Escherichia coli -Based Complementation Assay to Study the Chaperone Function of Heat Shock Protein 70
Summary
This protocol demonstrates the chaperone activity of heat shock protein 70 (Hsp70). E. coli dnaK756 cells serve as a model for the assay as they harbor a native, functionally impaired Hsp70, making them susceptible to heat stress. The heterologous introduction of functional Hsp70 rescues the growth deficiency of the cells.
Abstract
Heat shock protein 70 (Hsp70) is a conserved protein that facilitates the folding of other proteins within the cell, making it a molecular chaperone. While Hsp70 is not essential for E. coli cells growing under normal conditions, this chaperone becomes indispensable for growth at elevated temperatures. Since Hsp70 is highly conserved, one way to study the chaperone function of Hsp70 genes from various species is to heterologously express them in E. coli strains that are either deficient in Hsp70 or express a native Hsp70 that is functionally compromised. E. coli dnaK756 cells are unable to support λ bacteriophage DNA. Furthermore, their native Hsp70 (DnaK) exhibits elevated ATPase activity while demonstrating reduced affinity for GrpE (Hsp70 nucleotide exchange factor). As a result, E. coli dnaK756 cells grow adequately at temperatures ranging from 30 °C to 37 °C, but they die at elevated temperatures (>40 °C). For this reason, these cells serve as a model for studying the chaperone activity of Hsp70. Here, we describe a detailed protocol for the application of these cells to conduct a complementation assay, enabling the study of the in cellulo chaperone function of Hsp70.
Introduction
Heat shock proteins play an important role as molecular chaperones by facilitating protein folding, preventing protein aggregation, and reversing protein misfolding1,2. Heat shock protein 70 (Hsp70) is one of the most prominent molecular chaperones, playing a central role in protein homeostasis3,4. DnaK is the E. coli Hsp70 homologue5.
Various biophysical, biochemical, and cell-based assays have been developed to explore the chaperone activity of Hsp70 and to screen for inhibitors targeting this chaperone6,7,8. Hsp70 is a highly conserved protein. For this reason, several Hsp70s of eukaryotic organisms, such as Plasmodium falciparum (the main agent of malaria), have been reported to substitute for DnaK function in E. coli6,9. In this way, an E. coli-based complementation assay has been developed involving the heterologous expression of Hsp70s in E. coli to explore their cytoprotective function. Typically, this assay involves the utilization of E. coli cells that are either deficient for DnaK or that express a native DnaK that is functionally compromised. While DnaK is not essential for E. coli growth under normal conditions, it becomes essential when the cells are grown under stressful conditions such as elevated temperatures or other forms of stress10,11.
E. coli strains that have been developed to study Hsp70 function using a complementation assay include E. coli dnaK103 (BB2393 [C600 dnaK103(Am) thr::Tn10]) and E. coli dnaK756. E. coli dnaK103 cells produce a truncated DnaK that is non-functional, and as such, the cells grow adequately at 30 °C, while the strain is sensitive to cold and heat stress12,13. Similarly, the E. coli dnaK756/BB2362 (dnaK756 recA::TcR Pdm1,1) strain does not grow above 40 °C14,15. The E. coli dnaK756 strain expresses a mutant native DnaK (DnaK756) characterized by three glycine-to-aspartate substitutions at positions 32, 455, and 468, giving rise to compromised proteostatic outcomes. Consequently, this strain is resistant to bacteriophage λ DNA14. Additionally, E. coli dnaK756 exhibits elevated ATPase activity, while its affinity for the nucleotide exchange factor, GrpE, is reduced16. E. coli DnaK mutant strains serve as ideal models for investigating the chaperone activity of Hsp70 through a complementation approach. Since DnaK is only essential under stressful conditions, the complementation assay is typically conducted at elevated temperatures (Figure 1). Some advantages of using E. coli for this study include its well-characterized genome, rapid growth, and the low cost of culturing and maintenance17.
In this article, we describe in detail a protocol involving the use of E. coli dnaK756 cells to study the function of Hsp70. The Hsp70s we employed in the assay are wild-type DnaK and its chimeric derivative, KPf (made up of the ATPase domain of DnaK fused to the C-terminal substrate-binding domain of Plasmodium falciparum Hsp70-16,18). KPf-V436F was heterologously expressed as a negative control since the mutation essentially blocks it from binding substrates, thus abrogating its chaperone activity9.
Protocol
1. Transformation
NOTE: Use sterile glassware for culture, pipette tips, and freshly prepared and autoclaved media. Prepare cultures of the E. coli cells in 2x yeast tryptone (YT) [1.6% tryptone (w/v), 1% yeast extract (w/v), 0.5% NaCl (w/v), 1.5% agar (w/v)] agar. General reagents used in the protocol and their sources are provided in the Table of Materials.
- Label 2.0 mL microcentrifuge tubes and aliquot 50 µL of competent E. coli dnaK756cells, keeping the cells on ice.
- To the 2.0 mL microcentrifuge tubes with competent cells, aliquot 10-50 ng of pQE60/DnaK, pQE60/KPf, and pQE60/KPf-V436F plasmid DNA9 into separate tubes.
- Keep the 2.0 mL microcentrifuge tubes containing the competent cells and plasmid DNA on ice for 30 min.
- Heat shock the competent cells-DNA mix for 60 s at 42 °C and return the microcentrifuge tubes back on ice for 10 min.
- Add 950 µL of fresh 2x YT broth (pre-incubated at 37 °C) and incubate at 37 °C while shaking at 150 revolutions/min for 1 h. Leave the cells to grow for much longer to encourage their recovery if necessary.
NOTE: Avoid shaking the cells vigorously. - Pipette 100 µL of the cells and spread them onto 2x YT agar plates containing 50 µg/mL kanamycin, 10 µg/mL tetracycline for the respective strain, and 100 µg/mL ampicillin (see Table of Materials) for plasmid selection.
- Centrifuge the rest of the cells (whose volume is now approximately 900 µL) for 1 min at 5000 x g (at 4 °C) using a benchtop microcentrifuge.
- Decant about 800 µL of the broth and use the remaining medium to resuspend the pelleted cells.
- Plate the recovered cells onto the 2x YT agar plate.
- Incubate both agar plates overnight (or approximately 17 h) at 37 °C.
NOTE: These cells grow very slowly and may need to be incubated for much longer. Be careful to spot the colonies which may start off very small. The plate containing cells resuspended after centrifugation (step 1.7) serves as a back-up in case the transformation efficiency of the cells is poor, in which case the pelleted cells may improve the recovery of any transformed cells. However, if the transformation efficiency is excellent, the agar plate onto which concentrated cells were plated may be characterized by an overgrown culture upon incubation, making it difficult to identify single colonies. In that case, the other agar plate may be the one on which well-spaced colonies grow.
2. Cell plating
- Pick up a single colony from the transformants and inoculate it into 10 mL of 2x YT broth supplemented with 50 µg/mL of kanamycin, 10 µg/mL of tetracycline for the selection of the E. coli dnaK756 cells, and 100 µg/mL ampicillin for plasmid selection.
NOTE: Use flasks (≥50 mL) to ensure aeration when agitating the culture. Incubate the inoculum overnight (17 h) at 37 °C while shaking at 150 rotations/min. - Take an absorbance reading at OD600 the following morning.
- Clean the workbench surface using 75% ethanol to swab the surface in preparation for cell spotting.
- Using a 2 mL microcentrifuge tube, standardize the culture to an OD600 reading of 2.0 using 2x YT broth.
NOTE: Ensure that the OD readings are taken correctly as this step is important for standardizing cell density across the various samples. - Using 2 mL microcentrifuge tubes, prepare serial dilutions of the cells from 100 to 10-5.
- Incubate the agar plates to be used to spot the cells onto in an oven set at 40 °C to allow the plates to dry with lids partially open to ensure water vapor escapes.
NOTE: To ensure the cells are spotted at uniformly separated distances, draw lines on a piece of paper onto which a template of spotting sites is printed. - Spot 2 µL of the serially diluted cells onto the agar plates supplemented with 50 µg/mL kanamycin, 10 µg/mL tetracycline, 100 µg/mL ampicillin, and 0.5 mM IPTG (for the induction of expression of the recombinant proteins) (see Table of Materials).
- Spot each sample onto two separate plates (one to be incubated at 37 °C and the other at 43.5 °C).
NOTE: Avoid piercing the agar plate. - Spot control samples onto the same plate as the experimental samples to minimize environmental effects.
- Conduct the spotting quickly to ensure the process is completed before the cells start growing, as this may generate skewed growth patterns.
NOTE: It is important to avoid aerosols when spotting, as these would contaminate the plates. It is also important to keep the plates closed in between the spotting to avoid contamination. - Incubate one plate at 37 °C (permissive growth temperature) and the other at the non-permissive growth temperature of 43.5 °C.
NOTE: Incubate the plates facing upside down to avoid steam collecting onto the lid, as the condensed water would wash the spotted colonies off their positions. - Place all the plates in the incubators at the same time and avoid opening the incubator until the following morning.
NOTE: Opening the incubator door several times during incubation of the plates is discouraged. This is because the access of air into the incubator may result in temperature fluctuations that adversely impact cell growth.
3. Confirming expression of recombinant proteins
- Using a sterile loop, pick up part of the remaining cells from the same colony of transformed E. coli dnaK756 cells.
- Inoculate the cells into 10 mL YT broth supplemented with 50 µg/mL kanamycin, 10 µg/mL tetracycline, and 100 µg/mL ampicillin. Incubate overnight (17 h) at 37 °C while shaking at 150 revolutions/min.
- Transfer the culture into 90 mL of sterile 2x YT broth containing the necessary antibiotics as mentioned in step 3.2. Let the cells grow to mid-log phase (OD600 = 0.4-0.6).
- Take 2 mL of the sample culture before induction (0 h induction sample).
- Add IPTG to a final concentration of 1 mM to induce protein production and re-incubate the cells at 37 °C.
- Take a second 2 mL sample 6 h post-induction.
- Harvest the cells by centrifuging at 5000 x g for 10 min. Keep the centrifuge temperature at 4 °C.
- Discard the supernatant.
- Resuspend the pellet in PBS buffer (137 mM NaCl, 27 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4) and store at -20 °C.
4. SDS-PAGE and western blot analyses
- Prepare 2x 10% SDS gels as previously described19.
- Keep one of the SDS-PAGE gels for subsequent staining using Coomassie stain to view the protein bands. Use the second SDS-PAGE gel to conduct western blot analysis.
- Aliquot 80 µL of the resuspended samples and mix it with 20 µL of 4x Laemmli SDS loading buffer.
- Boil the suspension at 100 °C for 10 min. Then load 10 µL of each sample onto the precast SDS gel (see Table of Materials).
- Perform electrophoresis at room temperature for 1 h using a voltage of 120 V in a 1x solution of SDS running buffer (25 mm Tris, 250 mm glycine, 0.1% (w/v) SDS).
- Stain the gel with Coomassie stain (see Table of Materials) for 1 h, followed by destaining using destaining buffer (50% (v/v) methanol, 10% (v/v) acetic acid in distilled water) for 2 h.
- Visualize the protein bands present in the gel using a gel imaging system (see Table of Materials). Rerun another SDS-PAGE gel with the same samples following the above-mentioned protocol.
- When the electrophoretic run ends, take SDS-PAGE gels to conduct western blot analysis as previously described19.
- Wash the nitrocellulose membrane onto which the proteins were transferred three times in wash buffer (TBS-Tween, pH 7.4 [50 mM Tris, 150 mM NaCl, 1% (v/v) Tween]).
- Use α-DnaK (see Table of Materials) to detect DnaK and α-PfHsp70-17 to detect KPf and its mutant, KPf-V436F), respectively.
- Use the antibodies at a 1:2000 dilution in 5% non-fat milk. Incubate at 4 °C while shaking at 60 revolutions/min for 1 h.
- Remove non-specifically bound antibody by washing the nitrocellulose membrane in TBS-Tween 3 times in 15 min.
- Incubate the membrane in the secondary antibody (α-rabbit, see Table of Materials) under the same conditions as the previous step. This is followed by subsequent washing under the same conditions as the primary antibody.
- Resolve the bands using enhanced chemiluminescence (ECL) detection reagent.
- Visualize the bands using a gel imager.
Representative Results
Figure 2 presents an image of the scanned agar containing cells that were spotted and cultured at the permissive growth temperature of 37 °C and 43.5 °C, respectively. On the right-hand side of Figure 2, excised western blot components represent the expression of DnaK, KPf, and KPf-V436F in E. coli dnaK756 cells. As expected, all the E. coli dnaK756 cells cultured at the permissive growth temperature of 37 °C managed to grow. However, under the non-permissive growth conditions of 43.5 °C, only cells heterologously expressing DnaK and KPf managed to grow, as previously reported6,9,20(Figure 2). On the other hand, cells expressing KPf-V436F only grew at 37 °C but failed to grow at 43.5 °C. This demonstrates that DnaK and KPf were able to restore the growth defect of E. coli dnaK756 cells under heat stress conditions. The failure of cells heterologously expressing KPf-V436F to support the growth of cells at 43.5 °C demonstrates the lack of chaperone function of this protein. In this regard, KPf-V436F serves as an ideal negative control protein.
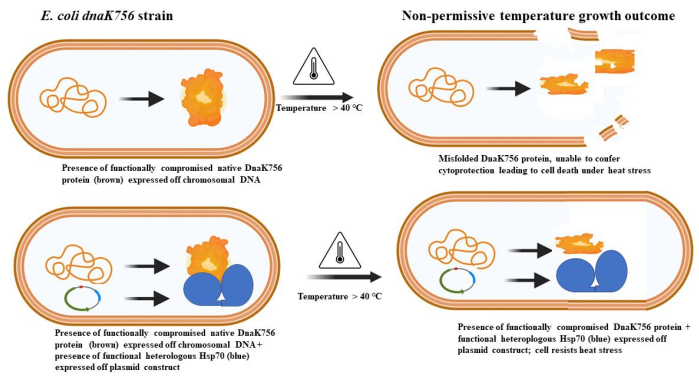
Figure 1: Principle of the complementation assay using E. coli dnaK756 cells to study the chaperone function of heterologously expressed proteins. E. coli dnaK756 expresses a native DnaK756 protein that is unable to protect the cells against heat stress. Introduction of functional heterologous Hsp70 rescues the cells from death upon exposure to heat stress. Please click here to view a larger version of this figure.
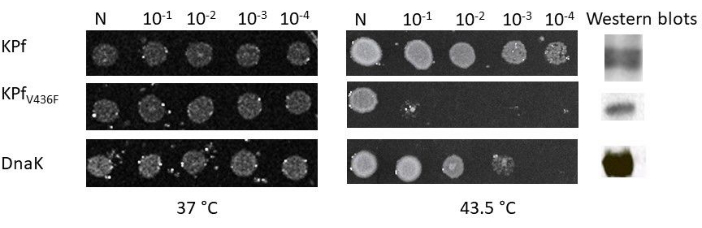
Figure 2: Complementation plate assay demonstrating the capabilities of DnaK and KPf to protect E. coli dnaK756 cells against heat stress. The transformed cells were cultured at 37 °C (permissive growth temperature) and 43.5 °C (non-permissive growth temperature). The cells were standardized and plated as serial dilutions. 'N' symbolizes 'Neat,' representing the first spot composed of undiluted cells. On the extreme right-hand side are the western blot excisions representing expression of the three proteins. Please click here to view a larger version of this figure.
Discussion
The protocol demonstrates the utility of E. coli dnaK756cells in exploring the chaperone function of heterologously expressed Hsp70. This assay could be adopted to screen inhibitors targeting Hsp70 function in cellulo. However, one limitation of this method is that Hsp70s unable to substitute for DnaK in E. coli are not compatible with this assay. Lack of post-translational modification21 of some non-native Hsp70s may account for their lack of function within the E. coli system. A yeast-based complementation assay22 may address some of the shortcomings of the E. coli-based assay.
Several key steps are crucial to ensuring reproducible results. These include ensuring that only cells transformed by the respective plasmid construct are used during plating. In addition, it is important to avoid contamination of the culture throughout the steps. Furthermore, since stressed E. coli DnaK cells are susceptible to excessive filamentation10, it is important to avoid vigorous shaking during culture, as this physical strain promotes extensive filamentation. Excessively filamented cells give higher false apparent growth readings at OD600, leading to an overestimation of culture growth and adversely impacting cell standardization prior to plating. Although Hsp70 is not essential for E. coli growth under normal conditions, cells lacking this protein are stress susceptible10. For this reason, E. coli dnaK756 cells grow much slower after being transformed or during their recovery from glycerol stocks. Additionally, they are extremely sensitive to temperature fluctuations. Therefore, it is important to avoid opening the incubator door once the plated agar plates are placed inside until it is time to view the plates.
The western blot data are important to confirm the expression of the respective recombinant Hsp70 proteins in E. coli dnaK756 cells. Failure to express the respective protein leads to a false-negative result for cells growing under non-permissive growth temperature.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The work was supported with grant funding obtained from the International Centre for Genetic Engineering and Biotechnology (ICGEB) grant number, HDI/CRP/012, Research Directorate of the University of Venda, grant I595, Department of Science and Innovation (DSI) and the National Research Foundation (NRF) of South Africa (grant numbers, 75464 & 92598) awarded to AS.
Materials
| 2-β-Mercaptoethanol | Sigma-Aldrich | 8,05,740 | Constituent for sample loading dye |
| Acetic acid | Labchem | 101005125 | Constituent of destainer |
| Acrylamide | Sigma-Aldrich | 8008300100 | Component of SDS |
| Agar | Merck | HG000BX1.500 | Constituent of medium and liquid growth assay |
| Agarose | Clever Scientific | 14131031 | Certified molecular biology agarose |
| Ammonium persulfate | Sigma-Aldrich | 101875295 | Constituent for SDS-PAGE gel |
| Ampicillin | VWR International | 0339—EU—25G | Selective antibiotic |
| Bis | Sigma-aldrich | 1015460100 | Component of SDS |
| Bromophenol | Sigma-Aldrich | 0449-25G | Constituent for sample loading dye |
| CaCl2 | Sigma-Aldrich | 10043-52-4 | For competent cells preparation |
| Coomassie brilliant blue | VWR International | 443293X | SDS-PAGE dye |
| Dibasic sodium phosphate | Sigma-Aldrich | RB10368 | Constituent of PBS buffer |
| ECL | Thermofischer Scientific | 32109 | Western blot detection reagent |
| Ethidium Bromide | Thermofischer Scientific | 17898 | DNA intercalating dye |
| Glycerol | Merck | SAAR2676520L | Constituent for sample loading dye |
| Glycine | VWR International | 10119CU | Component of SDS |
| IPTG | Glentham life sciences | 162IL | inducer |
| Kanamycin | Melford | K0126 | Selective antibiotic |
| Magnesium Chloride | Merck | SAAR4123000EM | Constituent of medium and liquid growth assay |
| Methanol | Labchem | 113140129 | Constituent of destainer |
| Monobasic potassium phosphate | Merck | 1,04,87,30,250 | Constituent of PBS buffer |
| Peptone | Merck | HG000BX4.250 | Constituent of medium and liquid growth assay |
| Potassium chloride | Merck | SAAR5042020EM | Constituent of PBS buffer |
| PVDF membrane | Thermofischer scientific | PB7320 | Western blot membrane |
| Sodium Chloride | Merck | SAAR5822320EM | Constituent of medium and liquid growth assay |
| Sodium dodecyl sulphate | VWR International | 108073 | To resolve expressed proteins |
| Spectramax iD3 | Separations | 373705019 | Automated plate reader |
| TEMED | VWR international | ACRO420580500 | Component of SDS gel |
| Tetracycline | Duchefa Biochemies | T0150.0025 | Selective antibiotic |
| Tris | VWR International | 19A094101 | Component of SDS gel |
| Tween20 | Merck | SAAR3164500XF | Constituent for Western wash buffer |
| Western transfer chamber | Thermofisher Scientific | PB0112 | Transfer of protein to nitrocellulose membrane |
| Yeast extract | Merck | HG000BX6.500 | Constituent of medium and liquid growth assay |
| α-DnaK antibody | Inqaba | BK CAC09317 | Primary antibody |
| α-rabbit antibody | Thermofischer scientific | 31460 | Secondary antibody |
References
- Bukau, B., Deuerling, E., Pfund, C., Craig, E. A. Getting newly synthesized proteins into shape. Cell. 101 (2), 119-122 (2000).
- Shonhai, A. Plasmodial heat shock proteins: targets for chemotherapy. FEMS Microbiol. Immunol. 58 (1), 61-74 (2010).
- Mogk, A., et al. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18 (24), 6934-6949 (1999).
- Edkins, A. L., Boshoff, A., Shonhai, A., Picard, D., Blatch, G. L. General structural and functional features of molecular chaperones. Heat shock proteins of malaria. Adv Exp Med Biol. , (2021).
- Bertelsen, E. B., Chang, L., Gestwicki, J. E., Zuiderweg, E. R. Solution conformation of wild-type E. coli. Hsp70 (DnaK) chaperone complexed with ADP and substrate. PNAS. 106 (21), 8471-8476 (2009).
- Shonhai, A., Boshoff, A., Blatch, G. L. Plasmodium falciparum heat shock protein 70 is able to suppress the thermosensitivity of an Escherichia coli DnaK mutant strain. Mol Genet Genomics. 274, 70-78 (2005).
- Shonhai, A., Botha, M., de Beer, T. A., Boshoff, A., Blatch, G. L. Structure-function study of a Plasmodium falciparum Hsp70 using three-dimensional modelling and in vitro analyses. Protein Pept Lett. 15 (10), 1117-1125 (2008).
- Cockburn, I. L., Boshoff, A., Pesce, E. -. R., Blatch, G. L. Selective modulation of plasmodial Hsp70s by small molecules with antimalarial activity. Biol Chem. 395 (11), 1353-1362 (2014).
- Makhoba, X. H., et al. Use of a chimeric Hsp70 to enhance the quality of recombinant Plasmodium falciparum s-adenosylmethionine decarboxylase protein produced in Escherichia coli. PLoS One. 11 (3), 0152626 (2016).
- Bukau, B., Walker, G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bact. 171 (5), 2337-2346 (1989).
- Makumire, S., Revaprasadu, N., Shonhai, A. DnaK protein alleviates toxicity induced by citrate-coated gold nanoparticles in Escherichia coli. PLoS One. 10 (4), 0121243 (2015).
- Spence, J., Cegielska, A., Georgopoulos, C. Role of Escherichia coli heat shock proteins DnaK and HtpG (C62. 5) in response to nutritional deprivation. J Bact. 172 (12), 7157-7166 (1990).
- Mayer, M. P., et al. Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat Struct Biol. 7 (7), 586-593 (2000).
- Georgopoulos, C. A new bacterial gene (groP C) which affects λ DNA replication. Mol Genet Genomics. 151 (1), 35-39 (1977).
- Tilly, K., McKittrick, N., Zylicz, M., Georgopoulos, C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 34 (2), 641-646 (1983).
- Buchberger, A., Gassler, C. S., Buttner, M., McMacken, R., Bukau, B. Functional defects of the DnaK756 mutant chaperone of Escherichia coli indicate distinct roles for amino-and carboxyl-terminal residues in substrate and co-chaperone interaction and interdomain communication. J Biol Chem. 274 (53), 38017-38026 (1999).
- Taj, M. K., et al. Escherichia coli as a model organism. Int J Eng Res. 3 (2), 1-8 (2014).
- Sato, S., Wilson, R. I. Organelle-specific cochaperonins in apicomplexan parasites. Mol Biochem Parasitol. 141 (2), 133-143 (2005).
- Molecular characterisation of the chaperone properties of Plasmodium falciparum. heat shock protein 70. Rhodes University Available from: https://commons.ru.ac.za/vital/access/manager/Repository/vital:3977?site_name=Rhodes+University (2007)
- Makumire, S., et al. Mutation of GGMP repeat segments of Plasmodium falciparum Hsp70-1 compromises chaperone function and Hop co-chaperone binding. Int J Mol Sci. 22 (4), 2226 (2021).
- Nitika, P. C. M., Truman, A. W., Truttmann, M. C. Post-translational modifications of Hsp70 family proteins: Expanding the chaperone code. J Biol Chem. 295 (31), 10689-10708 (2020).
- Knighton, L. E., Saa, L. P., Reitzel, A. M., Truman, A. W. Analyzing the functionality of non-native Hsp70 proteins in Saccharomyces cerevisiae. Bio Protoc. 9 (19), e3389 (2019).

