Quantitative Measure of Lung Structure and Function Obtained from Hyperpolarized Xenon Spectroscopy
Summary
The manuscript presents a detailed protocol for using hyperpolarized Xenon-129 chemical shift saturation recovery (CSSR) to trace pulmonary gas exchange, assess the apparent alveolar septal wall thickness, and measure the surface-to-volume ratio. The method has the potential to diagnose and monitor lung diseases.
Abstract
Hyperpolarized Xenon-129 (HXe) magnetic resonance imaging (MRI) provides tools for obtaining 2- or 3-dimensional maps of lung ventilation patterns, gas diffusion, Xenon uptake by lung parenchyma, and other lung function metrics. However, by trading spatial for temporal resolution, it also enables tracing of pulmonary Xenon gas exchange on a ms timescale. This article describes one such technique, chemical shift saturation recovery (CSSR) MR spectroscopy. It illustrates how it can be used to assess capillary blood volume, septal wall thickness, and the surface-to-volume ratio in the alveoli. The flip angle of the applied radiofrequency pulses (RF) was carefully calibrated. Single-dose breath-hold and multi-dose free-breathing protocols were employed for administering the gas to the subject. Once the inhaled Xenon gas reached the alveoli, a series of 90° RF pulses was applied to ensure maximum saturation of the accumulated Xenon magnetization in the lung parenchyma. Following a variable delay time, spectra were acquired to quantify the regrowth of the Xenon signal due to gas exchange between the alveolar gas volume and the tissue compartments of the lung. These spectra were then analyzed by fitting complex pseudo-Voigt functions to the three dominant peaks. Finally, the delay time-dependent peak amplitudes were fitted to a one-dimensional analytical gas-exchange model to extract physiological parameters.
Introduction
Hyperpolarized Xenon-129 (HXe) magnetic resonance imaging (MRI)1 is a technique that offers unique insights into lung structure, function, and gas exchange processes. By dramatically amplifying the magnetization of Xenon gas through spin-exchange optical pumping, HXe MRI achieves an order-of-magnitude improvement in signal-to-noise ratio compared to thermally polarized Xenon MRI2,3,4,5,6. This hyperpolarization enables the direct visualization and quantification of Xenon gas uptake into lung tissue and blood, which would otherwise be undetectable with conventional thermally polarized MRI7.
Chemical shift saturation recovery (CSSR) MR spectroscopy8,9,10,11,12,13 has proven to be one of the most valuable HXe MRI techniques. CSSR involves selectively saturating the magnetization of Xenon dissolved in lung tissue and blood using frequency-specific radiofrequency (RF) pulses. The subsequent recovery of the dissolved-phase (DP) signal as it exchanges with fresh hyperpolarized Xenon gas in the airspaces on a timescale of ms offers important functional information about the lung parenchyma.
Since its development in the early 2000s, the techniques behind CSSR spectroscopy have been progressively refined14,15,16,17,18,19,20,21,22,23. Further, advances in modeling Xenon uptake curves have enabled the extraction of specific physiological parameters, such as alveolar wall thickness and pulmonary transit times10,24,25,26. Studies have shown CSSR's sensitivity to subtle changes in lung microstructure and gas exchange efficiency in the form of pulmonary abnormalities found in clinically healthy smokers27, as well as in a range of lung diseases, including chronic obstructive pulmonary disease (COPD)18,27,28, fibrosis29, and radiation-induced lung injury30,31. CSSR spectroscopy has also been demonstrated to be sensitive to detect oscillations in the DP signal corresponding to pulsatile blood flow during the cardiac cycle32.
While significant progress has been made, practical challenges remain in implementing CSSR spectroscopy on clinical MRI systems. Scan times requiring single-dose breath holds approaching 10 s may be too long for pediatric subjects33,34 or patients with severe lung disease35,36. Additionally, the technique is susceptible to measurement biases if acquisition parameters such as the order of the saturation delay times or the efficacy of the dissolved-phase saturation are not properly optimized21. To address these limitations and make CSSR more accessible to the broader research community, clear, step-by-step protocols for both conventional breath hold and free-breathing acquisitions, currently under development, are needed.
The objective of this paper is to present a detailed methodology for performing optimized CSSR MR spectroscopy using HXe gas. The protocol will cover polarization and delivery of the Xenon gas, RF pulse calibration, sequence parameter selection, subject preparation, data acquisition, and key steps in data analysis. Examples of experimental results will be provided. It is hoped that this comprehensive guide will serve as a foundation for CSSR implementations across sites and help realize the full potential of this technique for quantifying lung microstructural changes in a range of pulmonary diseases.
Protocol
NOTE: While the hyperpolarized Xenon-129 CSSR MR spectroscopy technique described here is commonly used for animal and human imaging, the protocol below refers to human studies only. All imaging protocols adhered to FDA specific absorption rate (SAR) limitations (4 W/kg) and were approved by the Institutional Review Board at the University of Pennsylvania. Informed consent was obtained from each subject.
1. Pulse sequence design
- Decide whether to perform a breath hold or free breathing measurement.
NOTE: Breath hold acquisitions are technically simpler because they only require the inhalation of a single dose (500 – 1000 mL) of HXe gas followed by a 10 s breath hold during which the MRI data are collected. However, uncooperative subjects (e.g., young children) or patients with severe lung disease may be unable to hold their breath this long, so a free breathing acquisition entailing the inhalation of multiple, small doses (~50 mL) over the course of a few minutes may be advisable. - For a breath hold CSSR MR spectroscopy study, use variable delay times for maximum flexibility, and high excitation flip angles of up to 90° for maximum signal-to-noise ratios (Figure 1A).
- To saturate the DP magnetization on a 1.5 T MRI scanner, apply 5 rectangular 90° radio frequency (RF) pulses with center frequency, duration of 198 ppm, 2.5 ms and 218 ppm, 2.5 ms for 2 pulses and center frequency, duration of 208 ppm, 2.0 ms for the remaining 3 pulses. If permitted by the RF power amplifier, shorten the duration of the RF pulses for measurements at higher field strengths.
- Separate all RF pulses by 1 ms gradient spoilers, alternating along the x, y, and z-axes: 200 µs ramp time, 600 µs plateau time, 20 mT/m.
- After the final saturation pulse, wait for a delay time τi, where i refers to the i-th measurement in the breath hold. Use the following delay times in the prescribed order: 50, 2.5, 2.5, 2.5, 3.5, 5, 7.5, 50, 10, 15, 30, 60, 50, 80, 100, 120, 160, 50, 200, 250, 350, 500, 50, 5, 6, 8, 50, 12.5, 20, 40, 70, 50, 90, 110, 140, 180, 50, 225, 300, 400 ms.
- Apply a 1.2 ms Gaussian RF excitation pulse centered at 208 ppm. Set the flip angle to 90°. If the RF amplifier does not permit this, use the maximum flip angle that the amplifier allows. Scale the length of the RF excitation pulses inversely proportional to the field strength for measurements on high-field scanners.
- Sample the free induction decay for 30.72 ms (1024 sampling points). While the gas-phase T2* at 1.5 T is on the order of 15 ms, reduce the sampling duration significantly at higher field strengths without the need for additional signal apodization prior to processing.
- Apply a 5 ms gradient spoiler along the x-axis: 200 µs ramp time, 4.6 ms plateau time, 20 mT/m.
- Repeat steps 1.2.1 – 1.2.6 40x with a different τi during the same breath hold as described in step 1.2.3.
- For a free breathing CSSR MR spectroscopy study, conduct the following measurement continuously for approximately 3 min (Figure 1B), although the acquisition can be terminated earlier if the allotted HXe gas volume runs out.
- Repeat steps 1.2.1 and 1.2.2. Repeat step 1.2.4 with a flip angle to 7°. Sample the free induction decay for 10.24 ms (512 sampling points).
- Apply a 1 ms gradient spoiler along the x-axis: 200 ms ramp time, 600 ms plateau time, 20 mT/m. Repeat steps 1.2.3 – 1.2.5 40x with a repetition time of 12.6 ms.
- Repeat steps 1.2.1 – 1.2.6 until the end of the study.
2. Preparation for patient examination
- Prior to each study, ensure a clean facemask is prepared and connected to the gas delivery synchronization device using thin, flexible tubing.
- For free-breathing studies attach a bi-directional pneumotach for flow measurements.
- Perform a routine test using a glass syringe to mimic breathing in order to verify proper gas injection. The gas delivery device should detect the beginning of inhalation from the flow measurements of the pneumotach, enabling gas injection into the mask.
- Set up the optional physiological monitoring system that records breathing curves (flow and volumes) and real-time gas analysis (O2 and CO2) during imaging.
- Connect and test the MRI room headphones with the audio signal that guides the subject using an inhale-exhale audio recording. Adjust the playback speed of the audio track based on the normal breathing rate of each subject.
- Prepare the scanner bed with a clean head rest, leg support pillow, and blanket.
- Place the unfastened Xenon-129 chest vest coil on the table of the MRI scanner. Insert the connector plug of the coil and ensure that the MR scanner recognizes the coil.
3. Subject preparation and monitoring
- When the subject arrives at the imaging facility, obtain written informed consent using an IRB approved consent form. Once consent is obtained, screen the subject using an MRI safety questionnaire and a metal detector.
- Ask the subject to remove any metal or jewelry from their person and to change into a patient gown.
- Train the subject to adhere to the selected breathing protocol (breath hold or free breathing).
- For a free breathing study, introduce the subject to the inhale-exhale voice recording that will be played during imaging, and with which they should synchronize their breathing.
- Lead the subject into the MRI room and position them on the scanner bed: lying on top of the open Xenon vest coil.
- Once the subject is positioned, fasten the Velcro straps so that the vest coil is closed but does not constrict the subject's chest.
- For a free breathing study, place a face mask with a pneumotach over the patient's face and tighten the straps such that the mask fits snuggly over the nose and mouth without being too tight. After fitting, remove the mask and set aside for later, leaving the straps behind the subject's head.
- Place two pulse oximeters on the subject's right and left index fingers, respectively, to continuously monitor and record heart rate and blood oxygen saturation (SPO2) throughout the duration of the study.
- Place MRI-compatible headphones over the subject's ears.
- Move the MRI scanner table into the magnet bore such that the subject's lungs are positioned in the center of the field of view.
4. Hyperpolarized Xenon-129 polarization (Calibration gas)
NOTE: The following are the protocol steps for polarizing Xenon-129 gas using our polarizing device. Adjust according to the vendor-specific operating instructions for your installed gas polarizer.
- Approximately 2.5 h before the study starts, heat up the Xenon polarizer. Since Xenon gas, especially enriched >85% Xenon-129, is very expensive (currently ~$500 per L) and cannot be recaptured once polarized, the polarization process should only be started once the subject has arrived at the imaging site.
- Thread the connector tube of a 250 mL specialized PVF bag through a sealing clip. Ensure that the clip does not pinch the tube.
- Attach the specialized PVF bag to one of the four available polarizer dispense ports.
- On the polarizer touch screen, select the enriched Xenon tank, set the flow rate to medium, and set the polarization volume to 250 mL.
- Press the Start button to initiate the polarization process. The actual polarization procedure, Xenon freeze out, thawing, and dispensing into the specialized PVF bag, is fully automatic and takes about 15 min for 250 mL of Xenon.
- When the polarized Xenon gas has been dispensed, the polarizer will display a message on the touch screen stating that the bag can now be removed.
- Pinch the connector tube of the specialized PVF bag shut with the sealing clip. Disconnect the specialized PVF bag and quickly place it inside the bore of the MRI scanner to prevent rapid gas depolarization.
5. Hyperpolarized Xenon-129 inhalation for calibration
- Place a nose clip on the subject's nose to improve respiration through the mouth.
- At the end of normal expiration, insert the mouthpiece of the Xenon bag into the subject's mouth.
- Once the subject has inhaled 250 mL of Xenon dose from the bag, remove the mouthpiece and instruct the subject to continue inhaling room air until their lungs are full.
- At end inspiration, ask the subject to raise their thumb, and the nurse coordinator to verbally relay this information to the scanner operator to initiate the pulse sequence.
- For subjects that are unable to hold their breath, ask the nurse coordinator to observe the subject's chest motion and inform the operator when the subject has reached end exhalation and begins inspiration. While this approach decreases the measurement signal due to the partial exhalation of inspired Xenon gas, it ensures that the volume of Xenon within the subject's lungs remains fairly constant during acquisition of the calibration data.
- At the end of the data acquisition period (~5 s), instruct the subject to breathe normally again.
6. Gas frequency and radio frequency pulse voltage calibration
NOTE: Before executing a pulse sequence, modern MRI scanners usually calibrate the on-resonance frequency of the MR signal and the voltage to be applied to the transmit RF coil to achieve the desired flip angle for the excitation pulses. In conventional proton MRI, this calibration process is automatic and typically transparent to the user. However, this automatic calibration is not feasible for hyperpolarized Xenon-129 studies, as there is no signal source at thermal equilibrium available. Instead, the frequency and voltage for the RF pulses must be manually calibrated. On the MRI scanner used here, this manual calibration is done by supplying a reference voltage, which the scanner's software then uses to calculate the appropriate voltage for all subsequent RF pulses. Consult the vendor-specific operating instructions for the MRI system to understand how to input this calibration data into the measurement software.
- Load a proton scout pulse sequence. Select a field of view of 400 mm. Acquire 10 coronal slices (10 mm slice thickness, 20% gap).
- Review the proton images and ensure that the subject's lung is centered in the field of view. If necessary, reposition the subject and repeat step 1.
- Load the calibration pulse sequence. Use the HXe gas-phase (GP) frequency from the most recent human scan as a starting estimate of the receiver frequency.
- Set the reference voltage to a value such that the GP signal between the first and the last spectra acquired with the calibration sequence decreases by approximately 70% – 80% for most subjects. For the chest RF coil, set the initial reference voltage to 75 V.
- Start the sequence when the subject has inhaled the HXe calibration dose and is in breath hold or, if a breath hold cannot be achieved, when the subject is past the point of end expiration in the breathing cycle.
- Apply a 1.2 ms Gaussian RF excitation pulse centered at 0 ppm. Set the nominal flip angle to 90°. However, because the initial reference voltage is set far below its true value, the flip angle actually applied is around 15°.
- Sample the free induction decay for 30.72 ms (1024 sampling points). Apply a 20 ms gradient spoiler along the x-axis: 500 ms ramp time, 19 ms plateau time, 20 mT/m. Note that these gradient specifications are not optimized, shorter gradient durations are likely to suffice.
- Repeat steps 6.5.1.-6.5.2. 16 times with a repetition time of 55 ms. Again repeat steps 6.5.1-6.5.2. 16 times with a repetition time of 220 ms.
- Once data acquisition is complete, instruct the subject to return to normal breathing.
- Assess the subject's well-being by checking the SPO2 level and ask about any potential adverse reactions.
- Download the measured calibration data onto a USB drive, then transfer it to a laptop for further analysis.
- Use a MATLAB script to extract the center frequency of the GP peak, the flip angle of the RF excitation pulses, and the HXe gas T1 inside the lung.
- Load the 32 FIDs acquired by the calibration sequence. Use Fast Fourier Transforms (FFTs) to convert the FIDs to spectra.
- Phase the GP peaks to zeroth order. Fit a pseudo-Voigt line shape to the phased real component of the GP peaks.
- Calculate the GP frequency as the average over the center frequencies of the first 10 fits, as these have the highest signal-to-noise ratio. Output the frequency average on the screen.
- Integrate the area underneath all GP peaks. Fit mono-exponential decay functions to the first 16 and second 16 GP peak areas.
- Extract GP T1 and applied flip angle from the two fitted decay curves.
7. Hyperpolarized Xenon-129 polarization (measurement gas)
- For polarizing the measurement gas follow steps 4.2 – 4.7, with the following modifications:
- Use a 500 mL specialized PVF bag instead of a 250 mL bag.
- Set the polarization volume to 500 mL instead of 250 mL. The polarization process takes about 20 min for 500 mL.
8. Hyperpolarized Xenon-129 inhalation for measurement (Breath hold)
- Place a nose clip on the subject's nose to improve respiration through the mouth.
- At the end of normal expiration to functional residual capacity, insert the mouthpiece of the Xenon bag into the subject's mouth.
- Once the subject has inhaled 500 mL of Xenon gas from the Xenon bag, remove the mouthpiece and instruct the subject to continue inhaling room air until their lungs are full.
- At end inspiration, ask the subject to raise their thumb, and the nurse coordinator to verbally relay this information to the scanner operator to initiate the pulse sequence.
- At the end of the data acquisition period (~8 s), ask the subject to breathe normally again.
9. Hyperpolarized Xenon-129 inhalation for measurement (Free breathing)
- For the measurement scan, move the subject out of the MRI scanner, place the face mask over their nose and mouth, and connect the pre-fitted straps from behind the head to the mask, securing the mask in place. The pneumotach on the mask will detect the subject's successive inhalations and exhalations and will trigger the gas delivery system to dispense gas when an inhalation is detected.
- Move the subject back to their original position inside the scanner.
- Play the inhale-exhale audio recording so that the subject can synchronize their breathing pattern with the breathing protocol.
- Once the subject has fallen into rhythm with the breathing protocol, ask the nurse coordinator to inform the MRI operator to start the data acquisition. The nurse coordinator then opens the valves on the gas delivery system and the subject begins inhaling 50 mL of hyperpolarized Xenon-129 that mixes with the air flow inside the breathing mask.
- Ask the patient to continue for approximately 10 breaths until the Xenon gas volume has been used up for the imaging protocol.
10. Measurement data acquisition (Breath hold)
- Load the CSSR pulse sequence for breath hold, as outlined in step 1.2. Set the acquisition frequency according to the HXe GP frequency determined during the calibration scan in step 6.
- Adjust the reference voltage to match the value obtained from the calibration scan described in step 6.
- Choose Wait for User option, or its equivalent, for sequence execution, following the system vendor's operating instructions.
- Initiate the sequence. The MRI scanner will complete the sequence preparation, then pause and wait for the user to commence data acquisition.
- Start the data acquisition when the subject has inhaled the HXe measurement dose, flushed the airways by continuing to inhale room air until their lungs are filled, and has initiated breath hold. The latter should be performed as directed by the nurse coordinator and described in step 5 and step 8.
- Once data acquisition is complete, instruct the subject to return to normal breathing.
- Assess the subject's well-being by checking the SPO2 level and asking about any potential adverse reactions.
- Download the measured CSSR data onto a USB drive, then transfer it to a laptop for further analysis.
11. Measurement data acquisition (Free breathing)
- Load the CSSR pulse sequence for free breathing, as outlined in step 1.3.
- Set the acquisition frequency according to the HXe GP frequency determined during the calibration scan in step 6.
- Adjust the reference voltage to match the value obtained from the calibration scan described in step 6.
- Choose Wait for User option, or its equivalent, for sequence execution, following the system vendor's operating instructions.
- Initiate the sequence. The MRI scanner will complete the sequence preparation, then pause and wait for the user to commence data acquisition.
- Start the data acquisition once the nurse coordinator is ready to switch from room air to HXe gas/air mixture as described in step 9.4. Ensure the sequence is already running before the subject inhales the first dose of Xenon gas.
- Once data acquisition is complete at the end of 3 min of measurement or has been terminated when all the HXe gas has been used, remove the subject from the MRI scanner.
- Assess the subject's well-being by checking the SPO2 level and asking about any potential adverse reactions.
- Download the measured CSSR data onto a USB drive, then transfer it to a laptop for further analysis.
12. CSSR data analysis
NOTE: The acquired data consists of N x 40 free induction decays, where N is the number of times the acquisition was repeated with different delay times after saturation of the DP magnetization. Depending on whether the CSSR measurement was performed as a breath hold or a free breathing study, N is either 1 or the number of times the acquisition was repeated, respectively, and should total approximately 2 x the measurement time in s. However, the subsequent data analysis for both scenarios via MATLAB scripts is essentially identical except where indicated.
- Load the FIDs acquired by the CSSR sequence. Use Fast Fourier Transforms (FFTs) to convert the FIDs to spectra.
- Phase the GP peaks to zeroth order. Phase the DP peaks to first order.
- Fit a pseudo-Voigt line shape to the phased real component of the GP peaks.
- For free breathing measurements, divide all spectra by the area underneath the fitted GP peaks. Average all spectra with the same delay time.
- In all spectra, fit two pseudo-Voigt line shapes to the phased real components of the membrane peaks at ~196 ppm and the red blood cell peaks at ~217 ppm.
- Integrate the areas underneath the fitted DP peaks.
- For breath hold measurements, acquire the 50 ms delay time measurement repeatedly (see step 1.2.8), which allows for a more precise decay correction than normalization with the GP signal.
- Fit an exponential decay function to the membrane peak signal as a function of the acquisition index.
- Multiply all membrane and red blood cell peak signals by the inverse of the fitted exponential decay function for the respective acquisition index.
- Fit the corrected membrane and red blood cell signals as a function of their delay time to a Xenon gas uptake model. The two most commonly used models are the ones proposed by Patz et al.24 and Chang et al.25,37,38. We typically analyze data using the Patz model.
- Fit to either model to obtain the alveolar surface-to-volume ratio, the apparent alveolar septal wall thickness, and the capillary transit time. In addition, the model of Xenon exchange (MOXE) proposed by Chang et al. yields the thickness of the barrier between vessels and alveolar volume as well as the hematocrit.
Representative Results
Figure 2 illustrates a typical Xenon spectrum observed in the human lung during a breath hold, subsequent to the inhalation of 500 mL of Xenon dose. The spectrum displays two distinct regions, the GP resonance around 0 ppm, and the DP region, which consists of the membrane peak at approximately 197 ppm and the red blood cell peak at approximately 217 ppm. The relative peak amplitudes depend on a number of factors including the shape, duration, and center frequency of the RF excitation pulse as well as the delay time between saturation and excitation. Generally, the longer the delay, the larger the DP peaks are relative to the GP peak. This is because a longer delay allows more time for the Xenon magnetization to transfer from the alveolar volume to the lung parenchyma. Additionally, the red blood cell peak tends to decrease relative to the membrane peak with increasing age and disease severity.
For whole-lung Xenon spectra, the line shape of the observable resonances is not Lorentzian but can usually be approximated reasonably well by complex pseudo-Voigt functions as illustrated for the real and imaginary dissolved-phase signal components in Figure 3A-B. The area underneath all peaks in the spectra can therefore be calculated analytically. Over the course of a breath hold the Xenon concentration in the alveolar airspaces remains nearly constant except for a small quantity of Xenon that is removed via dissolution in the lung tissue and removal by the pulmonary circulation. However, the alveolar Xenon magnetization decreases considerably due to oxygen-induced T1 relaxation, and due to DP Xenon, which has been depolarized by the RF saturation pulses, exchanging back into the GP compartment. Consequently, the DP signal at the periodically acquired delay time of 50 ms shows an approximate exponential decrease. In healthy volunteers, it drops by 3%-4% per spectrum over the course of the measurements (see Figure 3C). After fitting the signal decrease with an exponential decay function, the DP signal for all delay times can be corrected by multiplying each acquisition with the inverse of the fitting function.
The breath-hold CSSR technique has been successfully used for over 20 years and applied to hundreds of human and animal subjects, primarily for detecting pathological increases in apparent alveolar septal wall thickness. The method has proven to be robust in practical use, not least because it eliminates the need for subject-specific parameter adjustments, requiring only accurate calibration as outlined in step 6. In contrast, the free-breathing protocol, which we are introducing for the first time in humans, aims to quantify lung function in individuals who cannot hold their breath; this approach is still under active development and has been primarily tested in a limited number of unpublished animal studies. In two example cases-a healthy 20-year-old female and a 69-year-old male lung transplant recipient-we utilized the breath-hold CSSR MR spectroscopy technique. Figure 4 illustrates the differences in the Xenon gas uptake curves for these 2 subjects. In the young, healthy subject the membrane magnetization rises very rapidly at short delay times due to a thin alveolar septal wall thickness of 6.1 µm (Figure 4A). Once the wall is saturated by the inflow of polarized Xenon gas, the signal buildup continues in an approximately linear fashion as the capillary blood flow transports increasing amounts of Xenon dissolved in the blood plasma downstream. In the older lung transplant recipient, the membrane signal build up is much more gradual, corresponding to a septal wall thickness of 10.3 µm (Figure 4B).
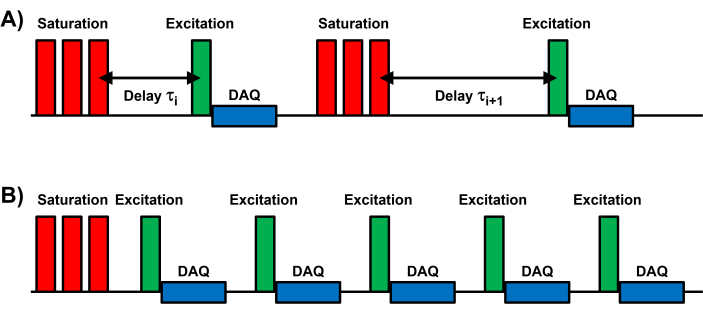
Figure 1: Schematic of the CSSR MR spectroscopy pulse sequence. All CSSR pulse sequences consist of three components: 1) Saturation of the DP magnetization; 2) subsequent RF excitation after variable delay times; 3) sampling of the free induction decay (DAQ). (A) Schematic for a breath-hold acquisition, where each measurement consists of an individual saturation-excitation module and an independent delay time τi. (B) Schematic for a free-breathing acquisition, in which each DP saturation is followed by a series of equidistantly spaced RF excitations and data sampling periods. Please click here to view a larger version of this figure.
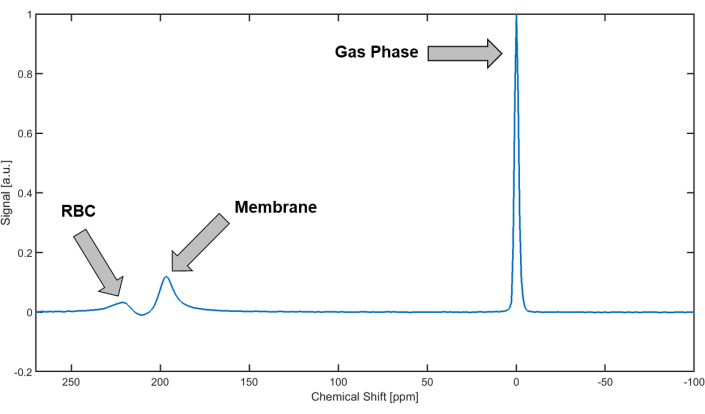
Figure 2: Xenon-129 spectrum from the human lung. In the human lung, Xenon-129 produces three distinct peaks: 1) The gas-phase peak at 0 ppm (by definition); 2) the membrane peak at approximately 197 ppm; 3) the red blood cell (RBC) peak of Xenon-129 bound to hemoglobin at approximately 217 ppm. Please click here to view a larger version of this figure.
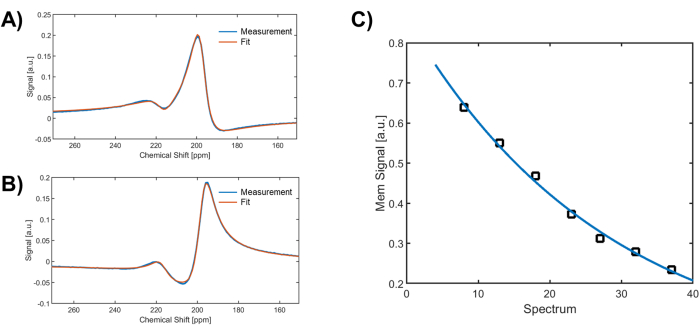
Figure 3: Fitting of the spectroscopic data. The two dissolved-phase peaks at 197 ppm and 217 ppm are usually fitted reasonably well by two complex pseudo-Voigt functions. (A) and (B) depict examples of fitting the real and imaginary components of the dissolved-phase resonances, measured at a 100 ms delay time in a healthy volunteer. (C) Periodic measurement of the membrane signal for the same 50 ms delay time enables correction of the Xenon-129 signal decay during a breath hold, providing a more robust method than normalizing with the GP signal. Please click here to view a larger version of this figure.
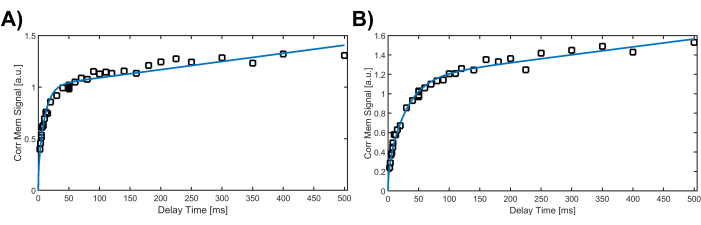
Figure 4: Corrected membrane signal as a function of the delay time after dissolved-phase saturation, fitted with the analytical Patz gas-uptake model. The increase of the corrected membrane signal at short delay times corresponds to the filling of the alveolar septal wall. (A) In the young, healthy female, the membrane signal increases rapidly due to the quick filling of thin walls. (B) In the older lung transplant recipient, the membrane signal rises more gradually, reflecting the longer time required to fill the thicker septal walls. Please click here to view a larger version of this figure.
Discussion
HXe CSSR MR spectroscopy is a powerful technique for assessing several pulmonary function metrics that would be difficult or impossible to quantify in vivo using any other existing diagnostic modality24. Nevertheless, the acquisition and subsequent data analysis are based on certain assumptions about physiological conditions and technical parameters that are never entirely achievable in living subjects. These limitations and their impact on the interpretation of the extracted metrics will be discussed below.
The CSSR technique is typically implemented as a global measurement without spatial encoding, as described in the protocol above. Thus, any Xenon-129 signal within the sensitive volume of the receiver coil, regardless of its origin, contributes to the measurement data and will be implicitly associated with the extracted pulmonary function parameters of the lung parenchyma. Due to the high surface-to-volume ratio within the alveolar airspaces, which facilitates high gas exchange rates between tissue and gas volumes, most of the Xenon-129 DP signal is indeed confined to this region. However, the same is not true for the GP signal. In fact, the conducting airways make up approximately 15% of the total lung volume and, depending on the subject's size and coil positioning, the volumes of the upper trachea, mouth, and nasal cavity may also need to be partially accounted for. The spatial distribution of the Xenon-129 gas at the time of data acquisition is relevant for several reasons, starting with the calibration. Since the B0 field of the main magnet and the B1 field of the RF transmit/receive coil vary spatially, signal contributions from outside the primary region of interest-i.e., the lung volume-can impact both the frequency and voltage calibration. To mitigate these issues, flushing the Xenon gas from the airways by chasing the Xenon dose with a subsequent inhalation of nitrogen or room air is highly advisable.
Accurate calibration faces further complications due to potential amplifier non-linearities and limitations in the maximum power output of the RF amplifier. The application of multiple high flip angle RF saturation pulses can lead to excessive SAR values, particularly when using large volume chest coils or operating at field strengths above 1.5 T. In our studies, the voltage requirements for the vest RF coil were low enough to achieve 90° flip angles using a 4 kW RF amplifier for all but the largest subjects, without exceeding SAR limitations. However, depending on site-specific hardware configurations, additional sequence optimizations may be necessary. These could involve increasing the length of the applied RF saturation pulses, altering the repetition time of the acquisition, or adjusting the flip angle of the pulses. The latter is of special concern because the quality of the CSSR acquisition relies heavily on the effective saturation of the DP signal through the application of one or more 90° RF saturation pulses. Significant deviations from this ideal value can result in artificially elevated DP signals at short delay times, thereby compromising the fit quality of the analytical gas uptake model.
Another potential problem is that the absolute HXe MRI signal amplitudes have no inherent meaning and can only be interpreted following some form of normalization. In practice, this is commonly achieved by dividing the acquired DP signals by the GP signal, under the assumption that all signals originate from the same location. Lastly, GP signal from outside the lung parenchyma has different temporal dynamics, mainly due to cardiogenic motion that periodically pushes Xenon gas up and down the major airways, causing the GP peak to fluctuate in amplitude and width39. However, many of these issues can be overcome in breath hold studies by instructing the subject to continue inhaling room air or nitrogen after the Xenon-129 gas bolus has been administered. In free breathing studies, on the other hand, the signal dynamics are dominated by the bulk motion of the Xenon-129 gas throughout the respiratory cycle and the repeated acquisition of the gas uptake during the measurement averages out many of the potential biases in a single breath hold measurement. Nevertheless, the free-breathing method is still under development and presents certain drawbacks. Compared to the breath-hold technique, it necessitates a more complex setup and consumes more Xenon gas. Given these limitations, this approach is likely to be reserved for cases where breath-hold studies are impractical, such as for critically ill patients, children under the age of 6, and long-term animal studies that struggle with maintaining a tight airway seal. To date, no comparative human studies have been conducted. However, existing rabbit studies suggest that we should not anticipate significant differences in metrics between the breath-hold and free-breathing methods21. The ventilation device employed for the free-breathing studies, while not commercially available, allows for the precise administration of small, well-defined Xenon doses. That said, this specialized equipment is not obligatory. A simpler approach using a continuous flow of Xenon gas mixed with room air or oxygen is also viable, albeit likely to result in greater wastage of hyperpolarized Xenon.
In contrast to free-breathing studies, where delay times after each saturation of the DP magnetization are sequentially ordered, breath-hold measurements offer greater flexibility in the timing of individual spectral acquisitions. Varying the order of short, intermediate, and long delay times over the course of a breath hold acquisition offers several advantages over sequential short-to-long or long-to-short arrangements. For one, even during a perfect breath hold, both heartbeat-induced bulk lung motion and pulsatile variations in blood volume and flow velocity gives rise to fluctuations in the DP signal that is partly averaged out by a non-sequential ordering of the delay times. Furthermore, in case the subject exhales before all data has been collected, a shorter minimum breath hold duration is required to obtain a sufficient number of sampling points along the uptake curve to allow fitting the analytical uptake model. Another potential source of bias is the heterogenous depolarization of the GP magnetization for intermediate and long delay times in regions with above average alveolar septal wall thickness compared to regions with thinner than average walls. As the measurement progresses, those lung volumes with elevated GP depolarization contribute less and less to the global spectroscopic signal, which means that measurement points acquired later in the breath hold correspond to a thinner septal wall thickness than those acquired earlier. This trend can to some extent be counteracted by periodically acquiring data at the same delay time. The changes of the DP-to-GP ratio over the breath hold can be used to compensate the drift towards thinner apparent septal wall thickness as described in step 12.9.
It is important to point out that apparent alveolar septal wall thickness cannot be extracted directly from CSSR measurements. Rather, the analytical uptake models fit L2/D, where L is the apparent septal wall thickness and D is the Xenon diffusion constant within that wall while D is usually estimated to be in the 3 – 3.3 • 10-6 cm2/s range, its exact value and distribution within the alveolar wall is not known. It is also not known whether or how much D changes with disease patterns such as interstitial edema or fibrotic tissue alterations. Moreover, the Xenon gas itself is likely not distributed homogeneously within the alveolar wall, as Xenon is lipophilic, and its chemical solubility varies among the different sub-compartments and structures that compose the septal wall. Further, pathological changes in the apparent septal wall thickness are likely to be spatially heterogeneous at the macroscopic level and CSSR spectroscopy only yields globally averaged metrics. Hence, while it has been demonstrated repeatedly that the CSSR-derived metric of apparent alveolar septal wall thickness is highly sensitive to pathological changes in the alveolar structure, when all these factors are considered together it becomes evident that the metric is sufficiently different from the histologically determined anatomic septal wall thickness, preventing a direct comparison or validation of these two parameters.
For those new to the field of hyperpolarized gas MRI, and CSSR MR spectroscopy in particular, there are several troubleshooting steps available if a measurement fails. A good starting point is to consult the position paper from the Xe-129 MRI clinical trials consortium40. Beyond the potential SAR and RF amplifier power issues already outlined, one common issue is the detection of little or no Xenon signal. To rule out polarizer malfunctions, refer to the operating instructions provided by the device vendor. As a quick quality control step, place the dispensed Xenon gas on top of the coil and perform a rudimentary pulse-and-acquire measurement using a low-voltage pulse within the sequence adjustment module on the scanner; this will confirm sufficient Xenon magnetization for imaging. If Xenon signal is detected but appears unusually low in the subject's lung, ensure that all valves on the ventilation device (if used) are fully open, and that the subject is executing the correct breathing maneuver. Additionally, test any remaining dispensed Xenon by placing it on the coil; the Xenon container might have been contaminated with oxygen, rapidly depolarizing the Xenon magnetization. Finally, as a quick check of dissolved-phase saturation quality, examine the measured Xenon uptake curves (see Figure 4). If the dissolved-phase signal at a delay time of approximately 100 ms is not 2.5x higher than at delay times less than 10 ms, the saturation was likely insufficient. Confirm that the voltage of the applied RF pulses is below the maximum permissible for the RF coil, as exceeding this limit could cap the flip angle below its nominal value.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH grants R01HL159898 and R01HL142258.
Materials
| Bi-directional Pneumotach | B&B Medical AccutachTM | ||
| Chest Vest Coil | Clinical MR Solutions | Adult Size | |
| Face Mask | Hans Rudolph | 7450 | |
| Matlab | Mathworks | Release 2018a | Optimization Toolbox required |
| Physiological Monitoring System | BIOPAC Systems Inc | ||
| Tedlar Bag | Jensen Inert Products | 250-mL and 500-mL; specialised PVF bag | |
| Xenon Polarizer | Xemed LLC | X-box E10 | |
| Whole-body MRI Scanner | Siemens | 1.5 T Avanto |
References
- Albert, M. S., et al. Biological magnetic resonance imaging using laser-polarized 129Xe. Nature. 370 (6486), 199-201 (1994).
- Happer, W. Optical Pumping. Rev Mod Phys. 44 (2), 169-250 (1972).
- Appelt, S., et al. Theory of spin-exchange optical pumping of He-3 and Xe-129. Phys Rev A. 58 (2), 1412-1439 (1998).
- Hersman, F. W., et al. Large production system for hyperpolarized 129Xe for human lung imaging studies. Acad Radiol. 15 (6), 683-692 (2008).
- Parnell, S. R., Deppe, M. H., Parra-Robles, J., Wild, J. M. Enhancement of Xe-129 polarization by off-resonant spin exchange optical pumping. J Appl Phys. 108 (6), 064908 (2010).
- Norquay, G., Collier, G. J., Rao, M., Stewart, N. J., Wild, J. M. ^{129}Xe-Rb spin-exchange optical pumping with high photon efficiency. Phys Rev Lett. 121 (15), 153201 (2018).
- Mugler, J. P., et al. MR imaging and spectroscopy using hyperpolarized 129Xe gas: preliminary human results. Magn Reson Med. 37 (6), 809-815 (1997).
- Ruppert, K., Brookeman, J. R., Hagspiel, K. D., Driehuys, B., Mugler, J. P. NMR of hyperpolarized (129)Xe in the canine chest: spectral dynamics during a breath-hold. NMR Biomed. 13 (4), 220-228 (2000).
- Butler, J. P., et al. Measuring surface-area-to-volume ratios in soft porous materials using laser-polarized Xenon interphase exchange nuclear magnetic resonance. J Phys Condens Matter. 14 (13), L297-L304 (2002).
- Mansson, S., Wolber, J., Driehuys, B., Wollmer, P., Golman, K. Characterization of diffusing capacity and perfusion of the rat lung in a lipopolysaccaride disease model using hyperpolarized 129Xe. Magn Reson Med. 50 (6), 1170-1179 (2003).
- Abdeen, N., et al. Measurement of Xenon diffusing capacity in the rat lung by hyperpolarized (129)Xe MRI and dynamic spectroscopy in a single breath-hold. Magn Reson Med. 56 (2), 255-264 (2006).
- Driehuys, B., et al. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A. 103 (48), 18278-18283 (2006).
- Patz, S., et al. Human pulmonary imaging and spectroscopy with hyperpolarized 129Xe at 0.2T. Acad Radiol. 15 (6), 713-727 (2008).
- Qing, K., et al. Assessment of lung function in asthma and COPD using hyperpolarized 129Xe chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR Biomed. 27 (12), 1490-1501 (2014).
- Stewart, N. J., et al. Reproducibility of quantitative indices of lung function and microstructure from 129 Xe chemical shift saturation recovery (CSSR) MR spectroscopy. Magn Reson Med. 77 (6), 2107-2113 (2017).
- Zhong, J., et al. Simultaneous assessment of both lung morphometry and gas exchange function within a single breath-hold by hyperpolarized (129) Xe MRI. NMR Biomed. 30 (8), (2017).
- Kern, A. L., et al. Regional investigation of lung function and microstructure parameters by localized (129) Xe chemical shift saturation recovery and dissolved-phase imaging: A reproducibility study. Magn Reson Med. 81 (1), 13-24 (2018).
- Kern, A. L., et al. Mapping of regional lung microstructural parameters using hyperpolarized (129) Xe dissolved-phase MRI in healthy volunteers and patients with chronic obstructive pulmonary disease. Magn Reson Med. 81 (4), 2360-2373 (2018).
- Xie, J., et al. Single breath-hold measurement of pulmonary gas exchange and diffusion in humans with hyperpolarized (129) Xe MR. NMR Biomed. 32 (5), e4068 (2019).
- Zanette, B., Santyr, G. Accelerated interleaved spiral-IDEAL imaging of hyperpolarized (129) Xe for parametric gas exchange mapping in humans. Magn Reson Med. 82 (3), 1113-1119 (2019).
- Ruppert, K., et al. Investigating biases in the measurement of apparent alveolar septal wall thickness with hyperpolarized 129Xe MRI. Magn Reson Med. 84 (6), 3027-3039 (2020).
- Zhang, M., et al. Quantitative evaluation of lung injury caused by PM(2.5) using hyperpolarized gas magnetic resonance. Magn Reson Med. 84 (2), 569-578 (2020).
- Friedlander, Y., et al. Hyperpolarized (129) Xe MRI of the rat brain with chemical shift saturation recovery and spiral-IDEAL readout. Magn Reson Med. 87 (4), 1971-1979 (2022).
- Patz, S., et al. Diffusion of hyperpolarized (129)Xe in the lung: a simplified model of (129)Xe septal uptake and experimental results. New J Phys. 13, 015009 (2011).
- Chang, Y. V. MOXE: a model of gas exchange for hyperpolarized 129Xe magnetic resonance of the lung. Magn Reson Med. 69 (3), 884-890 (2013).
- Stewart, N. J., Parra-Robles, J., Wild, J. M. Finite element modeling of (129)Xe diffusive gas exchange NMR in the human alveoli. J Magn Reson. 271, 21-33 (2016).
- Ruppert, K., Qing, K., Patrie, J. T., Altes, T. A., Mugler, J. P. Using hyperpolarized Xenon-129 MRI to quantify early-stage lung disease in smokers. Acad Radiol. 26 (3), 355-366 (2019).
- Kern, A. L., et al. Investigating short-time diffusion of hyperpolarized (129) Xe in lung air spaces and tissue: A feasibility study in chronic obstructive pulmonary disease patients. Magn Reson Med. 84 (4), 2133-2146 (2020).
- Stewart, N. J., et al. Experimental validation of the hyperpolarized (129) Xe chemical shift saturation recovery technique in healthy volunteers and subjects with interstitial lung disease. Magn Reson Med. 74 (1), 196-207 (2015).
- Fox, M. S., et al. Detection of radiation induced lung injury in rats using dynamic hyperpolarized (129)Xe magnetic resonance spectroscopy. Med Phys. 41 (7), 072302 (2014).
- Li, H., et al. Quantitative evaluation of radiation-induced lung injury with hyperpolarized Xenon magnetic resonance. Magn Reson Med. 76 (2), 408-416 (2016).
- Ruppert, K., et al. Detecting pulmonary capillary blood pulsations using hyperpolarized Xenon-129 chemical shift saturation recovery (CSSR) MR spectroscopy. Magn Reson Med. 75 (4), 1771-1780 (2016).
- Walkup, L. L., et al. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol. 46 (12), 1651-1662 (2016).
- Willmering, M. M., et al. Pediatric (129) Xe gas-transfer MRI-feasibility and applicability. J Magn Reson Imaging. 56 (4), 1207-1219 (2022).
- Amzajerdian, F., et al. Simultaneous quantification of hyperpolarized Xenon-129 ventilation and gas exchange with multi-breath Xenon-polarization transfer contrast (XTC) MRI. Magn Reson Med. 90 (6), 2334-2347 (2023).
- Niedbalski, P. J., et al. Utilizing flip angle/TR equivalence to reduce breath hold duration in hyperpolarized (129) Xe 1-point Dixon gas exchange imaging. Magn Reson Med. 87 (3), 1490-1499 (2022).
- Chang, Y. V. Toward a quantitative understanding of gas exchange in the lung. arXiv. , (2010).
- Chang, Y. V., et al. Quantification of human lung structure and physiology using hyperpolarized 129Xe. Magn Reson Med. 71 (1), 339-344 (2014).
- Collier, G. J., et al. Observation of cardiogenic flow oscillations in healthy subjects with hyperpolarized 3He MRI. J Appl Physiol. 119 (9), 1007-1014 (2015).
- Niedbalski, P. J., et al. Protocols for multi-site trials using hyperpolarized (129) Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the (129) Xe MRI clinical trials consortium. Magn Reson Med. 86 (6), 2966-2986 (2021).

