Gut Acidification Monitoring Assay: A Technique to Study the Acidification of the Intestinal Lumen in Drosophila using Bromophenol Blue Dye
Abstract
Source: Abu, F., et al. Monitoring Gut Acidification in the Adult Drosophila Intestine. J. Vis. Exp. (2021).
This video demonstrates an assay to monitor gut acidification in Drosophila flies. After the ingestion of food supplemented with a pH indicator dye, the acidification of the intestinal lumen results in a change in the color of the dye. Visual inspection of the digestive tract of the flies to examine the color change provides evidence of acidification.
Protocol
NOTE: The standard laboratory line Oregon R was used as a WT control. All flies were reared on standard cornmeal-molasses medium (containing molasses, agar, yeast, cornmeal, tegosept, propionic acid, and water) at room temperature with 12/12 h light/dark circadian rhythm.
1. Preparing for the assay
- Collect female flies (0-2 days old, non-virgin) under CO2 anesthesia and allow them to recover on standard cornmeal food for at least 3 days before experiments.
- Starve the flies for ~24 h at room temperature (~23 °C) in vials containing a laboratory wipe tissue soaked with ~2 mL of deionized water.
- Prepare the fly food with bromophenol blue (BPB) as follows:
- Melt the fly food in a microwave and then let it cool until it is lukewarm.
- Add 1 mL of 4% BPB to 1 mL of lukewarm food and mix well.
- Using a pipet, add the fly food containing BPB into a single dot (~200 µL) in the center of a Petri dish.
2. Gut acidification monitoring assay
- Transfer starved flies into a Petri dish containing single dots (200 µL) of fly food supplemented with 2% bromophenol blue (BPB). Allow the flies to forage for 4 h at room temperature while exposed to light.
- After 4 h, collect the flies and anesthetize them on ice; surgically isolate their guts.
- Perform the surgery in 1x phosphate-buffered saline (PBS) with forceps under a stereomicroscope (see the Table of Materials). Isolate the gut by holding the thorax with a pair of forceps and pulling down the abdomen with a second pair until the CCR of the gut is visible, taking care to ensure that the intestine remains attached at both ends.
- Determine acidification of the gut by examining the color of the CCR of the gut (Figure 1C; yellow indicates acidified, and blue indicates not acidified).
- Count only those flies that show robust BPB staining in their guts.
- Calculate the percentage using the following equation:
Percentage of flies with acidified guts = number of flies acidified × 100 / (number of flies acidified + number of flies non-acidified)
NOTE: A percentage of 0 indicates that no flies acidified their gut, whereas a percentage of 100 indicates all flies acidified their gut.
Representative Results
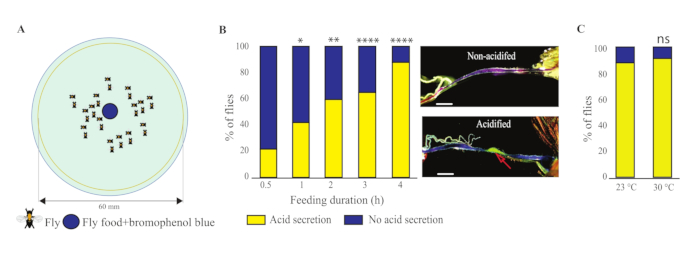
Figure 1: Gut acidification monitoring. (A) Schematic drawing of feeding arena. The blue dot represents fly food with bromophenol blue (a pH-indicating dye). Other spots represent fruit flies. (B) Graphical representation of percentage of flies showing gut acidification fed for different durations over 4 h. Representative gut images of an acidified gut and a non-acidified gut. The red arrow indicates acidic release in the copper cell region of the midgut. n = 4 experiments, 25-30 female flies per experiment. Scale bar = 500 µm each. Asterisks indicate significant differences from the control group (one-way ANOVA, followed by a Bonferroni test) *P < 0.05; **P < 0.01; ****P < 0.0001. (C) Flies were fed fly food with BPB for 4 h at 23 °C or 30 °C. Percentage (%) of flies showing gut acidification. n = 4 experiments, 25-30 female flies per experiment (unpaired t-test followed by non-parametric Mann-Whitney U test and Wilcoxon rank-sum test. Abbreviation: ns = not significant.
Disclosures
The authors have nothing to disclose.
Materials
| Bromophenol blue | Sigma-Aldrich | B0126 | |
| D. simulans | Drosophila Species Stock Center at the University of California | Riverside California1 (https://www.drosophilaspecies.com/) | |
| D. erecta | Drosophila Species Stock Center at the University of California | Dere cy1(https://www.drosophilaspecies.com/) | |
| D. pseudoobscura | Drosophila Species Stock Center at the University of California | Eugene, Oregon(https://www.drosophilaspecies.com/) | |
| D. mojavensis | Drosophila Species Stock Center at the University of California | Chocolate Mountains, California (https://www.drosophilaspecies.com/) | |
| Forceps | Inox Biology | Catalog# 11252-20 | |
| Kim wipes Tissue | Kimtech | ||
| Oregon R | Bloomington Drosophila Stock | (https://bdsc.indiana.edu/ # 2376) | |
| Petri dishes | Fisher Scientific | Catalog #FB0875713A | |
| Phosphate-buffered Saline (PBS) | HyClone | Catalog # SH30258.01 | |
| Stereomicroscope | Olympus SZ51 | Visual magnification |

