A Platelet-Rich Plasma Injection into the Sectioned Achilles Tendon of a Rat
Abstract
Source: Greimers, L., et al. Effects of Allogeneic Platelet-Rich Plasma (PRP) on the Healing Process of Sectioned Achilles Tendons of Rats: A Methodological Description. J. Vis. Exp. (2018).
This video demonstrates a technique for injecting platelet-rich plasma (PRP) into the sectioned Achilles tendon of rats. A small segment is excised from the Achilles tendon of a recipient rat, the site is sutured, and the rat is allowed to recover. Concurrently, whole blood is collected from a donor rat and centrifuged to obtain PRP, which is then activated and injected into the sutured site of the recipient rat to induce tendon repair.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Animal Preparation
- Use 132 2-month-old male Sprague-Dawley rats weighing 320-450 g (120 rats for experimentation and 12 rats for blood sampling, Figure 1). Fifteen rats for each group was enough for a power of 0.8 (80% chance of finding statistical significance if the specified effect exists).
- House all rats in classical cages under isolation conditions in a conventional facility equipped with a changing station. Instigate breeding in an IVC (individually ventilated cage) rack, with SPF (specific pathogen-free) rats being specifically purchased.
- Use the following housing conditions: temperature range from 19-24 °C; relative humidity: 40-60%; ventilation: frequency of air renewal: 10-15 per h; light/dark cycle: 12 h-12 h.
- Sterilize all caging equipment and use irradiated bedding. Systemically implement cage enrichment by placing sterile cardboard trays and towels inside the cages.
- Allow access to the zone under restricted conditions: new lab coat, mask, gloves, footwear, and hair bonnet at the entrance.
- Carry out colony health monitoring on sentinel animals housed on dirty bedding annually, according to the Felasa recommendations for conventional facilities. Keep four to five animals per cage with the appropriate irradiated diet and provide acidified water ad libitum. Monitor daily.
- Transfer rats elected for surgical intervention in filtering top cages to the surgical rooms 2 h before surgery.
2. Surgical Procedure
- Prepare the surgical instruments: fine scissors, a clamp, two hemostatic clamps, and a needle holder. During all the procedures, wear gloves, a mask, a hood, and a lab coat.
- Randomly divide the rats into two groups (PRP and saline solution).
- Take the rat out of its cage and weigh it. Use numbered ear tags to identify the rats. To avoid desiccation of the eyes, place waterdrops on the cornea.
- Anesthetize the rat intraperitoneally with xylazine (10 mg/kg of body weight) and ketamine (80 mg/kg of body weight). Place the animals under cardio-respiratory monitoring to confirm anesthetization and check for eye reflexes.
- Inject 50 µL of buprenorphine subcutaneously (0.01-0.05 mg/kg every 8-12 h) in the neck area as a painkiller.
- Shave the left hind limb with an electric shaver, disinfect it with 3 alternating scrubs of iso-betadine/alcohol (diluted 1:10) solution, and place the rat on a warm pad (20 °C) under a dissecting microscope. Place the rat on lateral decubitus with the leg to be operated on in a superior position. Hold the paw with surgical forceps.
- Using the scissors, make a small lateral incision (20-25 mm) in the skin surrounding the left Achilles tendon, and dissect the fascia using fine scissors to expose the Achilles tendon complex (Figure 2a).
- Remove the plantaris tendon with scissors (Figure 2b).
- Cut the Achilles tendon transversally 5 mm proximal to its calcaneal insertion and remove a 5-mm long portion using scissors. Do not suture the tendon (Figure 2c, 2d).
- Suture the fascia and the skin with resorbable yarn, doing an overjet suture (continuous suture). Monofilament sutures can also prevent wicking from the skin into the surgical incision.
- Place the rat under a heating lamp until awake, and then place the rat back in a cage (58 x 38 cm) (Nota bene: No immobilization is imposed).
3. PRP Preparation
- Anesthetize the donor rats with xylazine (10 mg/kg of body weight) and ketamine (80 mg/kg of body weight) by intraperitoneal injection. The PRP injection is carried out 2 h postoperatively without any re-anesthetic.
- Inject 50 µL of buprenorphine subcutaneously as a painkiller.
- Collect whole blood (20 mL per rat, final bleeding) by cardiac puncture into sterile tubes containing 3.2% buffered sodium citrate (0.109 M, anticoagulant). Then, euthanize the rat by intracardiac injection.
- To obtain PRP, centrifuge the blood for 10 min at 150 x g and room temperature. This initial low-speed centrifugation step yields two distinct phases: a lower phase consisting of red blood cells (RBCs) that accounts for about 80-90% of the total volume and an upper phase composed of platelet-rich plasma (PRP) (typically 10-20% of the blood sample).
- Collect the PRP (upper phase) in a secondary plastic tube using a plastic transfer pipette. As the interface between PRP and red blood cells is very loose, do not pipette too close to the interface. When handled properly, contaminating RBCs in PRP should be below 0.05 x 103 cells/µL. Discard the remaining lower phase and the primary blood collection tube.
- Determine the volume of collected PRP and measure the platelet count on a hematology analyzer. These values are required for calculations to adjust platelet concentration in the next step. A blood cell count is also helpful to evaluate potential contamination with RBCs and WBCs. Platelet concentration in PRP at this stage is typically 1 – 1.5 x106/µL.
- Perform a second centrifugation of the PRP for 10 min at 1,000 x g and room temperature. This high-speed centrifugation step yields a loose platelet pellet and a supernatant of autologous platelet-poor plasma (PPP). Using the values determined in step 3.6, calculate the volume of supernatant to be discarded to concentrate the PRP and reach a final concentration of 2.5 x106/µL.
- Gently collect the supernatant (PPP) in a secondary plastic tube using a plastic transfer pipette, leaving about two-thirds of the final volume calculated in step 3.7. As the pellet is loose, a significant quantity of platelets is lost when PPP is discarded, potentially reducing the desired concentration.
- Carefully resuspend the platelet pellet with the remaining supernatant by gentle repeated pipetting. Measure the platelet count on this concentrated PRP. If required, add the appropriate volume of autologous PPP to reach the final target concentration.
NOTE: Use the PRP within 3 h of preparation. - Add 50 µL of CaCl2 (11 mEq 10 mL) per mL of PRP to activate the platelets.
- Inject 50 µL of fresh PRP or saline solution with a 21G needle directly into the sutured operation site about 1 h after preparation. During this time, keep PRP at room temperature.
- Monitor the functional recovery of the rats closely during the days after surgery and before the removal of the Achilles tendon.
Representative Results
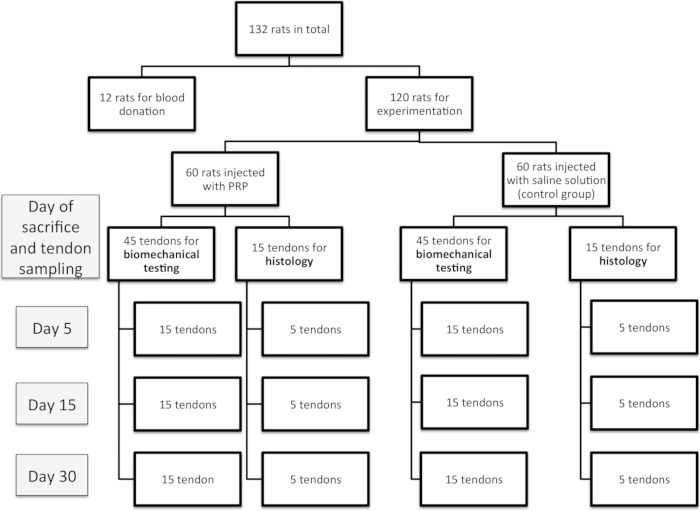
Figure 1: Experimental design of the study.
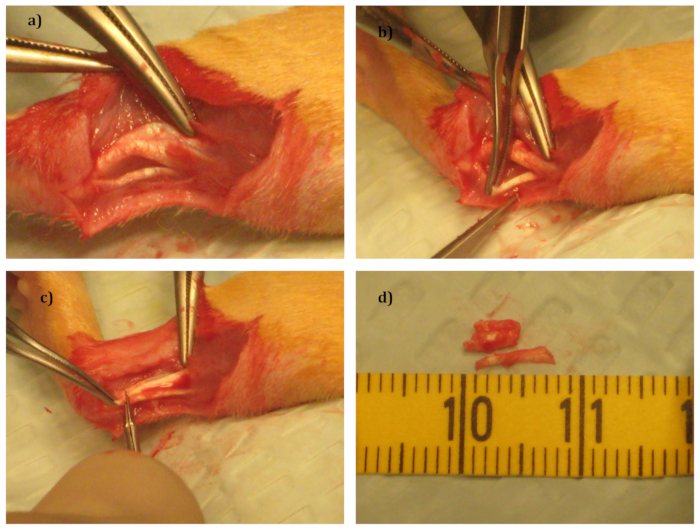
Figure 2: Surgical Procedure.
(a) The tendon complex after removal of the surrounding fascia.
(b) Removal of the plantaris tendon.
(c) Removal of a section of the Achilles tendon.
(d) The 2 tendons that have been removed.
Disclosures
The authors have nothing to disclose.
Materials
| Xylazine (Xyl-M) | VMD | none | anesthetic |
| Ketamin (Jétamine 1000 CEVA) | CEVA Santé Animale | none | anesthetic |
| Buprenorphin (Vetergésic Multidosis) | ALSTOE | none | Painkiller |
| iso-Betadine | MEDA-Pharma | none | Desinfectant |
| Resorbable yarn Vicryl 6/0 | Johnson & Johnson | ||
| Nembutal | CEVA Santé Animale | none | Anesthetic |
| Paraformaldehyde | Sigma-Aldrich | P6148 | Preserves structure of the tissue |
| Isopropanol 100% | VWR | 2,09,22,364 | |
| Ethanol 95% | VWR | 2,08,23,362 | |
| Xylene | VWR | 28973.363 | |
| Paraffin | VWR | LEIC3950.1006 | |
| Hematoxylin | Millipore | 1.15938.0025 | Colorant |
| Eosin | Millipore | 1.15935.0100 | Colorant |
| Eukitt | Sigma-Aldrich | 3989 | Mounting Medium |
| CaCl2 |
Tags

.
