Gavage Strategy for Decoction Formula of Traditional Chinese Medicine in Osteosarcoma Model Mice
Summary
The protocol provides a detailed standardization process of decoction formula and gavage technique with Yiqi Jiedu decoction in the osteosarcoma mouse model as an example. It describes animal protection and improves reliability of research data, providing effective strategies for investigating therapeutic efficacy and molecular mechanisms of decoction formulas in vivo.
Abstract
Decoction formula is the most commonly used dosage form in traditional Chinese medicine and applied in clinical practice for thousands of years by trans-oral administration, which is characterized by quick effect, easy absorption, and individualized treatment based on the specific syndromes of patients. The quality of the decoction formula is directly responsible for the clinical efficacy of traditional Chinese medicine; therefore, the standardization process of the decoction formula is important to avoid differences in decoction quality caused by subjective factors. Meanwhile, due to the limitations of performing clinical experiments, small animals bearing human diseases, such as mice, are often used in medical research to explore the therapeutic efficacy and comprehensive mechanisms of different interventions, including the decoction formula for traditional Chinese medicine. Consequently, as an important trans-oral administration method, the skilled gavage technique is particularly important to avoid potential esophagus damage and drug spillage, which will ensure an equal amount of medicine being administered to each model animal, leading to accurate experimental results. Furthermore, the standardized method of decoction formula preparation and skilled gavage strategy are necessary to protect animal welfare and minimize the number of animals used. Here, we reported a detailed standardization process of the decoction formula and gavage technique with Yiqi Jiedu decoction in osteosarcoma mouse model as an example. The efficacy was evaluated by the tumor volume. This protocol will maximize animal protection and improve the reliability of research data, therefore providing effective strategies for future investigating therapeutic efficacy and molecular mechanisms of decoction formula for traditional Chinese medicine in vivo.
Introduction
Decoction formula is the most commonly used dosage form of the traditional Chinese medicine and the liquid medicinal drugs1,2. The decoction formula of traditional Chinese medicine is processed by decocting the herbs in water after being soaked, followed by filtering to discard the dregs.
Because the decoction formula can be individualized based on clinical necessities, especially in line with the characteristics of evidence-based treatment in traditional Chinese medicine, this dosage form via trans-oral administration has been applied in clinical practice for thousands of years with specific and irreplaceable advantages3,4. The quality of the decoction formula is directly related to the clinical efficacy. Therefore, the decoction formula must be prepared based on the principles for the standardized decoction methods to ensure the active ingredients are maximally extracted and protected3. Meanwhile, decoction-ready medicines are the main form of raw Chinese herbs used in clinical practice; the quality standards and production methods of prescribed decoction-ready medicines must comply with the provisions of the Chinese Pharmacopoeia2. Moreover, the prescribed decoction-ready medicines must be soaked in cold drinking water for at least 30 min before being decocted in a special pharmaceutical pot by heating5. Studies have shown that most of the active ingredients can be extracted by decocting for two times, and the Code of Practice for the Administration of Decoction Rooms of Traditional Chinese Medicines in Medical Institutions also prescribes decoction to be done twice4,6,7. If special Chinese herbs, such as one with hard texture or tonics, are prescribed, the decoction time is recommended to be extended. The decoction utensils and storage containers that directly come in contact with the decoction formula, starting from the container in which the prescribed decoction-ready medicines are soaked, should be chemically stable and have lids, such as casseroles, ceramics, glass and stainless steel. However, aluminum and ordinary plastic products should not be used, while iron and other corrosive utensils should be prohibited to avoid possible chemical reactions, which will reduce therapeutic efficacy and even produce harmful effects4,6,7.
Due to the limitations of performing clinical experiments, small animals bearing the human diseases, such as mice, are often used in medical research to explore the therapeutic efficacy and comprehensive mechanisms of different interventions including the decoction formula of traditional Chinese medicine8,9; however, unlike humans, the decoction formulas should be prepared every day, mixed together, and freshly administered 2x a day in clinical practice. Animals are unable to voluntarily take their medications on time with the required dosage, and most of the animal experiments related to traditional Chinese medicine are gavage-based oral administration of the decoction formulas at present. The decoction formulas used for in vivo animal experiments should be prepared in advance, aliquoted, stored at -20 °C and thawed just before use3,4,10. Moreover, substandard animal immobilization and gavage needle manipulation increase the risk of unnecessary injuries and negative emotions. Therefore, as an important method of trans-oral drug delivery, the skilled gavage technique is particularly important to avoid possible esophageal injury and drug spillage, thus ensuring that each model animal receives an equal amount of prescribed herbal medicine to achieve accurate experimental results. Here, we provide a gavage protocol for the decoction formula of traditional Chinese medicine.
Protocol
This protocol describes the detailed gavage technique in osteosarcoma model mice by using the Yiqi Jiedu decoction as a sample. Four-week-old, male athymic mice (BALB/c nude) were used in this experiment and kept in the SPF-grade breeding room of Laboratory Animal Center in Shanghai University of Traditional Chinese Medicine. All animal experiments were approved by the Animal Management and Committee of Shanghai University of Traditional Chinese Medicine, complied with the Code of Ethics for Laboratory Animals (Animal Ethics Approval Number: PZSHUTCM2304060007), and conducted in strict accordance with the international requirements related to laboratory animals.
1. Materials
- Obtain the following materials: Chinese herbs (Ginseng 42 g, Hedyotis diffusa 52.5 g, Barbed skullcap herb 31.5 g), 1 mL syringe, and 12G/55 mm elbow gavage needle (Figure 1).
2. Dosage determination
- Calculate the daily dosage as follows: the daily dosage for adults with a standard body weight of 60 kg is Ginseng 12 g, Hedyotis diffusa 15 g, and Barbed skullcap herb 9 g, with a total of 36 g decoction-ready medicine, that is, 0.6 g decoction-ready medicines/kg body weight. Referring to the 12 times for equivalent dose, 6 times for low dose, and 24 times for high dose between the adults with standard body weight of 60 kg and the mice with standard body weight of 25 g according to the body surface area11, as well as our preliminary data, use a 20 times dose ratio of Yiqi Jiedu decoction for the mice in the current study, therefore, the dosage for mice was 12 g decoction-ready medicines/kg body weight.
- Calculate the dosage for each mouse by using the equation:
dosage for mouse (stand body weight) = dosage for adult (stand body weight) x 20 (designated equivalent dose ratio).
The daily dosage of each mouse is 0.3 g [(12 g decoction-ready medicines/1000 g body weight) x 25 g body weight] in 200 µL with a final concentration of 1.5 g/mL. - Calculate the total decoction-ready medicines for mice (n=7) in the Yiqi Jiedu decoction group with gavage administration for 28 days to be 58.8 g. Concentrate this to 39.2 mL to reach a final concentration of 1.5 g/mL.
- Considering possible drug loss during the decoction and the gavage stages, and for the convenience of calculation, prepare a total of 126 g (Ginseng 42 g, Hedyotis diffusa 52.5 g, Barbed skullcap herb 31.5 g) decoction-ready medicine, concentrate into a final volume of 84 mL.
3. Cell source and culture
- Purchase human-derived 143B osteosarcoma cells from ATCC cell bank.
- Seed 1 x 106 143B cells in 10 mL of MEM medium containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin in a 10 cm cell culture dish.
- Culture 143B cells at 37 °C in a 5% CO2 incubator until the cells are 80%-90% confluent.
4. Modeling methods
- Collect the 80% confluent cultured 143B cells, resuspend into 6 mL of 10% serum-containing MEM medium and mix with basement membrane matrix gel at a volume ratio of 1:1 to make a cell suspension with a final concentration of 1 x 107 cells/mL.
- Inject 10 µL of the cell suspension into the left tibia of each athymic mouse to establish an in situ xenograft osteosarcoma mouse model, as being described in our previous publication12. The steps in brief are as follows.
- Wash the cells 2x with PBS. Trypsinize the cells with 1.5 mL of 0.25% trypsin for 3 min. Add 6 mL of 10% serum-containing MEM media. Collect the cells in a 15 mL centrifuge tube.
- Aspirate 20 µL of cell suspension into the chamber of the cell counting plate and calculate cell concentration using an automatic cell counter. Centrifuge the cells at 800 x g for 5 min at room temperature.
- Discard the supernatant with a pipette, resuspend the cell pellet in 8.5 mg/mL basement membrane matrix, and place them on ice.
- Anesthetize the mice by exposing them to 2% isoflurane and 98% oxygen (oxygen flow rate, 2 L/min). Apply a small amount of vet ointment on the eyes to prevent dryness while under anesthesia.
- Perform the entire procedure in a well-ventilated area. Before osteosarcoma cell injection, ensure that each mouse is under deep anesthesia by gently touching the foot; if the mouse still has responses, such as twitch or jerk. Wait for a longer time if the mouse still has responses, such as twitch or jerk, until these responses disappear.
- Place the mouse in a supine position. Hold the ankle of the mouse using the thumb and index finger and disinfect the injection site of the tibia with three alternating rounds of betadine and 70% ethanol swab.
- Attach the needle to a 1 mL syringe and point the needle tip toward the injection site paralleling to the axis of the tibia. Percutaneously insert the needle to drill a hole through the tibia platform toward the distal end of the tibia.
- Load the osteosarcoma cell suspension previously being placed on ice into a microvolume syringe and replace the 1 mL syringe in the tibia with the osteosarcoma cell-loaded microvolume syringe. Slowly inject approximately 10 µL of (1 x 107 cells/mL) osteosarcoma cell suspension into each athymic mouse's tibia.
- Remove the microvolume syringe from the injection site, and press the injection site with the cotton swab for about 30 s. Place the mouse back into a clean cage and observe its recovery for 10 min.
5. Preparation of traditional Chinese medicine decoction formula for gavage ( Figure 2)
- Calculate the dosage as per step 2.
- Soak all the medicinal herbs for decoction, except for the expensive herb Ginseng, in drinking water with a level 3 cm higher than the medicine surface, for 30 min, and then perform the decoction 2x as described below.
- Perform the first decoction by heating to boiling over a strong fire, and then heating for 15-25 min over a mild fire. Mild fire refers to a low-power heat with temperatures typically ranging from 80 °C to 100 °C, while strong fire refers to a high-power heat typically ranging from 200 °C to 300 °C.
- Pour the decoction liquid into one container.
- Perform the second decoction by heating the medicinal herbs in drinking water, with a volume just enough to submerge the medicine surface, to boiling over a strong fire, and then heating for 15-25 min over a mild fire.
- Pour the decoction liquid into the same container as in step 5.4.
- Soak and decoct 42 g of Ginseng separately, with drinking water 3 cm higher than the medicine surface, since it is expensive.
- Perform the first decoction of soaked Ginseng by heating it over a strong fire for 60 min.
Pour the decoction liquid into the same container as in step 5.4. - Perform the second decoction of Ginseng in drinking water with a volume just enough to submerge the medicine surface, by heating over a mild fire for 40 min. Pour the decoction liquid into the same container as in step 5.4.
NOTE: The containers used for decoction should be chemically stable, such as casseroles (the most commonly used in clinical practice), ceramics, glass, and stainless steel to avoid possible chemical reactions with the medicinal substances. All decoction liquid obtained from different steps must be put in the same container. - Mix and filter the decoction liquid using three layers of gauze fixed at the mouth of a measuring cup with rubber bands.
- Boil the filtered decoction on a strong fire in a pot, then change to mild fire and keep the mild fire until reaching the target concentration (a final volume of 84 mL with a final concentration of 1.5 g/mL).
- Prepare 21 mL aliquots of the concentrated decoction according to the dose required for intragastric administration for 1 week, and store the aliquots in a refrigerator at -20 °C.
6. Gavage administration in mice by using a gavage device
- Place the mouse on the surface of the mouse cage or other rougher surface.
- Grasp the mouse's tail with the thumb and index finger of the right hand. Then, grasp the mouse's neck with the thumb, index finger, and middle finger of the left hand.
- Hold the mouse tail with the other two fingers of the left hand to make the head, neck, and body of the mouse in a straight line.
NOTE: Make sure to keep the mouse relaxed during the entire grasping process. If the mouse keeps struggling to move, the grasping process should be performed until the mouse is relaxed. - Hold the gavage device in the right hand and load 200 µL of decoction formula for a mouse with 20 g body weight (100 µL decoction formula/10 g body weight of mouse).
- Insert the gavage needle into the mouth from the corner and push the tongue against the upper jaw of mouse.
- Keep the mouse body in the same direction and gently push the gavage needle toward the stomach along the esophagus until about 10 mm of the gavage needle is left outside.
NOTE: There is a hollow feeling when the gavage needle enters into the esophagus.The length of the gavage needle inserted into the mouse should be measured to the top of the sternum. The total length of the gavage needle is 60 mm (Figure 3), therefore, about 50 mm of the gavage needle should be inserted into the body of mouse before the decoction formula is injected into the stomach of mouse. - Inject the decoction formula into the stomach of mouse. Meeting resistance or liquid coming out of the mouth corner means misplacement of the gavage needle, which must be avoided. In addition, the injection speed must be slowed down if the decoction formula is thick.
- Release the mouse and observe them for 10 min. The gavage is successful if no breathing abnormality is observed and return the mouse into the cage (Figure 4).
7. Course of treatment
- To explore the therapeutic efficacy of Yiqi Jiedu decoction, set up a control group and a Yiqi Jiedu decoction group. For mice in the control group, administer physiological saline, once a day for 28 days. For mice in the Yiqi Jiedu decoction group, administer the Yiqi Jiedu decoction once a day for 28 days.
8. Adverse reactions
- Closely observe the status of the mice after being placed back into the cage, including weight loss and decreased mental status due to the inability of food to enter into the stomach.
- Mice will experience difficulty in breathing and shortness of breath due to food-caused tracheal obstruction and will cough in an attempt to clear the trachea. If any of these symptoms is observed during gavage, immediately stop the gavage and euthanize the mice.
Representative Results
The inhibitory effect of Yiqi Jiedu decoction on the growth of osteosarcoma in vivo was determined in an intra-tibial xenograft osteosarcoma mouse model, which was prepared by injecting the 143B osteosarcoma cells into the tibia of athymic mice. The Yiqi Jiedu decoction was administered to the mice of the Yiqi Jiedu decoction group by gavage for 28 days starting from the second day of the 143B injection. Physiological saline was given to the mice in the control group. All mice were euthanized after 28 days. The results showed that the tumor volume in the Yiqi Jiedu decoction group was significantly reduced compared with that of the control group (Figure 5).

Figure 1: Decoction consumables. The decoction-ready medicine consists of (A) Ginseng, (B) Hedyotis diffusa, (C) Barbed skullcap herb, (D) Graduated measuring cup, (E) Rubber bands, (F) Gauze, (G) Fixing three layers of gauze at the mouth of measuring cup with rubber bands. Please click here to view a larger version of this figure.

Figure 2: Preparation of decoction formula for traditional Chinese medicine. (A) The decoction-ready medicines except for Ginseng being soaked for 30 min. (B) Ginseng being soaked separately for 30 min. (C) Ginseng being decocted separately. (D) Filtering decoction formula of traditional Chinese medicine. (E) Storage of concentrated decoction formula of traditional Chinese medicine. Please click here to view a larger version of this figure.
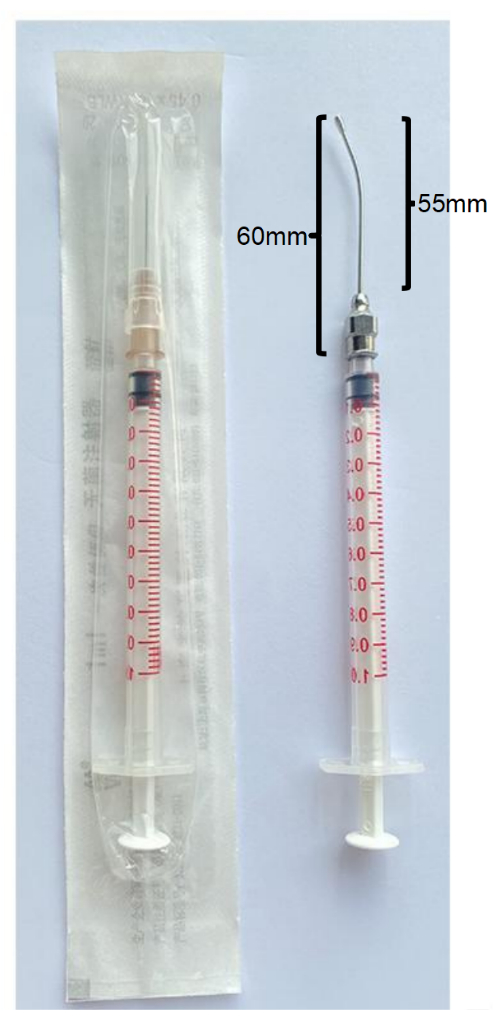
Figure 3: Gavage consumables. Regular 1 mL syringe with original needle (left panel), and 1 mL syringe with original needle being replaced by a No. 12/55 mm elbow gastric perfusion needle to set a gavage device (right panel). Please click here to view a larger version of this figure.
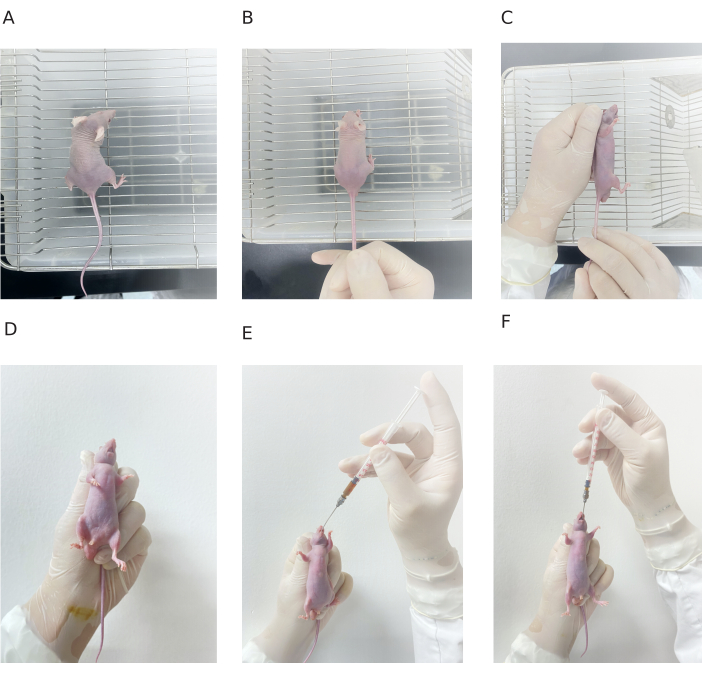
Figure 4: Procedure of gavage administration in mouse. (A) Mouse on the cage surface. (B) Mouse tail being grasped with one hand. (C-D) Mouse being fixed with one hand. (E) Needle being inserted from the mouse mouth. (F) End of gavage administration. Please click here to view a larger version of this figure.
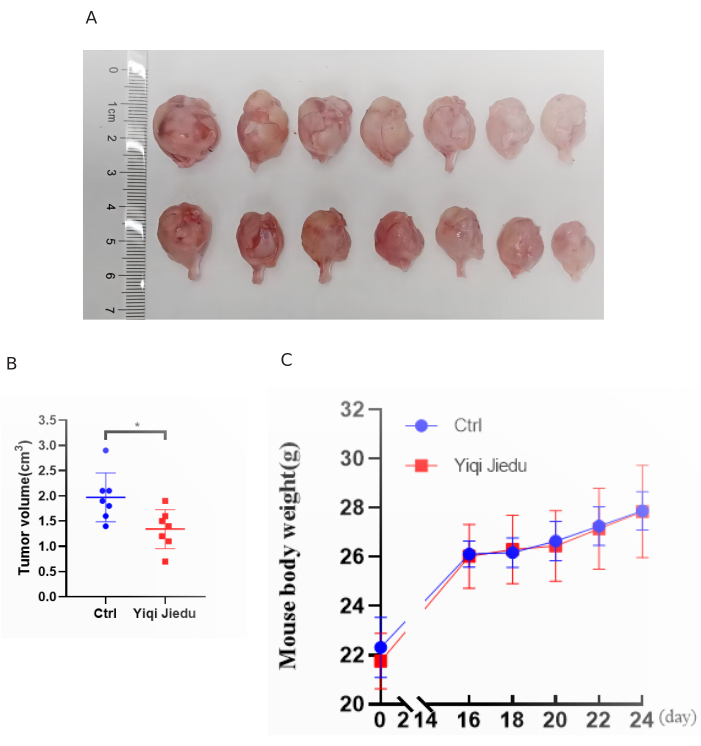
Figure 5: Inhibitory effect of the decoction in the osteosarcoma mouse model. (A) Osteosarcoma samples being collected from the control group and the Yiqi Jiedu decoction intervention group. Error bars stand for standard deviation (n=7) (B) Unpaired t test of tumor volume between control (Ctrl) group and Yiqi Jiedu decoction intervention group. Yiqi Jiedu decoction intervention for 28 days significantly inhibits the tumor volume (p<0.05). Error bars stand for standard deviation (n=7). (C) Body weight of mice in the Yiqi Jiedu decoction gavage group and the control group throughout the experimental period. Please click here to view a larger version of this figure.
Discussion
The clinical efficacy of the prescribed herbs in traditional Chinese medicine is largely associated with the administered dose and dosage form; therefore, the establishment of a standardized decoction strategy and a skilled administration process are necessary in clinical practice, as well as in improving the reliability of experimental results.
In this study, we first provided a detailed strategy for the standardized decoction of Chinese herbs used in clinical practice of traditional Chinese medicine, which facilitates the guidance of people to scientifically decoct medicines, also practically improves the quality of decoction formula and clinical efficacy of herbal medicines; therefore, once the standardization is achieved, it will improve the accuracy and consistency of the herbal medicine efficacy in clinical practice.
The gavage strategy has been widely applied as an oral intake method in preclinical studies and is applicable to various experimental animals, including mice, rats, and rabbits, which offers some advantages, such as ease of administration, accurate dosage control, and ability to mimic the clinical route of drug administration13. However, the animals will struggle if they are immobilized in an incorrect position without anesthesia, which may threaten the health of the researchers and reduce the reliability of the experiments. Therefore, the establishment of a standardized gavage method is important to reduce these possibilities and to avoid decoction entering the esophagus by mistake or causing esophagus damage, which in turn causes animal death and experimental failure.
Here, we chose the gavage route to deliver the decoction formula into the osteosarcoma model mice, as it allowed us to precisely measure the dosage and warrant consistent drug delivery throughout the experiment. To prepare the decoction formula, we first calculated the quantity of decoction-ready medicines for the mouse based on a ratio of 20 times the quantity used for adults with a body weight of 60 kg; soaked, and decocted 2x by heating to boiling; filter concentrated, and stored the decoction in aliquots at -20 °C. To perform the gavage administration, load 200 µL of the concentrated decoction formula into a gavage device, insert the gavage needle into the mouth, gently push the gavage needle toward the stomach and inject the decoction formula into the stomach of the mouse. One of our findings is the significant inhibition of tumor growth observed in the group receiving the decoction formula of Yiqi Jiedu decoction via gavage. We observed an obvious reduction in tumor volume in the Yiqi Jiedu decoction group compared to the control group. These results indicate that the gavage strategy effectively delivers the active components of the decoction formula to the target site, resulting in potent anti-tumor effects. Furthermore, we evaluated the safety profile of the gavage strategy by monitoring the general condition and body weight of the mice throughout the experimental period. We found no significant differences in body weight changes or signs of toxicity between the Yiqi Jiedu decoction gavage group and the control group, indicating that the gavage administration of the decoction formula is well-tolerated and does not cause obvious adverse effects.
Although the gavage strategy proved effective in delivering the decoction formula to the experimental animals, it is important to realize the potential adverse reactions and certain limitations associated with this administration method. To avoid food-caused trachea obstruction, closely observe mouse status during and after gavage, such as difficulty breathing, shortness of breath, and cough. Once any of them appear, immediately stop the gavage and perform chest compressions upon a horizontal hard desktop; furthermore, immediate veterinary help is necessary for any serious condition. Moreover, we need to pay close attention to the following limitations. Firstly, gavage may induce stress in the animals, potentially affecting their overall physiology and response to treatment. However, we took actions to minimize stress by using a small feeding needle and handling the mice gently during the gavage procedure. Moreover, gavage could only be performed by a person who has been specifically trained and qualified to ensure that a standardized technique is used for avoiding the possible harm to the animals14. Secondly, the exact mechanism by which the active components of the decoction formula exert their anti-tumor effects remains unclear. It is possible that the gavage strategy may alter the pharmacokinetics and pharmacodynamics of the herbal compounds, potentially influencing their bioavailability and therapeutic efficacy. Future studies should focus on elucidating the precise mechanisms underlying the anti-tumor effects of the decoction formula delivered through gavage. Furthermore, studies have reported that isoflurane anesthesia application during long-term gavage reduces the incomplete ingestion of decoction and the animal death due to the unskilled gavage technique15. Meanwhile, adding cooking oil into the decoction reduces the gavage associated animal stress16.
In conclusion, we provided the standardized decoction process of Chinese herbs and the skilled gavage strategy for mice. These efforts not only avoid possible esophageal injuries and death of the experimental mice, but also avoid drug overflows ensuring that each experimental animal administers equal quantity of herbal medicines, therefore, improve the accuracy and consistency of the experimental results, also the therapeutic effects. Our study demonstrates the efficacy and safety of the gavage strategy for administration of the traditional Chinese medicine decoction formula in an osteosarcoma mouse model. These results highlight the potential of this delivery method in the treatment of osteosarcoma and lay the foundation for further research in optimizing the gavage strategy of traditional Chinese medicine administration. Additionally, future studies should investigate the exact action mechanisms of the active components in the decoction formula and explore potential synergistic effects with other treatment modalities to enhance the therapeutic outcomes in osteosarcoma patients. Therefore, these findings shed light on the potential benefits and limitations of this administration method and provide valuable insights into its application in osteosarcoma treatment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The current work is supported by grants from the (1) National Nature Science Foundation (81973877 and 82174408), (2) National Key R&D Program of China (2020YFE0201600), (3) Shanghai Top Priority Research Center construction project (2022ZZ01009), (4) Shanghai Collaborative Innovation Center of Industrial Transformation of Hospital TCM Preparation, and (5) Research Projects within Budget of Shanghai University of Traditional Chinese Medicine (2021LK047).
Materials
| 143B cell line | ATCC | CRL-8303 | osteosarcoma cell line |
| Anesthesia machine | Shenzhen RWD Life Technology Co.,Ltd | R500IP | The Equipment of Anesthesia mice |
| Automatic cell counter | Shanghai Simo Biological Technology Co., Ltd | IC1000 | Counting cells |
| BALB/c athymic mice | Shanghai SLAC Laboratory Animal Co, Ltd. | Male | Animal |
| Barbed skullcap herb | Shanghai Yanghetang Traditional Chinese Medicine Tablet Co., Ltd. | 20230606 | Decoction-ready medicines |
| Basement Membrane Matrix | Shanghai Uning BioscienceTechnology Co., Ltd | 356234, BD, Matrigel | re-suspende cells |
| Centrifuge tube (15 mL) | Shanghai YueNian Biotechnology Co., Ltd | 430790, Corning | Centrifuge the cells |
| Elbow gavage needle (12-gauge/ 55 mm) | RWD Life Science Co., Ltd. | C21014-12 | Component of gastric perfusion device |
| Gauze | Haishi Hainuo Group Co., Ltd. | LC45 | Decoction filter |
| Ginseng | Shanghai Wanshicheng Pharmaceutical Co., Ltd. | 20220704-2 | Decoction-ready medicines |
| Hedyotis diffusa | Shanghai Yanghetang Traditional Chinese Medicine Tablet Co., Ltd. | 2023071107 | Decoction-ready medicines |
| isoflurane | Shenzhen RWD Life Technology Co., Ltd | VETEASY | Anesthesia mice |
| Micro-volume syringe | Shanghai high pigeon industry and trade Co., Ltd | 0-50 μL | Inject precise cells into the tibia |
| Phosphate-buffered saline | Beyotime Biotechnology | ST447 | wash the human osteosarcoma cells |
| Rubber bands | Shanghai Hengfei Biotechnology Co., Ltd. | XPJ | Fixing gauze on the measuring cup |
| Syringe (1 mL ) | Shanghai Mishawa Medical Industry Co., Ltd. | SBM0040 | Component of gastric perfusion device |
| Trypsin (0.25%) | Shanghai YueNian Biotechnology Co., Ltd | 25200056, Gibco | trypsin treatment of cells |
References
- Gao, S. Discussion on advantages and improvement of traditional Chinese medicine decoction. Chinese Community Physicians (Comprehensive Edition). (21), 12 (2005).
- Chinese Pharmacopoeia Commission. . Pharmacopoeia of the People’s Republic of China. , (2015).
- An, Y., et al. Interpretation on specification of traditional Chinese medicine decoction. Herald Med. 42 (11), 1648-1652 (2023).
- Chen, S., et al. Research strategies in standard decoction of medicinal slices. China J Chinese Materia Medica. 41 (8), 1367-1375 (2016).
- Luo, F. Effect of soaking traditional Chinese medicine before decoction on active components. Shanghai Yiyao. 40 (9), 63-64 (2019).
- Liu, F., Chen, M. On the standardization of decocting methods of modern decoction. Global Trad Chinese Med. 7 (5), 377-378 (2014).
- Li, X., et al. Influence of decocting times and instrument on the quality of decoction. J Trad Chinese Med. 50 (6), 550-552 (2009).
- Li, X., et al. Herbal decoctosome is a novel form of medicine. Sci China. Life Sci. 62 (3), 333-348 (2019).
- Lan, Y., Hu, Y., Huang, W., Tang, S. Reflections on PK – PD model based on animal experiments for study of Chinese medicine. Lishizen Med Materia Medica Res. 34 (2), 395-397 (2023).
- Li, Y., Bai, M., Song, Y., Guo, H., Miao, M. Research and reflection on standard decoction of Chinese materia medica. Chinese Trad Herbal Drugs. 49 (17), 3977-3980 (2018).
- . Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (2005)
- Chang, J., et al. Intratibial osteosarcoma cell injection to generate orthotopic osteosarcoma and lung metastasis mouse models. J Vis Exp. (176), 63072 (2021).
- He, C. Laboratory animal science. , (2006).
- Patten, A. R., Fontaine, C. J., Christie, B. R. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Fronti Pedia. 2, 93 (2014).
- Jones, C. P., Boyd, K. L., Wallace, J. M. Evaluation of mice undergoing serial oral gavage while awake or anesthetized. J Am Assoc Lab Animal Sci. 55 (6), 805-810 (2016).
- Recena Aydos, L., et al. Nonalcoholic fatty liver disease induced by high-fat diet in C57bl/6 models. Nutrients. 11 (12), 3067 (2019).

