Understanding the Effects of Non-Invasive Transauricular Vagus Nerve Stimulation on EEG and HRV
Summary
This protocol provides information on how to apply transcutaneous auricular vagus nerve stimulation (taVNS) in a clinical trial, including potential biomarkers such as EEG metrics and heart rate variability (HRV) to measure the effect of this treatment on the autonomic nervous system.
Abstract
Several studies have demonstrated promising results of transcutaneous auricular vagus nerve stimulation (taVNS) in treating various disorders; however, no mechanistic studies have investigated this technique’s neural network and autonomic nervous system effects. This study aims to describe how taVNS can affect EEG metrics, HRV, and pain levels. Healthy subjects were randomly allocated into two groups: the active taVNS group and the sham taVNS group. Electroencephalography (EEG) and Heart Rate Variability (HRV) were recorded at baseline, 30 min, and after 60 min of 30 Hz, 200-250 µs taVNS, or sham stimulation, and the differences between the metrics were calculated. Regarding vagal projections, some studies have demonstrated the role of the vagus nerve in modulating brain activity, the autonomic system, and pain pathways. However, more data is still needed to understand the mechanisms of taVNS on these systems. In this context, this study presents methods to provide data for a deeper discussion about the physiological impacts of this technique, which can help future therapeutic investigations in various conditions.
Introduction
Transauricular vagus nerve stimulation (taVNS) is a recent neuromodulation technique that does not require surgery and utilizes a non-invasive stimulation device placed at the concha or tragus of the ear. Consequently, it is more accessible and safer for patients1. In recent years, the taVNS field has rapidly expanded, primarily focusing on clinical trials demonstrating potential therapeutic advantages for various pathological conditions, including epilepsy, depression, tinnitus, Parkinson’s disease, impaired glucose tolerance, schizophrenia, and atrial fibrillation2. There is much to discuss about taVNS and its effects on biological processes in the central and peripheral systems. Ideally, a biological marker might demonstrate that the auricular branch of the vagus was stimulated, affecting intracranial structures and allowing researchers to analyze how taVNS affects physiological function. Nevertheless, without a trustworthy biomarker, it is not easy to understand what the taVNS data mean and how to interpret them effectively.
Electroencephalography (EEG) is an encouraging imaging tool to provide biomarkers for taVNS. It is a non-invasive, reliable, inexpensive approach to measure and quantify cortical activity3,4. Following this process, our group performed a systematic review, demonstrating elementary details that taVNS could influence cortical activity, mainly increasing EEG power spectrum activity in lower frequencies (delta and theta). However, diverse results in higher frequencies (alpha) and changes in early ERP components related to inhibitory tasks were also detected. High heterogeneity between the studies was found; therefore, more homogeneous, more significant, and well-planned studies are essential to make more robust conclusions about the effects of taVNS on brain activity measured by EEG3. Assessing EEG during taVNS could advance future research on integrating the two techniques for a mobile, closed-loop, monitoring, and non-invasive stimulation tool to affect brain oscillatory activity4.
Alpha asymmetry, which assesses the relative alpha band activity between the brain hemispheres, particularly at frontal electrodes, is a frequently researched EEG biomarker. Previous literature has used this biomarker to analyze the approach-withdraw hypothesis5,6, which holds that the right frontal side of the brain is associated with withdrawal behaviors. In contrast, the left frontal side is associated with approach behaviors. Since alpha is associated with low brain activity, an increase in alpha on the left side of the brain suggests lower activity and may show a lack of approach behavior. This concept helps to explain some results in the alpha band at the left side hemisphere in depressed patients7. Additionally, EEG electrodes record the activity of neuronal populations, examining Functional Connectivity (FC) or changes in large-scale brain networks, such as the default mode network (DMN)7,8.
Based on that, quantitative electroencephalography can be employed to assess the effects of taVNS on brain activity; however, more studies are required to systematically demonstrate the specific metrics and effects that would highlight the non-invasive stimulation through the auricular branch of the vagus nerve.
Peripherally, the vagus nerve and sympathetic nervous system mediate the heart’s contractile and electrical function9. This regulation promotes the heart’s pacemaker ability and controls it through physiological manifestations of the body, known as sinus depolarizations. Heart rate variability (HRV) records the changes per beat of sinus depolarization, thus non-invasively describing vagal influences on the sinus node10. Given this function, HRV has been seen and studied as a prominent neurocardiac function biomarker associated with an individual’s well-being and the likelihood of morbidity, mortality, and stress11,12.
In the context of taVNS, HRV has been recorded in many trials, and stimulation has been thought to modulate HRV9,11,12. Considering that decreased HRV has been related to the morbidity and mortality of different diseases through mechanisms such as over-activity of the sympathetic nervous system, inflammatory response, and oxidative stress, the vagal nerve modulation of taVNS is thought to directly impact HRV and its sinus regulation13,14. In fact, some trials have already indicated that taVNS can increase HRV in healthy subjects, thus supporting this hypothesis15,16. However, there is still a need to understand better whether different taVNS parameters can affect HRV differently.
Currently, no mechanistic studies have investigated the taVNS neural network and autonomic nervous system effects of this technique together. Therefore, this protocol aims to assess how taVNS can affect EEG metrics and HRV and evaluate its safety. Additionally, this also aims to identify predictors that can influence the response to taVNS. Understanding the variables associated with the response to taVNS can help design future clinical trials to maximize the effects of this intervention.
Protocol
All study procedures were performed at the Spaulding Neuromodulation Center/Spaulding Cambridge Hospital. Ethical approval for this protocol was obtained from Mass General Brigham IRB (Number Protocol #:2022P003200). Informed consent was obtained from all subjects using the encrypted Research Electronic Data Capture (REDCap) platform (see Table of Materials). Trial registration number: NCT05801809.
1. Subject selection and screening
- Identify potential subjects by several sources.
NOTE: For the present study, the human subjects were identified from (1) flyers in public areas across the Boston-land region, (2) internet and newspaper advertisements, (3) advertisements posted on public transportation (The T), (4) Via the Rally platform by Mass General Brigham Research (see Table of Materials). Forty-four healthy subjects were selected for the present study. - Contact eligible subjects or ask permission electronically to contact them to provide more information about the study.
- At the first point of contact (usually a phone call or Zoom Enterprise call), a study co-investigator administers an online pr-screening questionnaire. Once the online pre-screening process is completed, take the information gathered by the co-investigator to the PI of the study for further review to confirm eligibility. Then, store the data obtained from the pre-screening in an encrypted web-based platform (REDCap, see Table of Materials).
- Include subjects older than 18 years and naive to the stimulation (taVNS).
- Exclude pregnant women, the presence of medical conditions, and presence of any contraindication to transauricular vagus nerve stimulation.
2. Equipment details
- Use a Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) device (Figure 1), which consists of an earset (Figure 2) with conductive ear tips placed on the auricular concha of the ears (Figure 3).
- Connect the electrodes to a stimulator, and during active stimulation, stimulate both the cymbal conchae of the auricular at 30 Hz, 200-250 µs, for 60 min.
NOTE: For the commercial details of the device and the related accessories, please see Table of Materials.
3. taVNS procedure
NOTE: The protocol consists of two visits: Visit 1 (consent, screening, and collection of demographics information), and Visit 2 (assessments and intervention). The flow of the study can be found in Figure 4.
- On Visit 2, randomize the subjects to receive the intervention.
NOTE: The active group receives active taVNS, and the sham group receives sham taVNS. - Blind the subjects, intervention team (co-investigators/ CO-Is who performed the taVNS intervention), and outcome assessors (CO-Is who performed the assessments or analyzed the data) during the trial. Ensure that one non-involved staff member will generate the allocation sequence, seal the envelopes, and randomly assign individuals to interventions using external and visual display identical devices that differ on whether they are active (active current) or not (sham) by another staff member who is not involved in data collection or analysis.
- Collect the data for this study from subjects using an electronic format capture system (REDCap). The following assessments performed are displayed in Table 1.
- When the subject arrives, provide information regarding the procedure. First, assess the pain levels and pain modulation, using heat stimuli on the right forearm for the pain threshold, and cold water for the Conditioned Pain Modulation (CPM), following the adapted protocol suggested by Granot17 and Nirl18.
- First, determine the pain-60 test temperature (temperature that instigates pain experience at a magnitude of 60 on a 60-100 NPS) by applying a Peltier thermode (see Table of Materials) on the right forearm of subjects and deliver short heat stimuli (41-48 °C), each temperature lasting for 7 s starting from the time the stimulus intensity reaches the destination temperature.
- Ask the subjects to rate the level of pain intensity using a numerical pain scale (NPS) ranging from 0 = ''no pain'' to 100 = ''the worst pain imaginable''.
- Once the pain-60 temperature is determined, administer the test stimulus by applying the same for 30 s at that temperature, and ask the subjects to rate their levels of pain intensity 3 times: at 10 s, 20 s, and 30 s after the thermode reaches the pain-60 temperature (mean scores of the three pain rating will be calculated).
- 5 min after delivering the test stimulus, immerse the left hand of the subject in a bath of water set at 10 °C to 12 °C for 30 s for the conditioned stimulus. Then, apply the same pain-60 temperature on the right forearm of the subject (left hand will still be immersed) for 30 s and again ask the subject to rate their levels of pain intensity 3 times after the thermode reaches the pain-60 temperature: at 10 s, 20 s and 30 s.
NOTE: CPM (Conditioned Pain Modulation) response will be calculated as the difference between the average of pain ratings from the test stimulus minus the average of pain ratings during the conditioned stimulus.
- Ask subjects to place an HRV monitor (displayed in Figure 5 and Figure 6).
- Next, assess baseline HRV for 5 min (to analyze frequency HF, LF, LF/HF, and time domains metrics) recording with the monitor connected by Bluetooth to a tablet.
- Set up the EEG connected to a computer system, and start the assessments (resting and task-related), which lasts about 30 min.
- Next, set up the taVNS device.
- Examine, clean with a 70% alcohol pad, and prepare the ear skin of the subject to place the electrodes.
- Next, apply the saline solution to the eatips, place the electrodes on the ear, and start the stimulation, which lasts 60 min.
- When the taVNS reaches 30 min, record HRV and EEG again for 5 min only.
- After 60 min of stimulation, assess the subject for EEG, HRV, and pain, and repeat the pretrial procedures (as mentioned below):
- Perform EEG and HRV assessment, which lasts about 30 min.
- Perform CPM assessment following step 3.4.
- Perform assessments regarding side effects, fatigue and mood.
- Complete the session.
4. Follow up procedures
- After randomizing the subjects and completing the data collection, perform data analysis3.
Representative Results
We performed a preliminary descriptive analysis of the first randomized subject without unblinding the study. For this reason, which arms this subject was allocated to is unknown. The first subject is a 69-year-old woman, non-Hispanic, Caucasian, with a college degree, who did not report any adverse event during or after the stimulation session. The clinical data are displayed in Table 2.
Besides, a topographic distribution of scalp plots was created in resting-state EEG for theta, alpha, and beta bands in three time periods: baseline, during, and post-procedure (Figure 7). An asymmetric alpha pattern was noticed in the frontal region.

Figure 1: Main taVNS Device, with bilateral electrodes. Please click here to view a larger version of this figure.

Figure 2: Earset of the taVNS device. Please click here to view a larger version of this figure.

Figure 3: Conductive Ear tip of the electrodes of the taVNS device. Please click here to view a larger version of this figure.
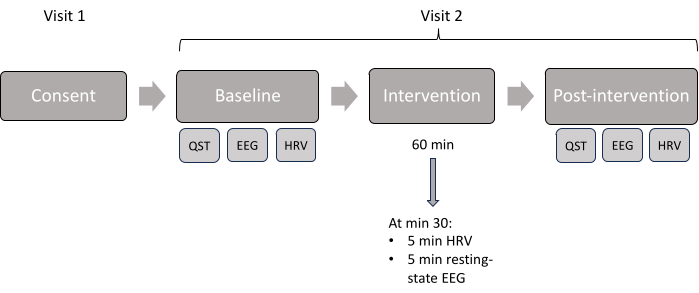
Figure 4: Study visit scheme. Displayed chronologic order of the assessments done in each visit. Please click here to view a larger version of this figure.
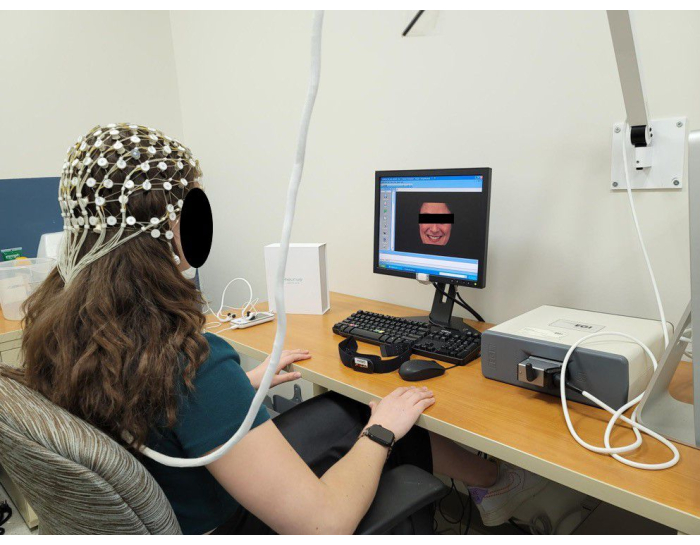
Figure 5: Representation of a subject during an EEG session using a 64-channel EGI system. Please click here to view a larger version of this figure.
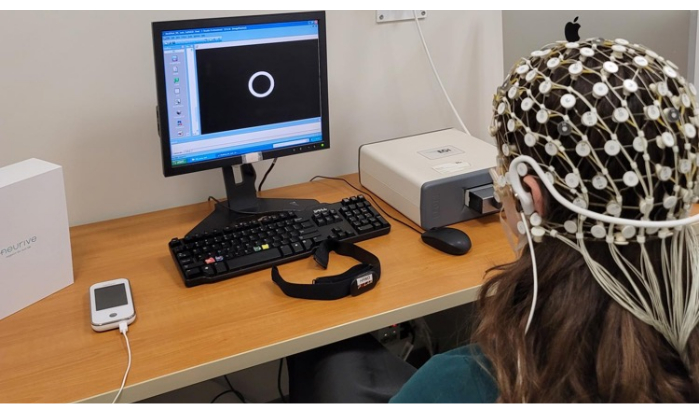
Figure 6: Representation of a subject during the EEG session plus taVNS stimulation. Please click here to view a larger version of this figure.
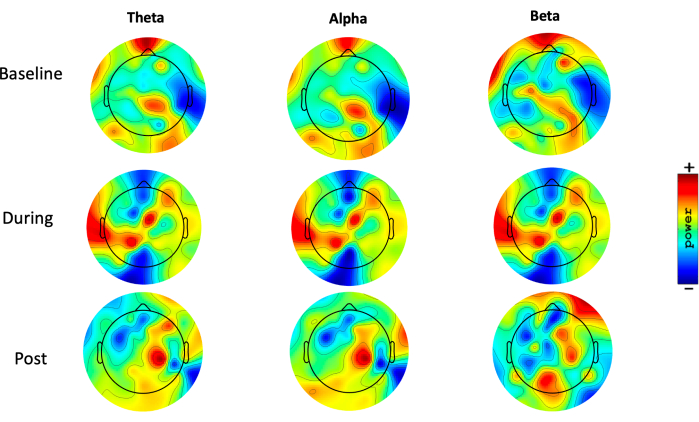
Figure 7: Topographic distribution. Topoplots, showing the topographic distribution of the theta (1-3.9 Hz), alpha (8-12.9 Hz), and beta (13-29.9 Hz) power (range 35 to 44 dB) (10 x log10 P), during resting-state EEG, in three different moments: pre-intervention (baseline), during intervention, and after intervention (post). Blue areas represent low activity, and red areas represent high activity. Please click here to view a larger version of this figure.
| Consent and screening | Baseline | Intervention | Post intervention | |
| Visit 1 (online) | Visit 2 | Visit 2 | Visit 2 | |
| Demographics | X | |||
| Medical History | X | |||
| Consent form | X | |||
| Beck depression inventory | X | |||
| EHI/SF | X | |||
| Pregnancy test | X | |||
| BMIS | X | X | ||
| VAS-F | X | X | X | |
| EEG | X | X | X | |
| HRV | X | X | X | |
| QST | X | X | ||
| Success of blinding questionnaire | X | |||
| Approximate visit time | 60 min | 60 min | 60 min | 30 min |
Table 1: Assessments scheme. "X" indicates that the procedures were done in each of the visits.
| Variable | Demographic data | Baseline | During intervention | After intervention |
| Age (years) | 69 | |||
| Gender | Female | |||
| Ethnicity | Non-Hispanic | |||
| Race | Caucasian | |||
| Education level | College degree | |||
| Pain 60 | 47 | . | 44 | |
| TS | 3 | . | 1 | |
| CPM | 4 | . | 5 | |
| Mood | -6 | . | 9 | |
| Fatigue | 5.7 | 6.6 | 6.76 | |
| HRV – HF | 0.046 | 0.066 | 0.584 | |
| HRV – LF | 0.073 | 0.043 | 0.037 |
Table 2: The clinical data representative of one subject.
Discussion
Transauricular Vagus Nerve Stimulation (taVNS) is emerging as a promising therapeutic avenue for addressing several neuropsychiatric conditions. Mood disorders, such as depression and anxiety, pose a significant global health burden, especially after the COVID-19 pandemic19. Recent studies exploring taVNS have exhibited the potential to alleviate symptoms associated with these disorders.
The vagus nerve plays a pivotal role in the brain-gut axis and the regulation of emotional responses20. Stimulation of the vagus nerve through taVNS is thought to modulate neurotransmitter activity, including serotonin and norepinephrine, which are crucial in regulating mood. Initial trials with healthy subjects have demonstrated the feasibility and safety of taVNS, hinting at its potential as a non-pharmacological treatment for mood disorders21. Moreover, the recent meta-analysis showed that approximately 80% of the studies used the parameters chosen in this protocol, with no severe adverse events reported. It also suggests that bilateral stimulation does not significantly increase the likelihood of cardiovascular events but can actually enhance the effects of vagus stimulation. We expect positive outcomes in this study that will bolster the foundation for further research in clinical populations, proposing a bilateral stimulation and providing a basis for developing individualized taVNS protocols to target specific mood-related neural pathways.
Similarly, chronic pain conditions, such as fibromyalgia and neuropathic pain, pose a significant challenge due to limited treatment options and the risk of opioid dependence. taVNS offers a ray of hope in this scenario. By stimulating the vagus nerve, taVNS can trigger the release of endogenous opioids and dampen pain signals through descending pain pathways22. Previous studies involving healthy subjects have revealed the potential of taVNS in reducing experimental pain perception23; however, the findings are still inconclusive. In future studies, we will assess the pain processing characteristics of healthy subjects with a comprehensive set of sensory tests, providing a crucial initial step in validating its protocols.
The limitation of the current study method is the absence of more sessions of intervention and follow-up assessments. Therefore, it is not possible to measure the effect size in a long-term period. Also, this study is limited to healthy subjects, which implies different results if tested in different populations with any medical or psychological disorder.
In conclusion, taVNS holds significant promise for addressing mood disorders and chronic pain conditions, among others. This study will provide insights into the safety, feasibility, and general effects of taVNS on physiological processes to establish a baseline understanding of how taVNS influences neurophysiological mechanisms, fostering confidence in the technique’s potential therapeutic value.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The author is grateful to the research team (Maria Fernanda Andrade, Allison Kim, Robin Heemels).
Materials
| Articulated arm | Electrical Geodesics, Inc. | 20090645 | |
| Baby shampoo | Dynarex | 1396 | |
| Charge Cable | NEURIVE Co. | HV12303003 | |
| Computer | Apple | YM92704U4PC | |
| Condutive eartip | NEURIVE Co. | HV12303003 | |
| Earset | NEURIVE Co. | HV12303003 | |
| EEG 64-channel cap | Electrical Geodesics, Inc. | H11333 | |
| Heart rate sensor | Polar | M311370175396 | |
| Monitor | Dell | REVA01 | |
| Net Amps 300 | Electrical Geodesics, Inc. | A09370244 | |
| Peltier thermode | Advanced Medical Systems, Ramat Yishai, Isreal | ||
| Potassium Chloride (dry) | Electrical Geodesics, Inc. | 820127755 | |
| Rally | Mass General Brigham Research | online platform | |
| Research Electronic Data Capture (REDCap) | Vanderbilt | web-based software platform | |
| Thermosensory Stimulator | Medoc Ltd | 1241 | |
| Transauricular vagus nerve stimulator | NEURIVE Co. | HV12303003 |
References
- Ben-Menachem, E., Revesz, D., Simon, B. J., Silberstein, S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 22 (9), 1260-1268 (2015).
- Johnson, R. L., Wilson, C. G. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 11, 203-213 (2018).
- Gianlorenco, A. C. L., et al. Electroencephalographic patterns in taVNS: A systematic review. Biomedicines. 10 (9), 2208 (2022).
- Ruhnau, P., Zaehle, T. Transcranial Auricular Vagus Nerve Stimulation (taVNS) and Ear-EEG: Potential for closed-loop portable non-invasive brain stimulation. Front Hum Neurosci. 15, 699473 (2021).
- Coan, J. A., Allen, J. J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 67 (1-2), 7-49 (2004).
- Davidson, R. J. Cerebral asymmetry, emotion, and affective style. Brain Asymmetry. , 361-387 (1995).
- de Aguiar Neto, F. S., Rosa, J. L. G. Depression biomarkers using non-invasive EEG: A review. Neurosci Biobehav Rev. 105, 83-93 (2019).
- Rao, R. P. N. . Brain-Computer Interfacing: An Introduction. , (2013).
- Machetanz, K., Berelidze, L., Guggenberger, R., Gharabaghi, A. brain-heart interaction during Transcutaneous Auricular Vagus Nerve Stimulation. Front Neurosci. 15, 632697 (2021).
- Spyer, K. M. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 474 (1), 1-19 (1994).
- Jarczok, M. N., et al. Investigating the associations of self-rated health: heart rate variability is more strongly associated than inflammatory and other frequently used biomarkers in a cross sectional occupational sample. PLoS One. 10 (2), 0117196 (2015).
- Shaffer, F., Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front Public Health. 5, 258 (2017).
- Haensel, A., Mills, P. J., Nelesen, R. A., Ziegler, M. G., Dimsdale, J. E. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. PNEC. 33 (10), 1305-1312 (2008).
- Wolf, V., Kühnel, A., Teckentrup, V., Koenig, J., Kroemer, N. B. Does transcutaneous auricular vagus nerve stimulation affect vagally mediated heart rate variability? A living and interactive Bayesian meta-analysis. Psychophysiol. 58 (11), e13933 (2021).
- Geng, D., Liu, X., Wang, Y., Wang, J. The effect of transcutaneous auricular vagus nerve stimulation on HRV in healthy young people. PLoS One. 17 (2), 0263833 (2022).
- Garrido, M. V., Prada, M. KDEF-PT: Valence, emotional intensity, familiarity and attractiveness ratings of angry, neutral, and happy faces. Front Psychol. 8, 2181 (2017).
- Granot, M., et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter. Pain. 136 (1-2), 142-149 (2008).
- Nirl, R. R., et al. A psychophysical study of endogenous analgesia: the role of the conditioning pain in the induction and magnitude of conditioned pain modulation. EJP. 15 (5), 491-497 (2011).
- Santomauro, D. F., et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 398 (10312), 1700-1712 (2021).
- Tan, C., Yan, Q., Ma, Y., Fang, J., Yang, Y. Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis. Front. Neurol. 13, 1015175 (2022).
- Kim, A. Y., et al. Safety of transcutaneous auricular vagus nerve stimulation (taVNS): A systematic review and meta-analysis. Sci. Rep. 12 (1), 22055 (2022).
- Martins, D. F., et al. The role of the vagus nerve in fibromyalgia syndrome. Neurosci. Biobehav. Rev. 131, 1136-1149 (2021).
- Frøkjaer, J. B., et al. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. J Neurogastroenterol Motil. 28 (4), 592-598 (2016).

