Shallow Water (Paddling) Variants of Water Maze Tests in Mice
Summary
Mice can swim, but many strains appear to find this activity stressful. To overcome this problem mazes have been devised where escape from shallow water is used to motivate behaviour. These have been demonstrated to support learning at least as good as the traditional and widely used Morris water maze.
Abstract
When Richard Morris devised his water maze in 19817, most behavioral work was done in rats. However, the greater understanding of mouse genetics led to the mouse becoming increasingly important. But researchers found that some strains of mutant mice were prone to problems like passively floating or diving when they were tested in the Morris water maze11. This was unsurprising considering their natural habitat; rats swim naturally (classically, the “sewer rat”), whereas mice evolved in the dry areas of central Asia.
To overcome these problems, it was considered whether shallow water would be a sufficient stimulus to provide escape motivation for mice. This would also avoid the problems of drying the small creatures with a towel and then putting them in a heated recovery chamber to avoid hypothermia, which is a much more serious problem than with rats; the large ratio of surface area to volume of a mouse makes it particularly vulnerable to rapid heat loss.
Another consideration was whether a more natural escape strategy could be used, to facilitate learning. Since animals that fall into water and swim away from the safety of the shore are unlikely to pass on their genes, animals have evolved a natural tendency to swim to the edge of a body of water. The Morris water maze, however, requires them to swim to a hidden platform towards the center of the maze – exactly opposite to their evolved behavior. Therefore the paddling maze should incorporate escape to the edge of the apparatus. This feature, coupled with the use of relatively non-aversive shallow water, embodies the “Refinement” aspect of the “3 Rs” of Russell and Burch8.
Various types of maze design were tried; the common feature was that the water was always shallow (2 cm deep) and escape was via a tube piercing the transparent wall of the apparatus. Other tubes (“false exits”) were also placed around the walls but these were blocked off. From the inside of the maze all false exits and the single true exit looked the same. Currently a dodecagonal (12-sided) maze is in use in Oxford, with 12 true/false exits set in the corners. In a recent development a transparent paddling Y-maze has been tested successfully.
Introduction
The Morris and Barnes Mazes
Since the beginnings of experimental psychology, studies of animal learning have relied heavily on mazes, generally constructed of opaque wood or metal. Inevitably, due to the extraordinary olfactory abilities of rodents, many studies using them were compromised to some extent; when the experimenter thought that he/she had succeeded in teaching an animal a visual or position discrimination, the rat or mouse had in fact been mainly using olfaction to solve the problem. This is epitomised by the controversy over whether rats with lesions of the hippocampus can perform spatial reference memory tasks, discussed in detail in 20024. Essentially, David Olton and colleagues, apart from in early work with the radial maze, do not appear to have always systematically rotated their mazes. This led to hippocampal lesioned animals solving the task, presumably by odor cues that made each arm distinctive. At Oxford, a (naturally) blind mouse was once observed on a six-arm radial maze. It left its start arm and turned right. Then it crossed the center and chose the opposite arm. Afterwards it fell into a stereotyped strategy of always turning right, but when it re-encountered the base of the first arm it had entered, it took a momentary sniff at it (~0.2 sec) then rejected it and moved to the next arm. If olfactory information on a single visit could be remembered, it is clear that testing over repeated trials on a static maze with the same arm always baited would lead to strong olfactory associations with the reward, and learning would easily occur even if the lesion prevented any purely spatial learning.
Problems such as these were the stimulus for Morris to develop his water maze7; water would not provide constant localized olfactory cues.
The paddling pool is essentially a hybrid between the traditional water maze designed by Morris7 and the dry Barnes1 maze. In the Morris water maze, the animal swims in deep water, escape from which is to the shallow water covering a slightly submerged platform located towards the maze center. In the Barnes maze, the animal is placed on a (dry) circular platform with exit holes around the periphery, only one of which offers escape to a box placed underneath.
The use of paddling as an escape motivator arose from problems reported when disproportionate numbers of some strains of transgenic mice failed to swim properly in the Morris maze. They either dived or passively floated11. Since this might represent a stress-related response, it was reasoned that reducing the water to paddling depth might suffice to overcome this problem, which indeed it did. It was also decided to allow escape to a dry tube set in the side of the apparatus, rather than to shallow water covering a platform. This would be a more “natural” escape response, and increase the spatial component of the test, since a lot of the initial learning in the Morris maze is procedural; the animals must first overcome their innate tendency to swim along the walls of the maze before spatial learning can begin.
The Barnes maze also can suffer from animals not being sufficiently motivated to escape the circular platform10,11.
Protocol
1. The Oxford Paddling Pools
The paddling pool has been made according to three successive designs, Mk 1-3. All employ shallow (2 cm) water contained in a white (to enhance its aversiveness) base, which is surrounded by transparent walls made of Perspex or clear acrylic plastic. These have true/false exits set in them. The false exits are occluded by black painted wooden plugs, whereas the true exit is open and joined to a black plastic pipe which can be removed with the mouse inside. The mouse is then swiftly and atraumatically returned to its home cage while inside the pipe. After the first publication of the paddling pool, it was reported that return to the home cage was also an effective motivator for learning the Lashley III maze2.
A water temperature of 20-25 °C is used. 20-21 °C is ideal; it has been noted that some mice seem less motivated at higher temperatures.
The first maze5 was circular. However, sometimes mice (particularly those with lesions to the hippocampus) would appear not to notice the open exit tube. An octagonal design, with the exit tubes placed in the corners so as to attract the attention of the mice, greatly ameliorated this problem. However, the error rate at chance performance would be 4/trial, as opposed to 6/trial with the 12-tube circular design. Therefore a dodecagonal pool is now used with 11 false and 1 true exit set in the angles made by the junctions of the walls.
1.1. Evolution and characteristics of the paddling pools
Mk 1: a circular pool 85 cm in diameter with 12 true/false exits (Figure 1).
Mk 2: an octagonal pool 86 cm in diameter with 8 true/false exits set in the corners.
Mk 3: a dodecagonal (12 sided) pool 120 cm in diameter with 12 true/false exit set in the corners (Figure 4).
In all the above (and the paddling Y-maze; see below) the exit tubes were 40 mm diameter. However, it was occasionally sensed that the mice were reluctant to enter these, and control performance in the Y-maze would sometimes dip after they had been responding well. Therefore, and considering how readily they entered the burrowing apparatus (see JOVE publication “Assessing burrowing, nest construction and hoarding in mice”), also that the exit holes in the Barnes maze are 50 mm diameter, it was considered if 40 mm was too small. A test was carried out in the home cage of six C57BL/6 mice. (They seem more ready to enter 40 mm tubes in the home cage as presumably stress is lower here than in a piece of apparatus, so doing the test in the home cage would minimize differences in entry if the smaller tubes were slightly aversive, making the test more conservative). Two 40 mm tubes and two 50 mm tubes were placed on the floor in an alternating pattern. As predicted, there were more entries into 50 mm tubes (28 entries) than 40 mm (9 entries). Therefore pilot tests are planned on a new Y-maze with 50 mm exit tubes; if this is successful the old 40 mm tubes on the dodecagonal pool and paddling Y-maze will be replaced with new 50 mm ones.
As the paddling pool, paddling Y-maze and spatial novelty Y-maze are all spatial tests, relying on the room cues which the mouse sees through the transparent walls of the apparatus, the room should be well furnished with distinctive cues (e.g shelves, cupboards, black plastic shapes on the walls).
2. Running the Paddling Pool
- Place a mouse in the center of the pool facing one of four positions on the perimeter (9, 12 or 3 o’clock if the escape tube is at 6 o’clock). Placement is semi-random; a maximum of three consecutive trials can be in the same direction. This can be difficult to achieve in practice, because as learning proceeds the mice tend to orientate themselves towards the correct exit as they are lowered into the pool by the tail. If they do this strongly no attempt should be made to force them into the planned position, since they are simply demonstrating learning.
- Release them when they are just above the water, as then they drop in and instantly know that they are no longer being held. Slowly releasing them results in them struggling to free themselves and this can impair initial orientation. This also applies to the paddling Y-maze.
- Maximum trial length is 60 sec. If the mouse fails to escape by then, manually guide it to the exit using a couple of clear Perspex paddles, each measuring about 30 x 20 cm. The measures taken are the time to find the exit (definition: all of the head within the tube) and errors. Errors were defined as coming within a head’s length from a tube (including the real exit). Passing close by the real exit, without entry, generally occurs infrequently after the first few trials. Although a case can be made that this is a problem, an alternative view is that mice impaired in spatial cognition do this because they don’t know there is an exit around there. Controls do know, and therefore investigate more fully. A ceiling of 11 errors/trial is imposed on the number of errors in the analysis, to prevent too much skew of the data. This would mitigate against a deficit being found in spatially impaired mice, i.e. it is a conservative measure. Mice which fail to reach the exit within 60 sec are assigned a score of 11 errors for that trial. So 60 sec times always have 11 errors.
3. The Paddling Y-maze
To simplify the paddling pool procedure and the apparatus it was decided to experiment with a Y-maze, operated according to the same principles as the paddling pools. The apparatus consists of three arms made of transparent polystyrene or Perspex, each 30 x 8 x 20 cm. This is mounted on a white base (Figure 7). White was chosen to maximize the aversiveness of the floor color and so encourage escape from the shallow (2 cm deep). As in the water maze, there is only one true exit, with the other two arms terminating in false exits which look the same from inside the maze (Figure 8).
Running the paddling Y-maze
Place a mouse at the end of one of the closed arms, facing away from the center. The sequence of arms chosen as the starting position is defined by a semi-random sequence; no more than three consecutive trials with the same position, and equal numbers of the left or right arms. Each trial lasts 60 sec. If the mouse fails to exit (whole head in exit tube) within this time encourage it to enter the arm with the aid of a piece of transparent Perspex. The exit arm’s base is blocked by the Perspex and the mouse allowed to finds the exit by itself; this should encourage better learning than pushing it right into the tube. But this may need to be resorted to if the mouse is reluctant to enter the tube after 15 sec. Take measures of the total time to exit and the number of errors (whole body excluding tail enters a blind arm).
Scoring the paddling Y-maze
There are two definitions of a correct trial: firstly one in which the mouse finds the exit in <60 sec and makes no entries into blind arms, with errors being scored if blind arms are entered and/or if the exit is not found within 60 sec. This analysis confounds failures to start moving with errors in arm entries. Secondly, a trial analysis that emphasizes cognitive ability can be performed by excluding, for each mouse, all trials where the mouse did not leave the start arm within 60 sec, (i.e. delete all 0+60 scores) then calculating the % correct of the remaining trials.
4. Two types of Y-maze Combined
1. Apparatus
When building a paddling Y-maze, it is useful to incorporate two doors to enable it to be run as a (dry) spatial novelty memory experiment 6 (Figure 13). A transparent guillotine door covers the exit hole in the end wall of the exit arm, while an opaque guillotine door can be inserted to seal of one arm from the other two during the sampling phase of the test. It is important that the runners for these doors are not within reach of the mice, as otherwise they may divert their attention because they appear potential escape routes. Ideally they should extend 7 cm down from the top of the maze wall.
2. Set-up
Place a thin (0.5 cm) layer of wood chip bedding material on the floor of the Y-maze. This is redistributed between the two test phases for individual mice, and between mice. For best practice, to make the olfactory environment more similar for the first versus subsequent mice, place one or two non-experimental mice in the maze for a few minutes before the experiment starts.
3. Procedure
With the exit arm blocked off by the opaque door and its exit hole blocked by the transparent door, place the mouse in the start arm (one of the two non-exit arms of the maze) and allow it to explore for 5 min. Then remove it, raise the opaque door to allow access to all three arms, replace it in the start arm and observe it for 2 min. Note the number of entries and the time spent in each arm. Control mice should remember that the first two arms, and spend more time in the previously inaccessible arm.
Representative Results
Hippocampal lesions greatly impair learning in the paddling pool5. Using a paddling Y-maze, an age-dependent deficit has been demonstrated in 10 and 14, but not 3 month old Tg2576 mice (a model of amyloid over-expression). Aged (21 month) control mice also performed poorly3. A spatial novelty memory experiment run in the dry Y-maze revealed impairments in mice with knockout of the glutamate receptor-A (GluR-A) AMPA receptor subunits9.

Figure 1. A mouse in the circular Mk 1 paddling pool.

Figure 2. The mouse finds the exit tube of the paddling pool.

Figure 3. The mouse escaping into the exit tube. The tube is then detached from the maze and the mouse returned to its home cage.
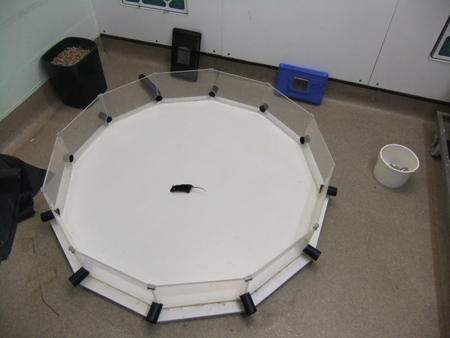
Figure 4. A mouse in the dodecagonal paddling pool.

Figure 5. A mouse about to commit an error as it approaches a false exit.
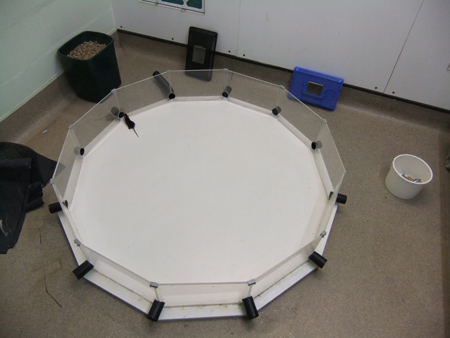
Figure 6. An error is committed as the mouse’s nose comes within a head’s distance of a false exit. The real exit is to its right, with the exit tube attached.
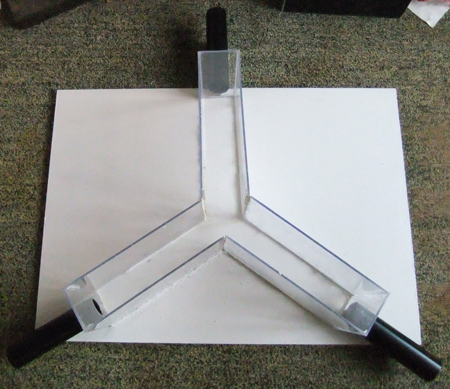
Figure 7. A paddling Y-maze.
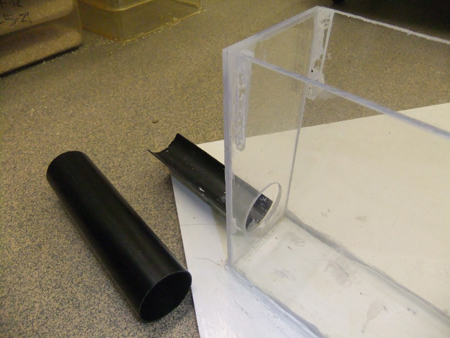
Figure 8. Detail of the exit arm end of the Y-maze. A shallow U-shaped trough adjoins the exit hole in the end wall. This supports the exit tube, shown below the support in the picture above. The exit tube can be removed from the support to take the mouse back to its home cage. All arms are fitted with this set-up but only one arm end wall has a circular hole cut into it to allow escape.
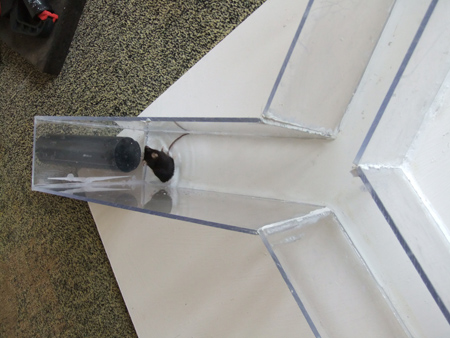
Figure 9. A mouse in a blind alley of the paddling Y-maze.

Figure 10. The mouse finds the exit tube.

Figure 11. A mouse, having escaped from the paddling Y-maze, waits to be returned to its home cage.

Figure 12. Mice can generally be relied upon to stay in the tube while they are carried back to their nearby home cage.

Figure 13. Combining a paddling Y-maze with a spatial novelty Y-maze configuration. An opaque guillotine door is inserted into a slide at the proximal end of one arm, and a transparent guillotine door seals off the exit hole.
Discussion
In conclusion, paddling seems a generally effective motivator for mice, and avoids the stress associated with deep water swimming. Unlike the path length and escape time measures in the Morris water maze, the error rate in the paddling pool for hippocampal lesioned mice remained constant throughout the training period5, so this represents a pure measure of spatial memory, as opposed to escape time or path length, which both decrease as the mice become acquainted with the non-spatial elements of the task. The errors measure also provides a greater magnitude of difference between impaired (e.g. hippocampal lesioned) mice and controls.
In the original publication, probe tests were conducted to check if the mice were truly using spatial cues5. In Probe 1, the maze was rotated 120 ° but the geographical location of the escape tube remained the same as in training. Performance remained virtually unchanged. In Probe 2, similar to the standard extinction test in the Morris maze, the exit tube was blocked. The time spent in the training quadrant of the maze, where the exit tube was previously located, was 50% for control mice, 25% for mice with hippocampal lesions. In a third probe test in which the position of the exit tube was changed, the controls again spent more time in the original training quadrant. These probe tests confirmed that the mice were not using intramaze cues but were guided by the spatial cues in the laboratory external to the maze. This lack of influence of intramaze cues puts the paddling pool at an advantage over the Barnes maze.
Although we have not tried rats in paddling mazes, this might be possible, although it might be an advantage to make the water colder than the 20-25 °C normally used in the Morris maze. However, since their bodies would not be immersed in the water this should not have adverse welfare effects.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The Wellcome Trust for providing Open Access funding to Oxford University. Robert Deacon is a member of Oxford OXION group, funded by Wellcome Trust grant WT084655MA.
References
- Barnes, C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74-104 (1979).
- Blizard, D. A., Cousino Klein, L., Cohen, R., McClearn, G. E. A Novel Mouse-Friendly Cognitive Task Suitable for Use in Aging Studies. Behav. Genetics. 33, 181-189 (2003).
- Deacon, R. M. J., Cholerton, L. L., Talbot, K., Nair-Roberts, R. G., Sanderson, D. J., Romberg, C., Koros, E., Bornemann, K. D., Rawlins, J. N. P. Age-dependent and -independent behavioral deficits in Tg2576 mice. Behav. Brain Res. 189, 126-138 (2008).
- Deacon, R. M. J., Bannerman, D. M., Kirby, B. P., Croucher, A., Rawlins, J. N. P. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav. Brain Res. 133, 57-68 (2002).
- Deacon, R. M. J., Rawlins, J. N. P. Learning impairments of hippocampal lesioned mice in a paddling pool. Behav. Neurosci. 116, 472-478 (2002).
- Dellu, F., Mayo, W., Cherkoaoui, J., Le Moal, M., Simon, H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 558, 132-139 (1992).
- Morris, R. G. M. Spatial localization does not require the presences of local cues. Learn. Motiv. 12, 239-260 (1981).
- Russell, W. M. S., Burch, R. L. . The principles of humane experimental technique. , (1959).
- Sanderson, D. J., Gray, A., Simon, A., Taylor, A. M., Deacon, R. M. J., Seeburg, P. H., Sprengel, R., Good, M. A., Rawlins, J. N. P., Bannerman, D. M. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav. Neurosci. 121, 559-569 (2007).
- Sunyer, B., et al. Barnes maze, a useful task to assess spatial reference memory in the mice. Protocol Exchange. , (2007).
- Wahlsten, D., Rustay, N. R., Metten, P., Crabbe, K. C. In search of a better mouse test. TINS. 26, 132-136 (2003).

