Multichannel Extracellular Recording in Freely Moving Mice
Summary
The protocol describes the methodology of extracellular recording in the motor cortex (MC) to reveal extracellular electrophysiological properties in freely moving conscious mice, as well as the data analysis of local field potentials (LFPs) and spikes, which is useful for evaluating the network neural activity underlying behaviors of interest.
Abstract
The protocol aims to uncover the properties of neuronal firing and network local field potentials (LFPs) in behaving mice carrying out specific tasks by correlating the electrophysiological signals with spontaneous and/or specific behavior. This technique represents a valuable tool in studying the neuronal network activity underlying these behaviors. The article provides a detailed and complete procedure for electrode implantation and consequent extracellular recording in free-moving conscious mice. The study includes a detailed method for implanting the microelectrode arrays, capturing the LFP and neuronal spiking signals in the motor cortex (MC) using a multichannel system, and the subsequent offline data analysis. The advantage of multichannel recording in conscious animals is that a greater number of spiking neurons and neuronal subtypes can be obtained and compared, which allows the evaluation of the relationship between a specific behavior and the associated electrophysiological signals. Notably, the multichannel extracellular recording technique and the data analysis procedure described in the present study can be applied to other brain areas when conducting experiments in behaving mice.
Introduction
The local field potential (LFP), an important component of extracellular signals, reflects the synaptic activity of large populations of neurons, which form the neural code for multiple behaviors1. Spikes generated by neuronal activity are considered to contribute to the LFP and are important for neural coding2. Alterations in spikes and LFPs have been proven to mediate several brain diseases, such as Alzheimer's disease, as well as emotions such as fear, etc.3,4. It is worth noting that many studies have highlighted that spike activity significantly differs between awake and anesthetized states in animals5. Although recordings in anesthetized animals offer an opportunity to assess LFPs with minimal artifacts in highly defined cortical synchronization states, the results differ to some extent from what can be found in awake subjects6,7,8. Thus, it is more meaningful to detect neural activity over long time scales and large spatial scales in various diseases in an awake brain state using electrodes implanted in the brain. This manuscript provides information for beginners on how to make the micro-drive system and set the parameters using common software for computing the spike and LFP signals in a fast and straightforward way in order to get the recording and analysis started.
Although the non-invasive recording of brain functions, such as by using electroencephalograms (EEGs) and event-related potentials (ERPs) recorded from the scalp, has been used extensively in human and rodent studies, EEG and ERP data have low spatial and temporal properties and, thus, cannot detect the precise signals produced by nearby dendritic synaptic activity within a specific brain area1. Currently, by taking advantage of multichannel recording in conscious animals, neural activity in the deeper layers of the brain can be recorded chronically and progressively by implanting a micro-drive system into the brains of primates or rodents during multiple behavioral tests1,2,3,4,5,6,7,8,9. Briefly, researchers can construct a micro-drive system that can be used for the independent positioning of the electrodes or tetrodes to target different parts of the brain10,11. For example, Chang et al. described techniques to record spikes and LFPs in mice by assembling a light and compact micro-drive12. In addition, micro-machined silicon probes with custom-made accessory components are commercially available for recording multiple single neurons and LFPs in rodents during behavioral tasks13. Although various designs have been used for assembling micro-drive systems, these still have limited success in terms of the complexity and weight of the whole micro-drive system. For example, Lansink et al. showed a multichannel micro-drive system with a complex structure for recording from a single brain region14. Sato et al. reported a multichannel micro-drive system displaying an automatic hydraulic positioning function15. The main disadvantages of these micro-drive systems are that they are too heavy for mice to move freely and are difficult to assemble for beginners. Although multichannel extracellular recording has been shown to be a suitable and efficient technology for measuring neural activity during behavioral tests, it is not easy for beginners to record and analyze the signals acquired by the complex micro-drive system. Given that it is difficult to get the entire operation process of the multichannel extracellular recording and data analysis started in freely moving mice16,17, this present article presents simplified guidelines to introduce the detailed process of making the micro-drive system using commonly available components and settings; the parameters in the common software for computing the spike and LFP signals in a fast and straightforward way are also provided. Additionally, in this protocol, the mouse can move freely due to the use of a helium balloon, which contributes to offsetting the weight of headstage and micro-drive system. Generally, in the present study, we describe how to easily build a micro-drive system and optimize the processes of recording and data analysis.
Protocol
All the mice were obtained commercially and maintained in a 12 h light/12 h dark cycle (light on at 08:00 A.M. local time) at a room temperature of 22-25 °C and a relative humidity of 50%-60%. The mice had access to a continuous supply of food and water. All the experiments were carried out in accordance with the Guidelines for Care and Use of Laboratory Animals of South China Normal University and approved by the Institutional Animal Ethics Committee. Male C57BL/6J mice aged 3-5 months old were used for the experiments.
1. Micro-drive system assembly
- Connect two computer-designed boards using two stulls and a screw that holds the movable micro-drive and attach the connector to one board (Figure 1A, Bi-iii). Drive the micro-drive by twisting a screw (0.5 mm/circle).
- Ensure the micro-drive can carry two sets of eight guide tubes (~3 cm long, ~50 µm internal diameter, ~125 µm external diameter) for each side of the MC region, and then cut it to the same length (at least 15 mm; Figure 1Biv, v).
- Cut 16 Ni-chrome wires (~5 cm long) with a 35 µm diameter, and then load them successively into the guide tubes, followed by applying the glue to fix them (Figure 1Bi, vi, vii).
- Strip the wire insulation, successively twine each exposed wire to each pin from the connector following the channel map, as well as the reference and ground electrodes, and then slowly coat conducting paint onto each pin (Figure 1Bviii-x).
- Cover the pins using epoxy resin (Figure 1Axi, xii), and then perform gold plating via an impedance tester to reduce the impedance of the electrode tips to ~350 kOhm (Figure 1Bxiii, xiv). Set the parameters of the impedance tester as follows: −10.08 µA direct current for 1 s with gold plating solution, including 5 mM PtCl4.
- Finally, move the micro-drive to the top by twisting a screw. Check the overall size of the micro-drive system modified as shown in Figure 1A (approximately 15 mm long, 10 mm width, 20 mm height, ~1 g weight). Check the detailed specifications of the computer-designed board and movable component in Figure 1Ai, ii.
2. Electrode array implantation
- Sterilize the surgery kits, wear sterile gloves and put on a doctor's sterile white coat before the operation starts.
- To manage the pain, use subcutaneous (s.c.) injection of meloxicam injectable (5 mg/kg) for the mouse in an induction chamber. Then anesthetize the mouse by an intraperitoneal (i.p.) injection of pentobarbital (80 mg/kg) in an induction chamber18,19. Apply a supplemental dose of pentobarbital (20 mg/kg/h) if the toe pinch reflex is still present.
- Fix the mouse in a stereotaxic apparatus and maintain its rectal temperature at 37 °C by using a temperature controller.
- Apply tetracycline eye ointment to both eyes of the mouse and change sterile gloves again before surgery.
- Shave the mouse's fur and disinfect the surgical site with three alternating rounds of betadine scrub and alcohol using a sterile cotton-tipped applicator in concentric circles starting at the center and moving outward (Figure 2i, ii). Make a small midline incision (~15 mm) to expose its skull. Immediately, apply the 1% lidocaine locally to the neck muscles for pain relief. Then, remove the residual tissue using scissors, and clean the skull using saline-coated cotton buds (Figure 2iii).
- Using a glass microelectrode filled with ink, mark the desired locations of the bilateral MC for implantation (Figure 2iv, v). Based on a previous study20, the locations of the bilateral MC are as follows: 0.74 mm anterior to the bregma, and 1.25 mm lateral to the midline.
- Implant four stainless steel screws (0.8 mm diameter) to protect the micro-drive system, and then link all the screws together with the reference and ground electrodes, followed by covering with mixed dental cement to form walls (Figure 2vi-xi).
- Carefully drill two small holes (~1.5 mm2) with a skull drill on both the left and right sides of the coordinated skull in the MC regions (Figure 2xii). Use the stereotaxic coordinates of the bilateral MC: 0.74 mm anterior to the bregma, 1.25 mm lateral to the midline, and 0.5 mm ventral to the dura.
- Remove the dura mater from the holes carefully with fine forceps (Figure 2xiii).
- Insert the micro-drive system into the center of the holes using a micromanipulator at 10 µm/s (Figure 2xiv-xvii).
- Fill the petroleum jelly into the dental cement walls after finishing the insertion of the micro-drive system (Figure 2xviii).
- Join the bottom plate of the micro-drive system and the dental cement walls with the mixed dental cement (Figure 2xix)
- Wash the incision with saline followed by local treatment with a gel containing lincomycin hydrochloride and lidocaine hydrochloride to relieve post-surgical pain.
- Wind the conductive copper foil tape around the implanted micro-drive system (Figure 2xx).
- Move the mouse into a cage kept at 31-33 °C and monitor the mouse for recovery from the anesthesia.
- Allow the mice to recover for 1 week with separate feeding. Check and treat the incision with 3 days of continuous application of a gel containing lincomycin hydrochloride and lidocaine hydrochloride.
3. Multichannel recording in the bilateral MC in free-moving mice
- Hold the head of an awake mouse lightly and carefully. Move down the electrode arrays (~0.1 mm depth) by twisting the screw on the movable part of the micro-drive system (Figure 1Aii) at least 1 day in advance.
- Hold the head of the awake mouse lightly and carefully. Link the center of the headstage and a helium balloon (filled with approximately 0.02 L of helium) with a thread to offset the weight of the headstage and the micro-drive system (Figure 3A, B).
- Capture raw signals using the recording electrodes and multichannel systems by sampling at 30 kHz in the recording software, and then digitize using a digital-analog (DA) converter from the multichannel systems.
- Extract the LFP signals from the raw data by resampling at 10 kHz in the recording software, and then use a notch filter from the recording software to remove the 50 Hz line noise.
- Record raw data in a stable state from a free-moving mouse for at least 60 s. After finishing the recording, slowly remove the connection between the headstage and the micro-drive system and return the mouse back to its home cage.
- Store the recorded data in the computer and analyze it offline (Figure 4 and Figure 5).
- After finishing the experiment, perform euthanasia as per the institute's guidelines and then confirm the locations of the electrodes by using the power supply at 3 V output to perform a 1 min electrolytic lesion, followed by performing histological analysis. Cut the mouse's brain into 30 µm slices using a freezing microtome, collect the MC sections, and then capture the images with a microscope (Figure 3C, D).
4. Spike sorting and analysis
- Click on File > Open > Nev files in the spike sorting software to open the spike data sampled at 30 kHz (Figure 4Ai).
- Click on Info to select the unsorted channel, and then select Sort > Change Sort Method > Use K-Means. Press the button Valley-Seeking Sort > K-Means Sorting to obtain the sorted units (Figure 4Aii, iii).
- Click on File > Save as, save the sorted spike data with a .nev filename extension, and select File > Export Per-Waveform Data to export the PCA results with a .txt filename extension (Figure 4Aiv).
- Click on File > Import Data > Blackrock File in the software for the neurophysiological data analysis to open the sorted spike file (Figure 4Bi).
- Click on Analysis > Autocorrelograms to obtain the autocorrelogram for the selected unit, and then set the parameters as follows: the X Minimum value at −0.05 s, the X Maximum value at 0.05 s, and the Bin value at 0.001 (Figure 4Bii, iii).
- Load the sorted spike data, click on Analysis > Interspike Interval Histograms to obtain the inter-spike interval histogram, and then set the parameters as follows: the Min. interval value at 0 s, the Max. interval value at 0.1 s, and the Bin value at 0.001 (Figure 4Biv, v).
- Click on Analysis > Crosscorrelograms to obtain the cross-correlogram between two sorted unit events, and then set the reference events and parameters as follows: the X Minimum value at −0.1 s, the X Maximum value at 0.1 s, and the Bin value at 0.001 (Figure 4Bvi, vii).
- Click on Results > Numerical Results to save the results of the autocorrelogram, inter-spike interval histogram, and cross-correlogram with .xls filename extensions (Figure 4Bviii, ix). Analyze the data, and draw the graph.
5. LFP analysis
- Click on File Import Data > Blackrock File in the software for the neurophysiological data analysis to open the continuous signal data sampled at 10 kHz (Figure 5Ai).
- Click on Analysis > Spectrum for Continuous to analyze the power spectrum for the LFP from the selected channel. Set the parameters as follows: the Number of Frequency Values at 8,192, the Multiple Tapers value at 3-5, the Normalization of the percentage of the total power spectral density (PSD), and the Frequency range from 1 Hz to 100 Hz (Figure 5Aii, iii).
- Click on Analysis > Coherence for Continuous to analyze the coherence for two LFPs from the left and right sides of the MC. Set the reference channel and parameters as follows: Calculate at Coherence Values, the Number of Frequency Values at 8,192, the Multiple Tapers value at 3-5, and the frequency range from 1 Hz to 100 Hz (Figure 5Aiv, v).
- Click on Analysis > Corr. with Cont. Variables to analyze the correlation between two LFPs from the left and right sides of the MC. Set the reference channel (LFP data) and parameters as follows: the X Minimum value at −0.1 s, the X Maximum value at 0.1 s, and the Bin value at 0.001 (Figure 5Avi, vii).
- Click on Results > Numerical Results to save the results of the PSD, coherence, and correlation with an .xls filename extension (Figure 5Aviii, ix).
- Select the channel for which the representative traces need to be extracted for each frequency band, click on Edit > Digital Filtering of Continuous Variables to obtain each frequency band, and then set the parameters as follows: the Filter Freq. Response as Bandpass, the Filter Implementation at infinite impulse response (IIR) Butterworth, and the Filter Order value at 2. Finally, set the frequency range of interest (Figure 5Bi-iv).
NOTE: The frequency ranges used here were as follows: delta (δ, 1-4 Hz), theta (θ, 5-12 Hz), beta (β, 13-30 Hz), low gamma (low γ, 30-70 Hz), and high gamma (high γ, 70-100 Hz) oscillations. - Analyze the data and draw the graph.
6. Correlations between the spike and LFP
- Click on File > Import Data > Blackrock File in the software for the neurophysiological data analysisto open the continuous signal data and spike data.
- Click on Analysis > Coherence Analysis to analyze the coherence between the spikes and LFP from the selected channel. Set the reference variable (spike timing) and parameters as follows: Calculate at Coherence Values, the Number of Frequency Values at 512, the Multiple Tapers value at 3-5, and the frequency range from 1 Hz to 100 Hz (Figure 5Ci, ii).
- Click on Results > Numerical Results to save the result of the spike field coherence with an .xls filename extension (Figure 5Ciii, iv).
- Analyze the data and draw the graph.
Representative Results
A high-pass (250 Hz) filter was applied to extract the multi-unit spikes from the raw signals (Figure 6A). Further, the recorded units from the MC of a normal mouse sorted by PCA were verified (Figure 7A–D), and the valley width and waveform duration of the units in the MC of the mouse were recorded. The results showed that both the valley width and waveform duration of the MC putative pyramidal neurons (Pyn) in mice are higher than those of the putative interneurons (IN) (Figure 7E,F; two sample Mann-Whitney test; for valley width, putative Pyn: 0.636 ms ± 0.004 ms, putative IN: 0.614 ms ± 0.001 ms, p = 0.002; for waveform duration, putative Pyn: 0.095 ms ± 0.004 ms, putative IN: 0.054 ms ± 0.002 ms, p = 1.402 x 10−16), corresponding to the characteristics of Pyn and IN in previous studies21. We also computed the cross-correlogram between putative Pyn and IN by setting the putative Pyn spikes as the reference and found a positive peak at ~18 ms (Figure 7G), indicating that the putative Pyn spiking occurs prior to the putative IN spiking with a window of ~18 ms.
Representative traces of each frequency band were filtered from the LFP by the IIR filter in the software for the neurophysiological data analysis (Figure 6A).In the LFP analysis, the LFPs of the left and right MC in normal mice were similar in the power spectrum, suggesting synchronized activities between the left and right MC (Figure 8A,B; two sample Mann-Whitney test; for δ, left MC: 50.71 ± 1.136, right MC: 50.47 ± 1.213, p = 0.70; for θ, left MC: 2.197 ± 0.187, right MC: 2.068 ± 0.193, p = 0.40; for β, left MC: 0.222 ± 0.058, right MC: 0.206 ± 0.055, p = 0.70; for low γ, left MC: 0.114 ± 0.034, right MC: 0.093 ± 0.018, p = 0.70; for high γ, left MC: 0.054 ± 0.027, right MC: 0.04 ± 0.015, p = 0.40). We then calculated the coherence and correlation between the left and right MC (Figure 8C,D; the left MC LFP follows within a window of ~1.2 ms after the right MC LFP, −1.167 ms ± 0.667 ms) and computed the magnitude of the putative Pyn or IN spiking synchronized with the LFP (1-100 Hz) in the left MC of a normal mouse (Figure 8E). This showed a stronger low γ coherence for the putative IN compared to the Pyn.
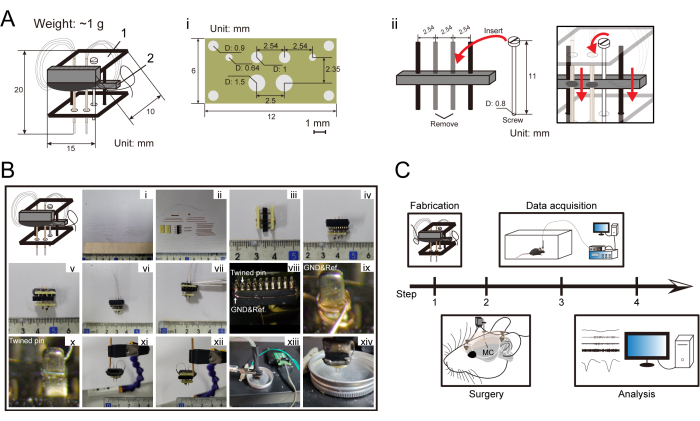
Figure 1: Diagram of the electrodes and multichannel recording system. (A) Illustration of the micro-drive system. i. Drawing and specification of the computer-designed board. ii. Schematic diagram of the movable micro-drive. (B) Micro-drive system and multichannel movable single electrode steps. i. The Ni-chrome wires; ii. The constituent parts of the electrode; iii. Assembly of the computer-designed boards; iv. Preliminary assembly of the electrodes, including the connectors and eight guide tubes; v. The other side of the micro-drive; vi,vii. The Ni-chrome wires are successively loaded into the guide tubes; viii-x. Each exposed wire is successively twined to each pin, followed by coating conducting paint onto each pin; xi,xii. The pins are covered using epoxy resin; xiii,xiv. Gold plating. (C) Experimental design of the extracellular recording in the MC of a free-moving mouse. Please click here to view a larger version of this figure.
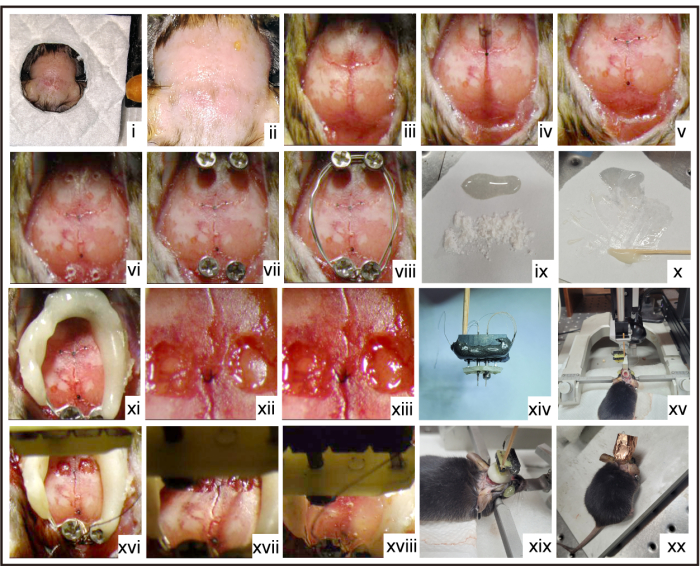
Figure 2: Step-by-step surgical procedure. i,ii. Shave the mouse's fur and disinfect the surgical site with three alternating rounds of betadine scrub and alcohol. iii. Clean the skull of the mouse. iv. Leveling. v. Mark the brain location. vi. Mark the positions of stainless-steel screws. vii. Insert stainless-steel screws. viii. Link the screws together with the reference and ground electrodes. ix,x. Mix the dental cement. xi. Build a wall with dental cement. xii,xiii. Drill two small holes above the bilateral MC, followed by removing the dura mater. xiv. Prepare the micro-drive system. xv-xix. Implant the micro-drive system followed by local treatment with a gel containing lincomycin hydrochloride and lidocaine hydrochloride to relieve post-surgical pain. xx. Protect the micro-drive system with conductive copper foil tape. Please click here to view a larger version of this figure.
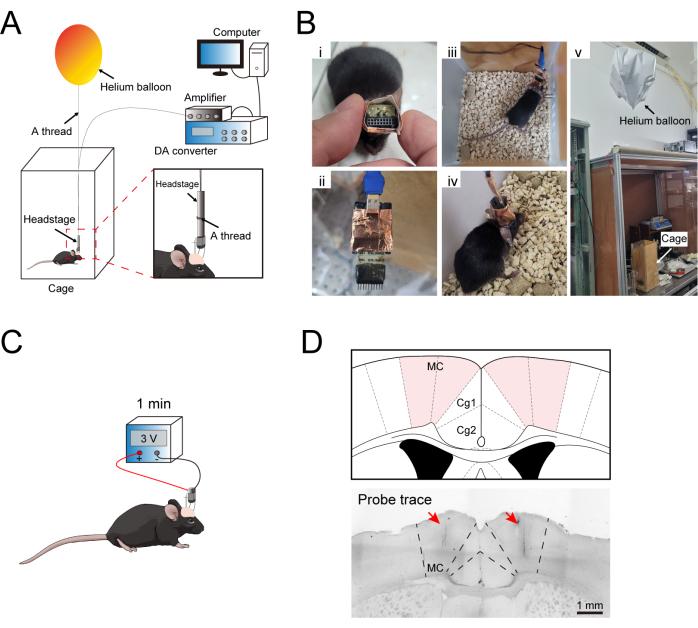
Figure 3: Illustration of a head-fixed recording in a conscious mouse. (A) Schematic diagram for free-moving recording. (B) Details of the images from the free-moving recording. i. Planform of the implanted micro-drive system; ii. Headstage; iii,iv. The micro-drive system and headstage are connected; v. The helium balloon is applied to offset the weight of the headstage and micro-drive system. (C) Illustration of verifying the location of the recording site using an electrolytic lesion. (D) The recording sites labeled by electrolytic lesions in the MC of a mouse. Please click here to view a larger version of this figure.

Figure 4: Illustration of spike sorting and analysis. (A) The parameters for clustering the spike data and exporting the results. i. Import the spike data; ii. Choose the sorting method; iii. Sort the spike data by using the κ-means algorithm; iv. Export the results from the sorted unit. (B) The process for analyzing the inter-spike interval histogram, autocorrelogram, and cross-correlogram of the sorted unit. i. Import the sorted spike data; ii. Conduct the autocorrelation analysis; iii. Set the parameters for the autocorrelogram; iv. Obtain the inter-spike interval histogram; v. Set the parameters for the inter-spike interval histogram; vi. Calculate the cross-correlation between the spikes from the sorted units; vii. Set the parameters for the cross-correlogram; viii,ix. Export the results. Please click here to view a larger version of this figure.

Figure 5: Illustration of continuous data analysis. (A) The process and parameters for analyzing the LFP signals that were calculated using the power spectrum of the LFPs, coherence, and correlation between two LFPs. i. Import the LFP data; ii. Calculate the power spectral density for the LFPs from the bilateral MC; Aiii. Calculate the power spectral density for the LFP; iv,v. Compute the coherence between LFPs; vi,vii. Calculate the correlation between two LFPs. viii,ix. Export the results. (B) The process for filtering each frequency range from the LFP signal. i. Extract the different frequency bands from the LFP data; ii,iii. View the filtered LFPs; iv. Save the filtered LFPs as an enhanced metafile. (C) The process for analyzing the coherence between the neuronal spikes and LFP. i,ii. Calculate the coherence between the LFP and sorted spikes; iii,iv. Export the results. Please click here to view a larger version of this figure.
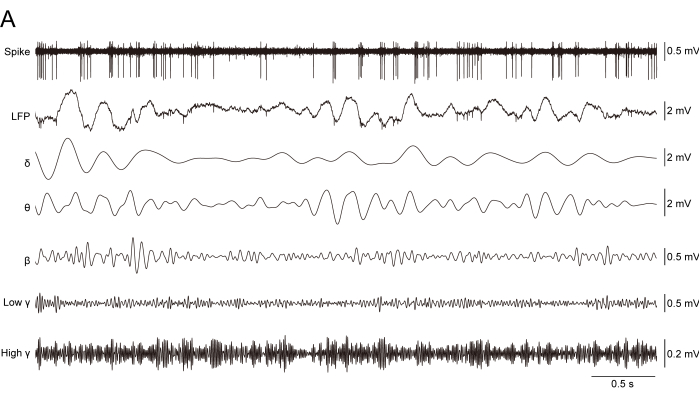
Figure 6: Representative traces of recorded signals. The spike was high-pass-filtered at 250 Hz from the raw data sampled at 30 kHz. The LFP was the raw data sampled at 10 kHz. δ was the delta frequency band bandpass-filtered at 1-4 Hz from the LFP. θ was the theta frequency band filtered at 5-12 Hz from the LFP. β was the beta frequency band filtered at 13-30 Hz from the LFP. Low γ was the low gamma frequency band filtered at 30-70 Hz from the LFP. High γ was the high gamma frequency band filtered at 70-100 Hz from the LFP. Please click here to view a larger version of this figure.
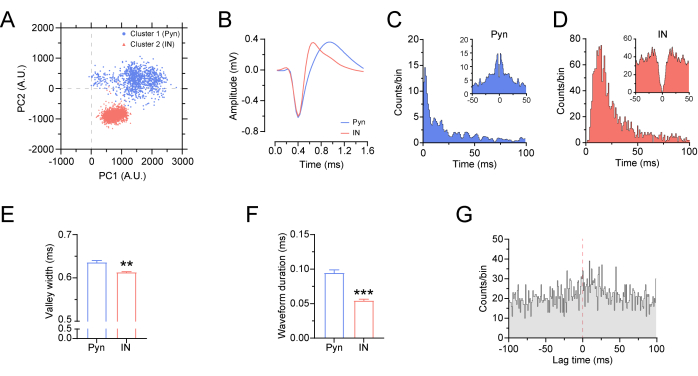
Figure 7: Characteristics of the sorted units and their firing pattern. (A,B) The sorted units were clustered using principal component analysis (PCA) from the same electrode. (C,D) Autocorrelations (top) and inter-spike interval histograms (bottom) for a putative excitatory neuron (Pyn) and a putative inhibitory neuron (IN). (E) The valley width of the putative Pyn was significantly higher than that of the putative IN (putative Pyn: n = 1,055 spikes, putative IN: n = 1,985 spikes). (F) The waveform duration of the putative Pyn was stronger than that of the putative IN (putative Pyn: n = 1,005 spikes, putative IN: n = 1,059 spikes). (G) The cross-correlation between the putative Pyn and IN. Statistical analysis with a Mann-Whitney test. All the data are presented as mean ± standard error of the mean, **p < 0.01, ***p < 0.001. Please click here to view a larger version of this figure.
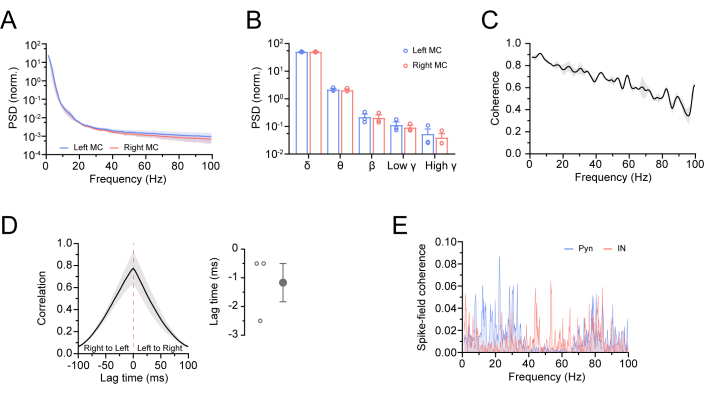
Figure 8: Analysis of two LFPs from the bilateral MC and the coherence between spike events and the LFP in mice. (A,B) Normalized power spectrums of the bilateral MC at each frequency band in mice (n = 3). (C) The curve of the coherence of two LFPs between the left and right MC (n = 3). (D) The cross-correlation curve of two LFPs showing a correlation between the left and right MC at ±100 ms time lags (n = 3). (E) The curve of spike-field coherence in the MC of a mouse. Statistical analysis with a Mann-Whitney test. All the data are presented as mean ± standard error of the mean. Please click here to view a larger version of this figure.
Discussion
Multichannel recording in free-moving mice has been deemed to be a useful technology in neuroscience studies, but it is still quite challenging for beginners to record and analyze the signals. In the present study, we provide simplified guidelines for making micro-drive systems and performing electrode implantation, as well as simplified procedures for capturing and analyzing the electrical signals via spike sorting software and software for neurophysiological data analysis.
Given that the quality of a custom-made micro-drive system greatly contributes to the acquisition of stable and qualitative signals in free-moving mice14,15,16,17, we designed and used a more sturdy and lightweight structure for the micro-drive system in this study, and beginners can easily and clearly follow the manufacturing steps for constructing the micro-drive system. In addition, the structure of the designed micro-drive system involves inexpensive materials that are easily available in hardware stores, unlike for the larger, heavier micro-drive systems used in previous studies14,15. This micro-drive system can decrease discomfort and withstand impact from free-moving mice during recordings. Meanwhile, we further improved the size and shape of the micro-drive system, which might be helpful for beginners by allowing them to observe, insert, and thus, move the tips of electrodes into the brain during surgery. Further, the simple slide structure applied in the micro-drive system is accurately progressed in the brain using a high-precision screw, meaning this system provides precise control in measuring multiple layers of the targeted brain area; indeed, this is extremely important for capturing extracellular signals in a free-moving animal over a long-term experimental period. Above all, the advantages of this micro-drive system are its simplicity and flexibility; however, the smaller number of channels and the use of an array of single electrodes should be further improved in a new version.
There are several improvements in the present study worth noting as well. Due to the smaller size and modified shape of the micro-drive system compared to previous systems, a broader vision and wider working space were supplied for the operation. Moreover, the walls on the mouse's skull were made of dental cement and stainless-steel screws, which allowed the micro-drive system to be strongly attached to the head of the mouse. In addition, the dental cement walls allowed for the loading of petroleum jelly to cover the holes in the mouse's skull before pouring the dental cement, which had protective effects on the brain surface without the dura and the movable part of the micro-drive system. Together, these improvements are useful, as they allow beginners to implant the micro-drive system easily and confidently into the mouse brain.
In multichannel extracellular recordings, it is widely believed that another difficulty lies in analyzing the recorded signals using a mathematically complex programming language17. Thus, we provide clear guidelines for beginners, particularly in terms of spike sorting, LFP data analysis, and computing the relationship between them using a commonly used software in electrophysiology. In addition, we strongly suggest that the recorded unit from a single-electrode assay clustered by PCA methods should have a high number of features for analysis, such as the sorted neuronal inter-spike intervals and the width between its valley and peak, as these values are useful for beginners to reduce bias when the units are clustered automatically with offline sorter software. Importantly, the relationship between signals that include spikes and the LFP is critical for mediating multiple behaviors. We also provide a series of straightforward illustrations for measuring spike-spike, LFP-LFP, and spike-LFP correlations using credible scripts in the software for neurophysiological data analysis; these illustrations will allow beginners to start processing and analyzing the recorded signals quickly in free-moving mice. Further, the results and data treated with this proprietary software can be used in conjunction with an open-source toolbox such as Fieldtrip for additional analysis in advance.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31871170, 32170950, and 31970915), the Natural Science Foundation of Guangdong Province (2021A1515010804 and 2023A1515010899), the Guangdong Natural Science Foundation for Major Cultivation Project (2018B030336001), and the Guangdong Grant: Key Technologies for Treatment of Brain Disorders(2018B030332001).
Materials
| 2.54 mm pin header | YOUXIN Electronic Co., Ltd. | 1 x 5 | Applying for the movable micro-drive which can slide on its stulls. |
| Adobe Illustrator CC 2017 | Adobe | N/A | To optimize images from GraphPad. |
| BlackRock Microsystems | Blackrock Neurotech | Cerebus | This systems includes headsatge, DA convert, amplifier and computer. |
| Brass nut | Dongguan Gaosi Technology Co., Ltd. | M0.8 brass nut | The nut fixes the position of screw. |
| Brass screw | Dongguan Gaosi Technology Co., Ltd. | M0.8 x 11 mm brass screw | A screw that hold the movable micro-drive. |
| C57BL/6J | Guangdong Zhiyuan Biomedical Technology Co., LTD. | N/A | 12 weeks of age. |
| Centrifuge tube | Biosharp | 15 mL; BS-150-M | To store mice brain with sucrose sulutions. |
| Conducting paint | Structure Probe, Inc. | 7440-22-4 | To improve the lead-connecting quality between connector pins and Ni-wires. |
| Conductive copper foil tape | 3M | 1181 | To reduce interferenc. |
| Connector | YOUXIN Electronic Co., Ltd. | 2 x 10P | To connect the headtage to micro-drive system. |
| DC Power supply | Maisheng | MS-305D | A power device for electrolytic lesion. |
| Dental cement | Shanghai New Century Dental Materials Co., Ltd. | N/A | To fix the electrode arrays on mouse's skull after finishing the implantation. |
| Digital analog converter | Blackrock | 128-Channel | A device that converts digital data into analog signals. |
| Epoxy resin | Alteco | N/A | To cover pins. |
| Excel | Microsoft | N/A | To summarize data after analysis. |
| Eye scissors | JiangXi YuYuan Medical Equipment Co.,Ltd. | N/A | For surgery or cutting the Ni-chrome wire. |
| Fine forceps | JiangXi YuYuan Medical Equipment Co.,Ltd. | N/A | For surgery. |
| Forceps | JiangXi YuYuan Medical Equipment Co.,Ltd. | N/A | For surgery or assembling the mirco-drive system. |
| Freezing microtome | Leica | CM3050 S | Cut the mouse’s brain into slices |
| Fused silica capillary tubing | Zhengzhou INNOSEP Scientific Co., Ltd. | TSP050125 | To serve as the guide tubes for Ni-chrome wires. |
| Glass microelectrode | Sutter Instrument Company | BF100-50-10 | To mark the desired locations for implantation using the filled ink. |
| GraphPad Prism 7 | GraphPad Software | N/A | To analyze and visualize the results. |
| Guide-tube | Polymicro technologies | 1068150020 | To load Ni-chrome wires. |
| Headstage | Blackrock | N/A | A tool of transmitting signals. |
| Helium balloon | Yili Festive products Co., Ltd. | 24 inch | To offset the weight of headstage and micro-drive system. |
| Ink | Sailor Pen Co.,LTD. | 13-2001 | To mark the desired locations for implantation. |
| Iodine tincture | Guangdong Hengjian Pharmaceutical Co., Ltd. | N/A | To disinfect mouse's scalp. |
| Lincomycin in Hydrochloride and Lidocaine hydrochloride gel | Hubei kangzheng pharmaceutical co., ltd. | 10g | A drug used to reduce inflammation. |
| Meloxicam | Vicki Biotechnology Co., Ltd. | 71125-38-7 | To reduce postoperative pain in mice. |
| Micromanipulators | Scientifica | Scientifica IVM Triple | For electrode arrays implantation. |
| Microscope | Nikon | ECLIPSE Ni-E | Capture the images of brain sections |
| nanoZ impedance tester | Plexon | nanoZ | To measure impedance or performing electrode impedance spectroscopy (EIS) for multichannel microelectrode arrays. |
| NeuroExplorer | Plexon | NeuroExplorer | A tool for analyzing the electrophysiological data. |
| NeuroExplorer | Plexon, USA | N/A | A software. |
| Ni-chrome wire | California Fine Wire Co. | M472490 | 35 μm Ni-chrome wire. |
| Offline Sorter | Plexon | Offline Sorter | A tool for sorting the recorded multi-units. |
| PCB board | Hangzhou Jiepei Information Technology Co., Ltd. | N/A | Computer designed board. |
| Pentobarbital | Sigma | P3761 | To anesthetize mice. |
| Pentobarbital sodium | Sigma | 57-33-0 | To anesthetize the mouse. |
| Peristaltic pump | Longer | BT100-1F | A device used for perfusion |
| Polyformaldehyde | Sangon Biotech | A500684-0500 | The main component of fixative solution for fixation of mouse brains |
| PtCl4 | Tianjin Jinbolan Fine Chemical Co., Ltd. | 13454-96-1 | Preparation for gold plating liquid. |
| Saline | Guangdong Hengjian Pharmaceutical Co., Ltd. | N/A | To clean the mouse's skull. |
| Silver wire | Suzhou Xinye Electronics Co., Ltd. | 2 mm diameter | Applying for ground and reference electrodes. |
| Skull drill | RWD Life Science | 78001 | To drill carefully two small holes on mouse's skull. |
| Stainless steel screws | YOUXIN Electronic Co., Ltd. | M0.8 x 2 | To protect the micro-drive system and link the ground and reference electrodes. |
| Stereotaxic apparatus | RWD Life Science | 68513 | To perform the stereotaxic coordinates of bilateral motor cortex. |
| Sucrose | Damao | 57-50-1 | To dehydrate the mouse brains after perfusion. |
| Super glue | Henkel AG & Co. | PSK5C | To fix the guide tube and Ni-chrome wire. |
| Temperature controller | Harvard Apparatus | TCAT-2 | To maintain mouse's rectal temperature at 37°C |
| Tetracycline eye ointment | Guangdong Hengjian Pharmaceutical Co., Ltd. | N/A | To protect the mouse's eyes during surgery. |
| Thread | Rapala | N/A | To link ballon and headstage. |
| Vaseline | Unilever plc | N/A | To cover the gap between electrode arrays and mouse's skull. |
References
- Buzsáki, G., Anastassiou, C. A., Koch, C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience. 13 (6), 407-420 (2012).
- Singer, W. Synchronization of cortical activity and its putative role in information processing and learning. Annual Review of Physiology. 55, 349-374 (1993).
- Arroyo-García, L. E., et al. Impaired spike-gamma coupling of area CA3 fast-spiking interneurons as the earliest functional impairment in the App(NL-G-F) mouse model of Alzheimer’s disease. Molecular Psychiatry. 26 (10), 5557-5567 (2021).
- Ozawa, M., et al. Experience-dependent resonance in amygdalo-cortical circuits supports fear memory retrieval following extinction. Nature Communications. 11 (1), 4358 (2020).
- Vinck, M., Batista-Brito, R., Knoblich, U., Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron. 86 (3), 740-754 (2015).
- Beck, M. H., et al. long-term dopamine depletion causes enhanced beta oscillations in the cortico-basal ganglia loop of parkinsonian rats. Experimental Neurology. 286, 124-136 (2016).
- Magill, P. J., Bolam, J. P., Bevan, M. D. Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. Journal of Neuroscience. 20 (2), 820-833 (2000).
- Magill, P. J., et al. Changes in functional connectivity within the rat striatopallidal axis during global brain activation in vivo. Journal of Neuroscience. 26 (23), 6318-6329 (2006).
- Rapeaux, A. B., Constandinou, T. G. Implantable brain machine interfaces: First-in-human studies, technology challenges and trends. Current Opinion in Biotechnology. 72, 102-111 (2021).
- Tort, A. B., et al. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proceedings of the National Academy of Sciences of the United States of America. 105 (51), 20517-20522 (2008).
- Yamamoto, J., Wilson, M. A. Large-scale chronically implantable precision motorized microdrive array for freely behaving animals. Journal of Neurophysiology. 100 (4), 2430-2440 (2008).
- Chang, E. H., Frattini, S. A., Robbiati, S., Huerta, P. T. Construction of microdrive arrays for chronic neural recordings in awake behaving mice. Journal of Visualized Experiments. (77), e50470 (2013).
- Vandecasteele, M., et al. Large-scale recording of neurons by movable silicon probes in behaving rodents. Journal of Visualized Experiments. (61), e3568 (2012).
- Lansink, C. S., et al. A split microdrive for simultaneous multi-electrode recordings from two brain areas in awake small animals. Journal of Neuroscience Methods. 162 (1-2), 129-138 (2007).
- Sato, T., Suzuki, T., Mabuchi, K. A new multi-electrode array design for chronic neural recording, with independent and automatic hydraulic positioning. Journal of Neuroscience Methods. 160 (1), 45-51 (2007).
- van Daal, R. J. J., et al. Implantation of Neuropixels probes for chronic recording of neuronal activity in freely behaving mice and rats. Nature Protocols. 16 (7), 3322-3347 (2021).
- Unakafova, V. A., Gail, A. Comparing open-source toolboxes for processing and analysis of spike and local field potentials data. Frontiers in Neuroinformatics. 13, 57 (2019).
- Mao, L., Wang, H., Qiao, L., Wang, X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-alpha, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators of Inflammation. 2010, 238321 (2010).
- Jin, Z., Zhang, Z., Ke, J., Wang, Y., Wu, H. Exercise-linked irisin prevents mortality and enhances cognition in a mice model of cerebral ischemia by regulating Klotho expression. Oxidative Medicine and Cellular Longevity. 2021, 1697070 (2021).
- Ding, X., et al. Spreading of TDP-43 pathology via pyramidal tract induces ALS-like phenotypes in TDP-43 transgenic mice. Acta Neuropathologica Communications. 9 (1), 15 (2021).
- Cao, W., et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron. 97 (6), 1253-1260 (2018).

