid formation process and to perform medium exchange or the addition of drugs/compounds10.
Micromolded non-adhesive hydrogels have been used as a strategy to form spheroids and also molds to guide the formation of larger microtissues10. However, the master molds used are mainly silicon, are unsuitable for long-term use, and might be expensive21. This study employed stereolithography (SLA) for the 3D printing technology to generate highly uniform master molds. SLA generates the prototype with speed, cost-effectiveness, flexibility, and precision22. Additionally, its advantages for customized manufacturing have been used in several fields, including tissue engineering and biomedicine in general22.
It is known that spheroid cell cultures are physiologically relevant in vitro study models that can closely resemble human features. Hence, they have been used as a model to study the onset of diseases and toxicology and are already commercialized as a bioproduct for regenerative medicine23,24,25. Accordingly, they are used as the raw material for forefront technologies such as 3D bioprinting3,26 and organs-on-chips24,27, continuously showing how significant spheroids cultures are and the importance of developing new strategies to generate them on a large scale.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Foundation for Research Support of the State of Rio de Janeiro (FAPERJ, Brazil), the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), and the Brazilian National Council for Scientific and Technological Development (CNPq, Brazil). We thank Bioedtech for providing the stamp-like devices used in this study and Professor Bartira Bergmann from the Immunopharmacology Laboratory for the use of their cell culture facilities.
Materials
| 6 well plate | Merck | CLS3516 | |
| Agarose | Promega | V3121 | |
| Biodevice | Bioedtech | ||
| Biological Safety Cabinet | ThermoFisher | 51029701 | |
| Centrifugue | ThermoFisher | 75004031 | |
| Corning 50 mL centrifuge tubes | Merck | CLS430829-500EA | |
| Corning cell culture flasks surface area 75 cm2 | Merck | CLS430641 | |
| Draft Resin | FormLabs | FLDRBL01 | |
| Dulbecco′s Modified Eagle′s Medium – low glucose | Merck | D6046 | |
| Fetal Bovine Serum (FBS) | ThermoFisher | 16000044 | |
| Form 2 | FormLabs | ||
| Incubator | ThermoFisher | 51033782 | |
| L929 cell lines | Stablished in the lab | ||
| Penicillin and Streptomycin (PS) | ThermoFisher | 15140122 | |
| Phosphate-Buffered Saline (PBS) | Merck | 806552 | |
| Trypsin with EDTA | Merck | T3924 |
References
- Laschke, M., Menger, M. Life is 3D: Boosting spheroid function for tissue engineering. Trends in Biotechnology. 35 (2), 133-144 (2017).
- Mekhileri, N., et al. Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication. 10 (2), 024103 (2018).
- Itoh, M., et al. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One. 10 (12), 0145971 (2015).
- Kronemberger, G., et al. The hypertrophic cartilage induction influences the building-block capacity of human adipose stem/stromal cell spheroids for biofabrication. Artificial Organs. 45 (10), 1208-1218 (2021).
- Mironov, V., et al. Organ printing: Tissue spheroids as building blocks. Biomaterials. 30 (12), 2164-2174 (2009).
- Skardal, A., Shupe, T., Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discovery Today. 21 (9), 1399-1411 (2016).
- Garcez, P., et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 352 (6287), 816-818 (2016).
- Charelli, L., et al. Biologically produced silver chloride nanoparticles from B. megaterium modulate interleukin secretion by human adipose stem cell spheroids. Cytotechnology. 70 (6), 1655-1669 (2018).
- Cui, X., Hartanto, Y., Zhang, H. Advances in multicellular spheroids formation. Journal of the Royal Society Interface. 14 (127), 20160877 (2017).
- Achilli, T., Meyer, J., Morgan, J. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opinion on Biological Therapy. 12 (10), 1347-1360 (2012).
- Rolver, M., Elingaard-Larsen, L., Pedersen, S. Assessing cell viability and death in 3D spheroid cultures of cancer cells. Journal of Visualized Experiments. (148), e59714 (2019).
- Quan, H., et al. Photo-curing 3D printing technique and its challenges. Bioactive Materials. 5 (1), 110-115 (2020).
- Rodriguez-Salvador, M., Perez-Benitez, B., Padilla-Aguirre, K. Discovering the latest scientific pathways on tissue spheroids: Opportunities to innovate. International Journal of Bioprinting. 7 (1), 331 (2021).
- Baptista, L., et al. Adult stem cells spheroids to optimize cell colonization in scaffolds for cartilage and bone tissue engineering. International Journal of Molecular Sciences. 19 (5), 1285 (2018).
- Shakeri, A., Khan, S., Didar, T. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab on a Chip. 21 (16), 3053-3075 (2021).
- vander Valk, J. Fetal bovine serum (FBS): Past – present – future. ALTEX. 35 (1), 99-118 (2018).
- vander Valk, J., Brunner, D., et al. Optimization of chemically defined cell culture media – Replacing fetal bovine serum in mammalian in vitro methods. Toxicology in Vitro. 24 (4), 1053-1063 (2010).
- Smyrek, I., et al. microtubules and FAK dominate different spheroid formation phases and important elements of tissue integrity. Biology Open. 8 (1), 037051 (2018).
- McMillen, P., Holley, S. Integration of cell-cell and cell-ECM adhesion in vertebrate morphogenesis. Current Opinion in Cell Biology. 36, 48-53 (2015).
- Tung, Y., et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst. 136 (3), 473-478 (2011).
- Guo, X., Li, S., Ji, Q., Lian, R., Chen, J. Enhanced viability and neural differential potential in poor post-thaw hADSCs by agarose multi-well dishes and spheroid culture. Human Cell. 28 (4), 175-189 (2015).
- Andréa Dernowsek, J., Rezende, R., Lopes daSilva, J. The role of information technology in the future of 3D biofabrication. Journal of 3D Printing in Medicine. 1 (1), 63-74 (2017).
- Garcez, P., et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 352 (6287), 816-818 (2016).
- Skardal, A., et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Scientific Reports. 7, 8837 (2017).
- Armoiry, X., et al. Autologous chondrocyte implantation with chondrosphere for treating articular cartilage defects in the knee: An evidence review group perspective of a NICE single technology appraisal. PharmacoEconomics. 37 (7), 879-886 (2018).
- Nakamura, A., et al. Bio-3D printing iPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication. 13 (4), 044103 (2021).
- Mesquita, C., Charelli, L., Baptista, L., Naveira-Cotta, C., Balbino, T. Continuous-mode encapsulation of human stem cell spheroids using droplet-based glass-capillary microfluidic device for 3D bioprinting technology. Biochemical Engineering Journal. 174, 108122 (2021).

.
