A Modified Murine Heterotopic Heart Transplant Protocol Matching Contemporary Standards of Aseptic Technique, Anesthesia, and Analgesia
Summary
The present paper describes a modified technique for heterotopic vascularized cardiac transplantation with updated aseptic technique, analgesia, and anesthesia.
Abstract
The development of experimental models of cardiac transplantation in animals has contributed to many advances in the fields of immunology and solid organ transplantation. While the heterotopic vascularized murine cardiac transplantation model was initially utilized in studies of graft rejection using combinations of mismatched inbred mouse strains, access to genetically modified strains and therapeutic modalities can provide powerful new preclinical insights. Fundamentally, the surgical methodology for this technique has not changed since its development, especially with respect to important factors such as aseptic technique, anesthesia, and analgesia, which make material impacts on postsurgical morbidity and mortality. Additionally, improvements in perioperative management are expected to provide improvements in both animal welfare and experimental outcomes. This paper reports upon a protocol developed in collaboration with a subject matter expert in veterinary anesthesia and describes the surgical technique with an emphasis on perioperative management. Additionally, we discuss the implications of these refinements and provide details on troubleshooting critical surgical steps for this procedure.
Introduction
We owe much of our understanding of immunology and transplantation to research based on experimental models of solid organ transplantation using animal subjects. Since the first description of vascularized cardiac transplantation in mammals1, such models have contributed to knowledge in wide-ranging domains, including the therapeutic application of hypothermia2, the benefits of using specialized sutures3, and techniques for total lung and heart homotransplantations4. The development of cardiac transplantation models in rats5,6 provided broader scope for immunological experimentation due to the availability of different breeding lines. The substantially wider range of available inbred and mutant mouse strains led Corry et al.7 to develop a technique of murine heterotopic cardiac transplantation due to the considerable advantages that this range brings to transplantation research. This model has been used widely and has contributed to a greater understanding of graft rejection8 and therapeutics9. Since its first description, however, the technique has remained largely unchanged other than some minor technical details such as adjustments to the position of anastomotic sites10,11.
Since the integration of the technique of Corry et al.7 into our experiments, we have identified areas of promise for improving the protocol, namely those of aseptic technique, anesthesia, and analgesia. Improvements in these areas were expected to offer a positive impact on experimental outcomes and improve animal welfare. This has previously been shown when aseptic technique is used in small animal surgeries as it aids in the reduction of postoperative infections12, which not only impacts morbidity and mortality but can also compromise experiments designed to assess the immune response following transplantation surgery. From an anesthesia and analgesic point of view, the use of a refined regimen helps to reduce the cost to animals and balance the ethical argument of this surgical model by mitigating the pain and suffering of experimental subjects. Further, appropriate anesthesia and analgesia limit the pain-associated stress response, improving the quality of postoperative recovery and, ultimately, increasing the surgical success rate13.
With the aim of improving both animal welfare and experimental outcomes, a protocol was developed with adjustments to bridge these gaps. This protocol has been adapted from that originally described by Corry et al.7 with consultation from a veterinary anesthetist and with due consideration for both the effects and duration of effects of the pharmacological interventions utilized in the anesthetic and analgesic regimen. The approach was based on the principles of balanced anesthesia and multimodal analgesia to ensure appropriate perioperative care14. In addition to the application of aseptic technique, the opioid buprenorphine and the local anesthetic bupivacaine were pre-emptively administered. General anesthesia was performed using the inhalant anesthetic agent isoflurane.
Protocol
This research was performed in accordance with the Code of Practice for the Care and Use of Animals for Scientific Purposes15 and approved under Animal Ethics Protocols RA/3/100/1568 and AE173 (The University of Western Australia Animal Ethics Committee and The Harry Perkins Institute of Medical Research Animal Ethics Committee, respectively). See the Table of Materials for details regarding all materials, instruments, and animals used in this protocol.
1. Preparation of the animal for surgery
NOTE: Personnel are dedicated to either the role of performing surgery or monitoring anesthesia throughout the procedure.
- For preoperative analgesia, administer a dose of buprenorphine (0.05-0.1 mg/kg, diluted to 0.03 mg/mL with sodium chloride 0.9%) subcutaneously to the recipient mouse at least 1 h prior to the initiation of recipient surgery. Enter all details related to the administration of drugs, their dose, the time of administration, and their effects in the anesthetic record.
NOTE: This approach is not necessarily required for the donor as it is a non-recovery surgery, where the donor is euthanized under general anesthetic immediately after harvest of the organ. - Induction of anesthesia
- Place the mouse in the induction chamber of the anesthetic breathing system with an oxygen flow of 1-2 L·min−1 with 4% isoflurane. Confirm adequate anesthesia by observing recumbency, loss of the righting reflex, and a decreased respiratory rate.
- Once adequately anesthetized, remove the mouse from the induction chamber and closely shave the ventral abdomen using clippers to remove hair. In the case of the donor, shave the area extending from the genitalia to the upper margin of the ventral thorax. In the case of the recipient, shave the area extending from the genitalia to the costal margin. In both cases, ensure that the area shaved reaches the midaxillary line laterally.
- To maintain anesthesia, position the mouse in dorsal recumbency to receive anesthetic and oxygen from the nose cone of the breathing system (non-rebreathing), which delivers oxygen at a rate of 1 L·min−1 and isoflurane (1.5%-2.5%).
NOTE: The surgical work surface is a surgical board over a heating pad, and each limb of the mouse is secured using micropore tape. - Given the difficulty in comprehensively monitoring the physiological changes associated with anesthesia in mice, monitor and record limited parameters. Monitor the temperature, depth of anesthesia, and respiratory rate at least every 5 min for the duration of anesthesia.
- To prevent severe hypothermia and hyperthermia (from active warming by the heat pad), monitor the body temperature throughout the procedure. Insert a clean, lubricated rectal probe into the rectum of the animal and then secure it to the surgical board using micropore tape.
NOTE: This probe feeds back to a dynamic system (a feature of the anesthetic delivery system), which modifies the heating pad temperature to manage body temperature. - Have the person responsible for anesthesia assess the anesthetic depth by observing responses to stimulation of the paw or tail from pressure applied by atraumatic forceps, the palpebral reflex, and muscle tone.
- Measure the respiratory rate by observing movement of the chest wall while observing the respiratory effort subjectively to assess tidal volume. Calculate the respiratory rate by counting breaths over a 10-15 s period and multiplying by 6 or 4, respectively, to determine a breath rate/min.
- To prevent severe hypothermia and hyperthermia (from active warming by the heat pad), monitor the body temperature throughout the procedure. Insert a clean, lubricated rectal probe into the rectum of the animal and then secure it to the surgical board using micropore tape.
- To prepare the skin, disinfect the surgical site using sterile cotton-tipped applicators. Apply chlorhexidine in a circular, expanding motion working from the center of the surgical site to the edges. Repeat this process 3x (with a new cotton-tipped applicator each time) before a final application of a combination of chlorhexidine and ethanol with a new sterile cotton-tipped applicator in the same pattern, moving from the center of the surgical site to the edge.
- Have the surgeon apply an ethanol-based hand gel prior to donning a sterile surgical gown and sterile surgical gloves.
- To prepare the surgical field, place sterile surgical drapes (precut to 25 cm x 25 cm) on either side of the surgical board, serving as the site to place sterile instruments. Use sterile scissors to fenestrate a wider 25 cm x 40 cm sterile drape to cut a small (slightly longer than the incision site), oval-shaped opening. Lay this drape over the top of the animal such that the fenestration is located at the proposed incision site. Ensure that the lateral ends of this third drape overlap the two smaller drapes on either side to create a continuous surgical field.
2. Donor surgery
NOTE: See Supplemental Figure S1 for the key aspects of donor surgery.
- Perform the donor surgery with the assistance of a surgical binocular microscope. To begin, use a magnification of 8x, and perform a ventral midline skin incision using a surgical scalpel blade (#23). Ensure that the incision spans from the caudal end of the shaved area to the costal margin with an intact margin of prepared skin at either end.
NOTE: The starting magnification of 8x is chosen to enable sufficient visualization of the macrostructure of the subject at the beginning of surgery. From this point on, magnification is at the operator's discretion and should be selected to provide an appropriate balance between the situational awareness provided by lower magnification and the fine detail that can be visualized with higher magnification. - Using two sterile cotton-tipped applicators moistened with warmed normal saline, shift the small bowel to expose the abdominal aorta and inferior vena cava (IVC). Use the applicators to bluntly dissect these vessels from the surrounding tissue.
- Use a 3.0 mL syringe with a 30 G, 0.5 in needle to draw up 2.5 mL of 100 IU·mL−1 of heparinized sodium chloride 0.9% solution (maintained at 4 °C until required during surgery). Using straight-tipped, round-body suture forceps with the non-dominant hand to secure the abdominal aorta in the infra-diaphragmatic area, use the dominant hand to inject 1.5 mL of solution into the aorta in the direction of the heart. Seal the resultant aortotomy with pressure from a cotton-tipped applicator.
- Use straight-tipped microsurgical scissors to transect the IVC to allow exsanguination.
- Perform a thoracotomy using surgical scissors to make two incisions in the bilateral midaxillary lines. At this point, confirm the death of the animal and turn off the isoflurane vaporizer.
- Secure the resultant median segment of the thoracic wall using a micro-bulldog clamp. Pass this to the non-sterile surgical assistant who can secure this to the nose cone using micropore surgical tape.
NOTE: The goal is to provide traction on this segment of thorax, which aids with exposure of the cardiac tissues. - Using round body suture forceps, identify and mobilize the intra-thoracic IVC.
NOTE: Ideally, the straight-tipped forceps should be in the non-dominant hand and the curved forceps in the dominant hand. - With the IVC secured in the non-dominant hand's forceps, use the dominant hand to inject the remaining 1.5 mL of 100 IU·mL−1 heparinized sodium chloride 0.9 % solution into the heart.
- Using both sets of forceps, ligate the IVC using 7/0 braided silk of 2 cm length. Use an instrument tie surgeon's knot with two additional throws for security. Make this knot as proximal along the vessel to the heart as possible.
- Secure the two ends of this knot using artery forceps. Position these forceps so that they provide gentle traction of the heart in the caudal direction to facilitate optimal vessel positioning for subsequent dissection.
- Identify the thymus at the anterosuperior aspect of the heart. Use forceps to dissect this organ from the donor to identify the superior vena cava (SVC).
- Remove the adventitia and associated tissues of the SVC using forceps. Use curved forceps to bluntly dissect and make a small channel posterior to the vessel. Ensure that this channel is as proximal to the heart as possible.
- Pass a piece of 7/0 braided silk of 2 cm length through this channel using forceps and then tie it using the aforementioned technique.
- At a point approximately 2 mm from this ligation (on the side opposite to the heart), divide the SVC using curved microsurgical scissors.
- Using cotton-tipped applicators, flip the heart to the anatomical right.
- Identify the azygous vein on the anatomical left of the heart. Using forceps, bluntly dissect it from surrounding structures. As before, use curved-tipped forceps to create a small channel posterior to this vessel.
- Use a third piece of 7/0 braided silk cut to 2 cm to ligate the azygous vein at maximal proximity to the heart using the same knot-tying technique. Cut the vessel 2 mm from the ligation on the side away from the heart.
- Using cotton-tipped applicators, flip the apex of the heart back to the anatomical left. Use forceps to identify and mobilize the ascending aorta. Pass the curved forceps underneath the aortic arch to create a channel between the ascending and descending aorta.
- Using straight-tipped microsurgical scissors, transect the aortic arch proximal to its branches.
- Using forceps, identify and mobilize the pulmonary artery. Using curved forceps, make a channel posterior to the vessel.
- Using straight-tipped microsurgical scissors, transect the artery at a point just proximal to its bifurcation.
- Use a Rycroft irrigating cannula to gently inject 2 mL of 10 IU·mL−1 of heparinized sodium chloride 0.9% through the pulmonary artery and ascending aorta to flush any remaining blood from the heart.
NOTE: An adequate flush is indicated by the clearance of visible blood from the coronary vessels. - Using a 3 cm piece of 7/0 braided silk, ligate the remaining posterior vessels (the pulmonary veins) en bloc using a surgeon's knot with two subsequent throws. Separate the heart from the posterior thoracic wall by carefully cutting using surgical scissors.
- Gently remove the heart from the thorax, submerge it in University of Wisconsin Solution (UWS), and then place it on ice for storage (at 4 °C).
3. Recipient surgery
- Following preparation of the animal as described in section 1., apply eye lubricant. Inject a weight-based dose (8 mg/kg) of bupivacaine (0.25% diluted to 0.625 mg/mL in sodium chloride 0.9% solution) into the subcutaneous tissue of the ventral abdomen along the planned incision site. Use a 29 G insulin syringe for this injection and look for a straight line of visible blebbing that covers the extent of the planned incision (Supplemental Figure S2A-C).
NOTE: Five-seven minutes should be given to allow time for peak effect of the local anesthetic. - With the microscope set to 8x magnification, make a ventral midline skin incision using a sterile surgical scalpel blade (#23). Ensure that the laparotomy spans from the lower abdomen to the costal margin. Insert a retractor to maximize the surgical field (Supplemental Figure S2D).
- Moisten a 5 cm x 5 cm segment of sterile gauze with warmed 0.9% sodium chloride solution and position it at the superior aspect of the surgical site. Using dampened sterile cotton buds, gently eviscerate the intestines, position them on top of this gauze, and wrap the gauze around the organ (Supplemental Figure S3A).
NOTE: This procedure helps to reduce insensible fluid loss during surgery and aids in retraction. - Free and mobilize the abdominal aorta and IVC from surrounding tissues using a blunt dissection technique. Use a combination of cotton-tipped applicators and round-body suture forceps for this step. Ensure that the area of clearance is between the infra-renal aspect of the vessels and just above the bifurcation of the aorta (Supplemental Figure S3B).
NOTE: Appropriate visualization at this point will facilitate high-quality vascular anastomoses. - Identify the posterior abdominal vessels. Using forceps, gently traction the aorta in a direction away from the vertebral column (i.e., longitudinal to the axis of the abdominal vessels).
NOTE: It is important that only the aorta and not the IVC is handled in such a manner due to the friability of the latter. - Ligate each abdominal vessel identified in the planned anastomotic zone. Make a channel on either side of these vessels cephalocaudally by passing the curved forceps posterior to the abdominal vessels on either side. Ligate each vessel identified and mobilized in this manner using lengths of 10/0 nylon tied with instruments into surgeon's knots with one additional throw (Supplemental Figure S3C).
- Isolate the anastomotic site from circulation. To do so, install a surgical clamp both at the head and then the caudal ends of the abdominal vessels (importantly, in that precise order). Ensure that the clamps cross both vessels to a sufficient degree to ensure complete occlusion.
- Using forceps in the non-dominant hand to steady the aorta, perform an aortotomy using a 30 G needle at the anterior aspect of the aorta. Extend it using straight-tipped microsurgical scissors (Supplemental Figure S3D).
- Perform a venotomy. Using straight forceps, apply gentle anterior traction to the IVC at the point in line with the middle of the aortotomy. Use curved microsurgical scissors with the concave side facing anteriorly to remove a segment of IVC of equal length to the aortotomy (Supplemental Figure S4A).
- Using 10 IU·mL−1 heparinized sodium chloride solution, wash the interiors of the opened vessels of remaining blood.
- Place the donor heart into the abdomen. Ensure that the positioning is such that the ascending aorta is directly alongside the abdominal aortotomy and the heart is rotated so that the pulmonary artery can be drawn across for the second anastomosis.
- Using 10/0 nylon, place a stay suture between the 12 o'clock position of the aortotomy and the corresponding extremity of the lumen of the ascending aorta. Perform this using straight-tipped forceps and a microsurgical needle holder and tie it using a surgeon's knot with three subsequent throws. Cut the ends to leave approximately 2 mm of suture.
- Place a second stay suture between the 6 o'clock position of the aortotomy and the corresponding aspect of the ascending aorta. As this suture will also serve as the base for subsequent running sutures, leave at least 10 mm of the tail for the ultimate tie-off.
- Place a continuous running suture of 10/0 nylon in an ascending manner to oppose the anatomical right edge of the aortotomy and the corresponding free edge of the ascending aorta. Use approximately four throws for this line.
- Pass the free end of the suture around the distal stay suture before placing a second continuous running suture down the anatomical left side to affect apposition with the remaining free edge of the aortotomy. Tie off the suture to the tail using a surgeon's knot with two additional throws.
- Place a stay suture between the 12 o'clock position of the IVC venotomy and the corresponding extremity of the lumen of the pulmonary artery.
- From this anchor point, place a continuous running suture in a descending manner between the anatomical left edge of the pulmonary artery and the corresponding edge of the venotomy. Use an average of four throws for this line followed by one between the 6 o'clock position of the venotomy and the corresponding extremity of the pulmonary artery lumen. Make four more throws to draw the final free edges of the pulmonary artery and venotomy together.
- Tie off the free end of the suture to the anchor end using an instrument tie surgeon's knot with two additional throws.
- Reposition the heart to sit centrally in the abdomen using cotton buds. Check the vessels for twisting, which would interfere with blood flow.
- Position gel foam over all the suture lines (Supplemental Figure S4B). Place and mold two pieces of approximately 2 mm each around them such that all the visible suture lines are covered.
- Release the vascular clamps: first the caudal clamp, and then the cephalo-clamp. As a small amount of hemorrhage is to be expected, pre-emptively position cotton-tipped applicators over the anastomotic sites to provide pressure.
- Once free from observable leaks, assess the heart for pulsation (Supplemental Figure S4C). If this is not occurring, check to ensure that no twisting of the cardiac vessels has occurred (especially for the IVC).
- Reposition the intestines now over and around the heart. If appearing dry, moisten the peritoneal cavity using warmed sodium chloride solution.
- Close the abdominal cavity using non-absorbable 6/0 prolene monofilament by layers:
first the muscular layer, and then the skin (Supplemental Figure S4D). Use the continuous non-interrupted technique. - Gently remove the recipient from the surgical board and take it off the anesthetic.
- Administer 1 mL of warm saline subcutaneously and place the recipient in a preprepared cage with warming for observation as per postoperative recovery protocols (Supplemental Figure S4E).
4. Postoperative care
- Immediately after surgery, place the recipient in a clean cage on a heating pad under close observation for at least 3 h. During this period, monitor various parameters (activity, body posture, coat condition, facial expression, gait, ventilation, appearance of the surgical site, presence of a palpable abdominal heartbeat) at least every 30 min. Attribute a score to each parameter (0 = normal, 1 = slightly or intermittently abnormal, 2 = moderately or consistently abnormal).
NOTE: Interventions are triggered by the sum of the monitored parameters exceeding welfare scores specified by the ethics protocol related to this model. - Move the recipients to a warmed cabinet kept at 25 °C, where they remain until post-op day 7. During the first 3 days, monitor them at least 2x daily. Over the remaining 4 days, monitor them at least 1x daily. For post-operative analgesia, administer a dose of buprenorphine (0.5-0.1 mg/kg, diluted to 0.03 mg/mL with sodium chloride 0.9%) subcutaneously to the recipient mouse on the evening post-surgery and twice daily for the next 3 postoperative days.
- Once removed from the warmed cabinet, monitor the recipients at least 2x weekly until the appropriate experimental endpoint.
Representative Results
To determine the effectiveness of the surgical technique in promoting good outcomes of wound healing and mouse recovery, early experiments in the laboratory determined the survival characteristics of a range of heart grafts of variable immunogenicity to the recipient. These included congenic (n = 5) and syngeneic (n = 5) grafts, which share the same major histocompatibility complex (MHC) markers as the recipient, and major mismatch grafts (n = 9), in which the graft and the recipient have different MHC markers. We used direct palpation of the heterotopic abdominal heartbeat to assess ongoing graft function and viability, which serves as a proxy marker of rejection versus tolerance.
In both control groups, all grafts were viable at the experimental time endpoint of 100 days (mean undefined). The mismatched group had a mean survival time of 9 days. Figure 1 presents Kaplan-Meier survival curves demonstrating the stark contrast in graft survival between mismatched and control heart grafts16. These data are suggestive of the technique being sufficient to promote an appropriate healing response following the procedure. In the presence of pathological inflammation, however, in this case represented by graft rejection in the mismatch condition, tissue destruction leads to rapid loss of function.
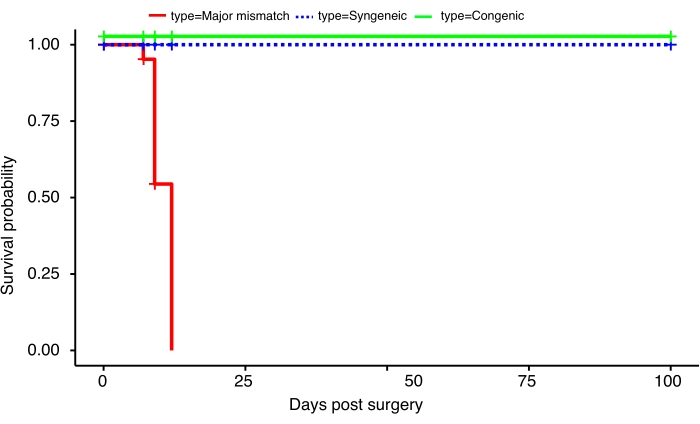
Figure 1: The influence of mismatch on the survival of orthotopic heart transplants. Survival curves illustrating the full recovery and acceptance of syngeneic (n = 5) and congenic (n = 5) heterotopic murine heart transplants for at least 100 days post-surgery in contrast to the rapid rejection of major mismatched (n = 7) heterotopic murine heart transplants from as early as day 7 post surgery. These data were published in Prosser et al.16. Please click here to view a larger version of this figure.
| Surgery Stage | Cold Ischemia time | Warm Ischemia time |
| Donor | 13 – 15 min | |
| Storage 4 °C | 20- 25 min | |
| Recipient | 22 – 25 min |
Table 1: Range of warm and cold ischemia times for donor and recipient surgeries associated with the orthotopic heart transplant.
Supplemental Figure S1: Key aspects of donor surgery. (A) Isoflurane anesthesia; (B) heparin injection; (C) donor heart exposed; (D) flush of the heart with heparinized saline; (E) tying of the vessel; (F) donor heart for cold ischemia storage. Please click here to download this File.
Supplemental Figure S2: Key aspects of recipient surgery-preparation and cauterization of cut skin vessels. (A) Recipient surgical site preparation; (B) bupivacaine injection; (C) sterile surgical draping of the surgical site; (D) cauterization of the cut skin vessels. Please click here to download this File.
Supplemental Figure S3: Key aspects of recipient surgery-from repositioning of intestines to aortotomy. (A) Temporary repositioning of intestines; (B) inferior vena cava exposed and clamped; (C) placing the stay suture; (D) first phase: aortotomy. Please click here to download this File.
Supplemental Figure S4: Key aspects of recipient surgery-from venotomy to recovery. (A) Second phase: venotomy; (B) placing the gel foam; (C) reperfusion; (D) surgical closure; (E) recovery. Please click here to download this File.
Discussion
The murine orthotopic heart transplant model is a robust preclinical model used primarily to investigate the effects of MHC mismatch on the level and nature of immunological rejection and, more recently, the effect of transplantation on the retention of graft tissue-resident immunity16. While initially closely following the Corry et al.7 protocol, we have refined the protocol to incorporate best-practice standards of aseptic technique, analgesia, and anesthesia. The updating of these new practices was achieved via additional training, the provision of sterile surgical gloves, gowns, and surgical drapes, the application of additional anesthesia, and the updating of the analgesia dosing. Such changes led to a small increase in surgical setup time and additional costs per surgery.
The use of animals to address important research concerns is permitted under a contract between researchers and an animal ethics committee (AEC) to maintain a social license to undertake such work. Decisions of an AEC are based on clear ethical guidelines15, with an overriding principle of balancing the costs to the animal against the benefits to society. The concept of the three Rs (reduction, replacement, and refinement) is vital in addressing how the costs of a project are mitigated.
Minimizing the harm to the animals involved by the adoption of species-appropriate, perioperative analgesia and anesthesia has an irreplaceable role in animal models of surgery and is an example of refinement. Additionally, care and techniques that reduce the risk of environmental and behavioral vectors of infection to the surgical recipient have positive implications for both reducing the harm to the animal in terms of morbidity and mortality and helping to minimize the financial costs associated with repeating failed surgeries. Although the cleanliness of the experimental animal "operating theater" does not closely approach that of a hospital equivalent, it should not be an afterthought in such work.
From a scientific perspective, postoperative infections necessarily influence the profile of inflammatory cytokines and immune cells, which are the typical readouts for experiments assessing transplant recovery or rejection. Maximal effort should, therefore, be made to control for postoperative infection, given the adverse impact this can have on the validity of the research. The focus on analgesia is important from an animal welfare point of view. Animal transplantation surgeries are major procedures, and great effort should be made in reducing unnecessary pain and suffering of the subjects. To return to the practical outcomes of this focus, an additional practical benefit of effective pain control is the reduced likelihood of animals being removed from the experimental protocol due to pain-associated signs of distress.
Since this procedure was first described, several authors have reported the troubleshooting of common problems that occur during the procedure10,11. The control of hemorrhage following the release of the clamps is well described and mirrors techniques used in human surgeries, namely the use of pressure to the site of hemorrhage, further suturing, and hemostatic agents. We have noticed that bleeding often occurs from one of two main sites: the anastomosis sites or damage to the myocardium. Approaches to halt bleeding from the heart have been reported by Niimi10, who controlled bleeding from the heart through ligation of the atrium. In our experience, the stemming of blood flow from the myocardium itself is exceptionally challenging due to its rich vascularization.
Due care must be exercised, therefore, to avoid such injury, which is caused most commonly by a miscontrolled forceps tip contacting the heart muscle during surgery. We, therefore, seek to only ever directly contact the heart muscle using moistened cotton-tipped applicators. To reduce direct contact in the manipulation of the heart, the free ends of the final silk ligation can be used to move the heart, such as when moving it from UWS to the thoracic cavity.
A second major challenge is the prevention of postoperative hind-limb paralysis, a complication that mandates euthanasia. Anecdotally, we have found that a warm ischemic time of >30 min is associated with a higher risk of this paralysis occurring. Our ischemic times are strictly monitored and recorded as an informal standard of performance. It should be noted, however, that ischemic time does not seem to reliably predict this complication. Niimi10, for example, a surgeon of substantial operative experience (over 3,000 surgeries), reported that ischemic times of up to 2 h are acceptable.
Perhaps even more startling than this, Abbott et al.5, who developed a similar technique in rats but used an end-to-end anastomotic setup in the abdomen (i.e., the IVC and abdominal aorta were ligated permanently), reported on two rats kept as long-term survivors lasting over 100 days without any apparent ill effects. These intergroup differences in outcomes are perhaps explained by subtly different techniques or, alternatively, by the genetic differences between different strains of mice. For example, we note that Ly5.1 mice are much more susceptible to this complication than BALB/c mice. To improve clarity on the effects of ischemic time on incidences of hind-limb paralysis, the effect of abdominal vessel occlusion time length could be investigated.
In summary, this described protocol provides straightforward refinements to established techniques using readily available drugs and materials. These refinements align the standard to which this surgical model is performed to that of clinical veterinary standards and benefit the animals and, ultimately, the research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the superb efforts of the animal care staff of the University of Western Australia and of the Harry Perkins Institute of Medical Research, whose dedication and expertise contributed to the feasibility and success of these surgeries.
Materials
| 2030 Rycroft irrigating cannula 30 G | McFarlane | 56005HU | |
| Braided surgical silk 7-0 | |||
| Bulldog clamp curved – 35 mm | Roboz | RS-7441-5 | |
| Bupivacaine 0.25% | |||
| Buprenorphine | |||
| Castroviejo needle holder catch curved - 145 mm | Haag-Streit | 11.62.15 | |
| Chlorhexidine 5% solution | Ebos | JJ61371 | |
| Cotton-tipped applicator – 7.5 cm | Dove | SN109510 | |
| Ethanol 70% solution | Ebos | WH130192EE | |
| Gauze 5 x 5 cm white | Aero | AGS50 | |
| Gelfoam 80 mm x 125 mm | Pfizer | 7481D | |
| Hair clipper | Wahl | 9860L | |
| Heparin 1,000 IU in 1 mL | |||
| Iris SuperCut scissors straight – 11.5 cm | Inka Surgical | 11550.11 | |
| Isoflurane vaporiser | Darvall | 9176 | |
| Micro bulldog clamp – 3.7 cm | Greman | 14119-G | |
| Micro scissors curved 105 mm | |||
| Micropore plain paper surgical tape – 2.5 cm wide | Ebos | 7810L | |
| Microsurgical scissors – curved tip | |||
| Monofilament polyprolene suture – 5/0 | Surgipro | P-205-X | |
| Myweigh i101 Precision Scale 100 g x 0.005 g | Myweigh | Kit00053 | |
| Needle – 30 G x 0.5 inch | BD | BD304000 | |
| Needleholder 15 cm curved "super fine" | Surgical Specialists | ST-B-15-8.2 | |
| Nylene 10/0 x 15 cm on 3.8 mm 3/8 circle round bodied taper (diam 0.07mm) CV300 | |||
| Round body suture forceps curved 0.3 mm 120 mm | B. Braun | FD281R | |
| Round body suture forceps straight 0.3 mm 120 mm | B. Braun | FD280R | |
| Round handled vannas spring scissors-str/12.5 cm | 15400-12 | ||
| Spring scissors-Cvd Sm blades | 15001-08 | ||
| Stevens scissors blunt straight 110 mm | |||
| Surgical backboard | Rigid laminated cardboard. 15 x 15 cm | ||
| Surgical drapes | Cut into two sizes. 25 cm x 25 cm, and 25 cm x 40 cm | ||
| Surgical microscope | |||
| Syringe – 1 mL | BD | 592696 | |
| Syringe – 3 mL | Leica | M651 | |
| Toothed forceps | BD | 309657 | |
| University of Wisconsin Solution | |||
| Warming pad | Far infrared warming pad 20 x 25 cm | ||
| Westcott spring scissors | |||
| Yasargil clip applier bayonet | Aesculap | FE582K | |
| Yasargil titanium clip perm 6.6 mm | Aesculap | A19FT222T | |
| Mouse usage | |||
| Strain/SEX/Weight | Donor | Recipent | |
| BALB/c, female, 19-23 g | 7 | 21 | |
| C57BL/6, female, 17-20 g | 7 | 0 | |
| CD45.1 BALB/c, female, 17-21 g | 5 | 0 |
References
- Mann, F. C., Priestley, J. T., Markowitz, J., Yater, W. M. Transplantation of the intact mammalian heart. Archives of Surgery. 26 (2), 219-224 (1933).
- Neptune, W. B., Cookson, B. A., Bailey, C. P., Appler, R., Rajkowski, F. Complete homologous heart transplantation. A.M.A. Archives of Surgery. 66 (2), 174-178 (1953).
- Downie, H. G. Homotransplantation of the dog heart. A.M.A. Archives of Surgery. 66 (5), 624-636 (1953).
- Blanco, G., Adam, A., Rodriguezperez, D., Fernandez, A. Complete homotransplantation of canine heart and lungs. A.M.A. Archives of Surgery. 76 (1), 20-23 (1958).
- Abbott, C. P., Lindsey, E. S., Creech, O., Dewitt, C. W. A technique for heart transplantation in the rat. Archives of Surgery. 89, 645-652 (1964).
- Ono, K., Lindsey, E. S. Improved technique of heart transplantation in rats. The Journal of Thoracic and Cardiovascular Surgery. 57 (2), 225-229 (1969).
- Corry, R. J., Winn, H. J., Russell, P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 16 (4), 343-350 (1973).
- Joffre, O., et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nature Medicine. 14 (1), 88-92 (2008).
- Gregori, S., et al. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. The Journal of Immunology. 167 (4), 1945-1953 (2001).
- Niimi, M. The technique for heterotopic cardiac transplantation in mice: Experience of 3000 operations by one surgeon. The Journal of Heart and Lung Transplantation. 20 (10), 1123-1128 (2001).
- Hasegawa, T., Visovatti, S. H., Hyman, M. C., Hayasaki, T., Pinsky, D. J. Heterotopic vascularized murine cardiac transplantation to study graft arteriopathy. Nature Protocols. 2 (3), 471-480 (2007).
- Hoogstraten-Miller, S. L., Brown, P. A. Techniques in aseptic rodent surgery. Current Protocols in Immunology. 82, 1-14 (2008).
- Navarro, K. L., et al. Mouse anesthesia: The art and science. ILAR Journal. , (2021).
- Adams, S., Pacharinsak, C. Mouse anesthesia and analgesia. Current Protocols in Mouse Biology. 5 (1), 51-63 (2015).
- Australian code for the care and use of animals for scientific purposes. NHMRC Available from: https://www.nhmrc.gov.au/about-us/publications/australian-code-care-and-use-animals-scientific-purposes (2013)
- Prosser, A., et al. Dynamic changes to tissue-resident immunity after MHC-matched and MHC-mismatched solid organ transplantation. Cell Reports. 35 (7), 109141 (2021).

