Odorant-induced Responses Recorded from Olfactory Receptor Neurons using the Suction Pipette Technique
Summary
Olfactory receptor neurons (ORNs) convert odor signals first into a receptor current that in turn triggers action potentials that are conveyed to second order neurons in the olfactory bulb. Here we describe the suction pipette technique to record simultaneously the odorant-induced receptor current and action potentials from mouse ORNs.
Abstract
Animals sample the odorous environment around them through the chemosensory systems located in the nasal cavity. Chemosensory signals affect complex behaviors such as food choice, predator, conspecific and mate recognition and other socially relevant cues. Olfactory receptor neurons (ORNs) are located in the dorsal part of the nasal cavity embedded in the olfactory epithelium. These bipolar neurons send an axon to the olfactory bulb (see Fig. 1, Reisert & Zhao1, originally published in the Journal of General Physiology) and extend a single dendrite to the epithelial border from where cilia radiate into the mucus that covers the olfactory epithelium. The cilia contain the signal transduction machinery that ultimately leads to excitatory current influx through the ciliary transduction channels, a cyclic nucleotide-gated (CNG) channel and a Ca2+-activated Cl– channel (Fig. 1). The ensuing depolarization triggers action potential generation at the cell body2-4.
In this video we describe the use of the “suction pipette technique” to record odorant-induced responses from ORNs. This method was originally developed to record from rod photoreceptors5 and a variant of this method can be found at jove.com modified to record from mouse cone photoreceptors6. The suction pipette technique was later adapted to also record from ORNs7,8. Briefly, following dissociation of the olfactory epithelium and cell isolation, the entire cell body of an ORN is sucked into the tip of a recording pipette. The dendrite and the cilia remain exposed to the bath solution and thus accessible to solution changes to enable e.g. odorant or pharmacological blocker application. In this configuration, no access to the intracellular environment is gained (no whole-cell voltage clamp) and the intracellular voltage remains free to vary. This allows the simultaneous recording of the slow receptor current that originates at the cilia and fast action potentials fired by the cell body9. The difference in kinetics between these two signals allows them to be separated using different filter settings. This technique can be used on any wild type or knockout mouse or to record selectively from ORNs that also express GFP to label specific subsets of ORNs, e.g. expressing a given odorant receptor or ion channel.
Protocol
1. The Recording Setup
The recording chamber is mounted on a Nikon TE2000U Eclipse inverted microscope with phase contrast optics which is fitted on an air table and electrically shielded using a Faraday cage. The Plexiglass recording chamber consists of two sections partially separated by a barrier and glued onto a silanized glass slide. One section of the chamber is used to settle the cells while the other is used for stimulus-exposure during the recording to minimize premature exposure of settled but as yet unused cells to odorant. The recording setup consists of a suction pipette (see below) mounted in a pelleted pipette holder and a ground electrode. The suction pipette is positioned using a micromanipulator. Stimulus solutions are applied using a triple-barrel glass square tube, with each square tube being 0.7 mm x 0.7 mm. The triple-barrel glass was connected to a motorized fast-step perfusion system mounted on a manipulator (Sutter SF-77B, Warner Instruments) and entered the recording chamber at an angle of ~45°. The end of the triple barrel glass was beveled at an angle of again 45° such that the final face (opening) of the triple-barrel glass was vertical to allow close positioning of the pipette.
The suction pipette electrode filled with standard mammalian Ringer solution and the ground electrode are connected to the 1 GΩ headstage of a patch clamp amplifier (PC-501A, Warner Instruments) in voltage clamp mode. The analog signal from the amplifier (low pass filtered at 5 kHz) is displayed on a digital oscilloscope to monitor the signal in real-time and also connected to an 8-pole Bessel filter (Krohn-Hite) where the signal is low pass filtered at 50 Hz. Both signals (low pass filtered at 5 kHz and 50 Hz) are fed into an A/D converter (Cambridge Electronic Design Micro 1401 mkII) which is connected to a PC for data acquisition. The data is recorded using Signal 3 acquisition software (Cambridge Electronic Design) at a sampling frequency of 10 kHz. The signal is recorded at the two different bandwidths to first record the receptor current together with the superimposed action potentials and then the receptor current alone (see Fig. 2). The perfusion system is used to switch rapidly between Ringer (control) solution and odorant stimuli or any solution of desired composition and is controlled by the PC and Signal 3 software. The solutions are contained in 60 ml syringes and the flow rate is controlled by medical- grade intravenous flow regulators. Typically, the flow rate is set to 1 ml/min.
The suction on the recording pipette is controlled in the following way: The side port of the pipette holder is connected to an oil line which in turn is connected to a height-adjustable oil reservoir. Raising or lowering the reservoir ensures that the pipette pressure and therefore Ringer flow through the tip of the pipette is minimal and slightly positive. Additional suction or pressure can be applied through a tube connected to the airspace of the reservoir with a mouthpiece at the other end to suck the cell body of an isolated ORN into the tip of the recording pipette.
The experiments are performed at 37 °C in order to closely simulate the native physiological condition of the cell. The temperature is controlled by passing the solutions through a custom-made heating unit which consists of ceramic resistors through which stainless steel tubes containing the solutions are passed10. The temperature is monitored using a thermocouple thermometer (Fluke).
2. Solutions
Mammalian Ringer’s solution (mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 0.01 EDTA, 10 HEPES, 10 Glucose. The pH was adjusted to 7.5 with NaOH. A 10 % diluted Ringer’s solution was used to measure the junction current. Odorant solutions: Acetophenone and eugenol were prepared daily in Ringer’s solution from 20 mM DMSO stocks.
3. Making Electrodes
- Place an unfilamented borosilicate glass capillary in a Sutter P-97 micropipette puller and pull a micropipette with a long taper.
- Transfer the pipette to a custom-made micropipette holder mounted on a Nikon E200 Eclipse microscope which is in turn fitted with a reticle in the eyepiece and a movable diamond knife mounted on the stage.
- Observe the tip of the pipette under the 20x objective and using the reticle as a guide; gently scribe the pipette at a 90° angle using the custom-made diamond knife where the pipette is 10 μm wide (outer diameter).
- Move the diamond knife further towards the tip and gradually apply pressure on the tip with the diamond knife. The pipette tip should break cleanly at the point where it was scribed.
Alternatively steps 3.1 to 3.4 can be combined and pipettes can be pulled with desired dimensions directly without having to cut the pipette tip, although it can prove harder to pull reliably pipettes with such a large tip diameter and cleanly cut edges.
- Under a 40x objective, fire polish the tip of the pipette to an inner diameter of 5 μm using an electrically heated filament. Once finished, the micropipette is ready to be used for recording currents from mouse ORNs. The open pipette resistance should be around 1 MΩ when filled with Ringer.
4. Isolation of Olfactory Receptor Neurons
- Sacrifice a mouse following institutional guidelines and regulations. Remove the head, peel away the skin overlying the skull and medially bisect the head along the midline.
- Place the two hemi-heads under a dissecting stereomicroscope, pull off the nasal septum and remove the olfactory turbinates. Place all tissue in a Petri dish with glucose containing mammalian Ringer solution.
- Peel the olfactory epithelium off the underlying cartilage from two turbinates and transfer the tissue into an Eppendorf tube containing 250 μl Ringer. We use tissue from 2 turbinates to get the right density of cells for the size of our recording chamber. Store the remaining tissue at 4 °C for later use.
- Vortex the Eppendorf tube containing the olfactory epithelium twice briefly for 1 sec at medium speed. This step leads to mechanical dissociation of the ORNs from the epithelium.
- Place the Ringer solution containing the dissociated ORNs in the recording chamber. Remove large pieces of tissue also present in the suspension using fine forceps.
- Let the ORNs settle for 20 min before beginning continuous superfusion with Ringer and proceed to the recordings.
5. Recordings
- Lower the suction electrode connected to the oil line into the recording chamber and regulate the height of the oil reservoir to establish slightly positive pressure at the pipette tip. This can be done by observing e.g. cell debris moving away from (positive pressure) or towards (negative pressure) the pipette. Try not to contaminate the tip of the pipette with cell debris.
- Scan the recording chamber for an isolated ORN which can be recognized by its typical bipolar morphology using a magnification of 20-40x. Move the recording electrode into close proximity of the ORN cell body. Gently start sucking so that the cell body enters into the tip of the pipette. Carefully and slowly continue to apply suction until the entire cell body is drawn into the tip of the suction pipette, leaving the dendrite and cilia exposed to the bath solution. For images of sucked ORNs and pipette shapes see7,11. Ensure that once no suction is applied, the ORN does not move in or out of the pipette. If so, adjust the height of the oil reservoir accordingly.
- Using the micromanipulators, move the suction pipette from the section containing the settled ORNs to the recording section of the chamber. Carefully place the suction pipette in front of the 3-barrelled tube for solution exchange. The suction pipette should be placed close enough to the opening of the 3-barrelled tube so that the ORN is exposed to a laminar flow thus avoiding mixing turbulence and to achieve fast solution exchange. The solution exchange is achieved by stepping the interface between parallel streams of flowing solution across the tip of the suction pipette. The time course of the solution exchange is typically around 20 ms, measured from the junction current evoked by stepping the cell between solutions of different ionic composition (see Fig. 2). Solutions are delivered by gravity.
6. Representative Results
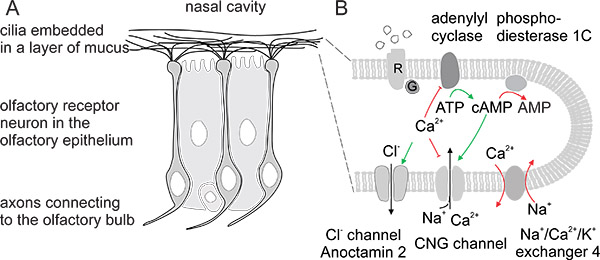
Figure 1. A) Bipolar receptor neurons in the olfactory bulb. B) Signal transduction machinery in the cilia ultimately leads to excitatory current influx. The ensuing depolarization triggers action potential generation at the cell body.
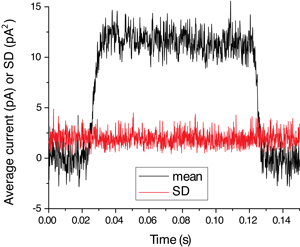
Figure 2 shows the speed and reliability of solution exchange. A pipette was stepped from normal into 10 % diluted Ringer for 100 ms and the junction current resulting from the dissimilar ion concentration was recorded. Black trace is the average of 5 trials, red trace its SD. The little change in SD during the solution exchange demonstrates reliability of solution exchange from trial to trial and the lack of excess noise in either solution stream.
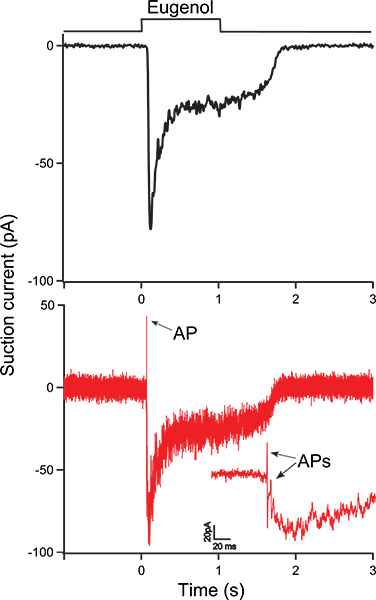
Figure 3 shows a eugenol-induced response from an ORN using the suction pipette technique. The ORN was exposed to 100 μM eugenol for 1 second as indicated by the bar above the recording. In Fig. 3A (black trace) the receptor current was filtered at a bandwidth 0 – 50 Hz to only display the receptor current. Fig. 3B shows the same recording, now filtered at the wide bandwidth of 0 – 5000 Hz (red trace) to also display action potentials (APs, indicated by arrows). The inset shows the same trace at an expanded timescale to visualize APs more clearly at the onset of the response.
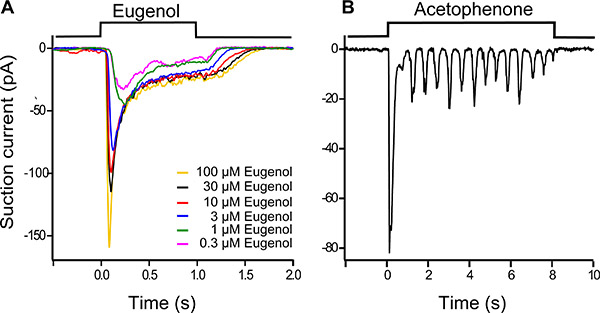
Figure 4. Dose response family of a eugenol-responsive ORN (A). Eugenol was used at concentrations ranging from 0.3 μM to 100 μM, the stimulus duration was 1 s and traces were filtered at 0 – 50 Hz. A progressive increase in odorant concentration led to a larger and faster response, which also terminated more slowly once odorant exposure was terminated.
ORNs can display an oscillatory response pattern during long stimulations at intermediate odorant concentrations (Fig. 4B). Here an acetephenone (3 μM) responsive ORN was stimulated for 8 seconds. After a fast and transient peak response at the onset of stimulation a series of smaller, recurring responses are observed. The recording bandwidth was 0 – 50 Hz.
Discussion
The suction pipette technique is a electrophysiological method which is used to record the odor-induced slow receptor current and the fast biphasic action potentials from an ORN simultaneously. Since the plasma membrane of the cell is not breached, this method leaves the intracellular milieu undisturbed ensuring that the odorant responses are not altered due to changes of the cytoplasmic ion concentrations or dilution of intracellular factors. Cells can be recorded from for long durations (up to 4 h in frog and 1 h in mouse). Another advantage of this technique is, since the cell body is inside the recording pipette, that the stimulus is delivered only to the apical part of the ORN, i.e. the cilia and dendritic knob (as is the case in vivo) thus eliminating any non-specific effects that might arise if the entire cell were exposed. Also, as the cell is relatively well protected by sitting stably in the tip of the pipette, high solution flow rates and thus fast solution changes can be achieved compared to a tight-seal recording configuration where high flow rates can easily result in loss of seal. Conversely, since intracellular access is not gained, this technique cannot be used to voltage-clamp the cell to e.g. study voltage-gated currents. Also, because it is a loose-seal technique, the recordings are sensitive to electrical noise that might arise from various sources. Care should be taken to ensure that the electrode holder is clean and not coated in salts/ringer solution so as to prevent it from being electrically conductive which can be a potential noise source. Also, all grounding of the equipment and the Faraday cage has to be done carefully.
The size and consistency of the pipette tip is very important as any lack of symmetry in the opening can result in a poor seal between the cell body and the pipette. Also, the size of the opening is crucial. A tip too large for the sucked ORN can result in a very loose seal leaving the recordings even more vulnerable to noise and to the ORN also being lost during the recording. A tip too small can squeeze the cell too tight, causing damage and short recording durations. The same pipette can be used for recording from multiple cells; if the tip gets blocked by debris, it should be cleaned using an ultrasonicator, otherwise a new pipette must be used.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH DC009613, the Human Frontiers Science Program and a Morley Care Fellowship (to JR).
Materials
| Name of the material | Type | Company | Catalogue / Model number |
Comments |
| Air table | equipment | Newport | ||
| Air Pump | equipment | Newport | ACGP | |
| Pipette Puller | equipment | Sutter | P-97 | |
| Borosilicate glass | equipment | WPI | 1B150-4 | |
| Nikon Eclipse Inverted microscope | equipment | Nikon | TE2000U | Equipped with Hg lamp, GFP filter and objectives 20X and 5X at least |
| Amplifier PC-501A | equipment | Warner | 64-0008 | Headstage 1 GΩ |
| Diamond knife | Equipment | Custom-made | ||
| Digitizer Mikro1401 A/D | equipment | Cambridge Electronic Design | ||
| Filter unit 3382 | equipment | Krohn Hite corporation | ||
| Signal | software | Cambridge Electronic Design | ||
| Molded Ag/AgCl Pellet | equipment | WPI | 64-1297 | |
| Pipette holder | equipment | Warner | 64-0997 | Custom modified to fit headstage |
| Recording chamber | Equipment | Custom-made | ||
| Micromanipulator MP85-1028 |
equipment | Sutter Instrument | Micromanipulator MP85-1028 |
|
| Mineral oil | Solution | Sigma | 330779-1L | |
| Oscilloscope TDS 1001 | equipment | Tektronix | ||
| Three-barreled square glass tube | Equipment | Warner | 64-0119 | 0.6 mm ID , 5 cm long |
| Valve | equipment | The Lee Company | ||
| Valvelink 8.2 | equipment | Automate Scientific | ||
| SF-77B Perfusion fast step | equipment | Warner |
References
- Reisert, J., Zhao, H. Perspectives on: Information and coding in mammalian sensory physiology: Response kinetics of olfactory receptor neurons and the implications in olfactory coding. J. Gen. Physiol. 138, 303-310 (2011).
- Kaupp, U. B. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11, 188-200 (2010).
- Tirindelli, R., Dibattista, M., Pifferi, S., Menini, A. From pheromones to behavior. Physiol. Rev. 89, 921-956 (2009).
- Kleene, S. J. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem. Senses. 33, 839-859 (2008).
- Baylor, D. A., Lamb, T. D., Yau, K. W. Responses of retinal rods to single photons. J. Physiol. 288, 613-634 (1979).
- Wang, J., Kefalov, V. J. Single-cell Suction Recordings from Mouse Cone Photoreceptors. J. Vis. Exp. (35), e1681 (2010).
- Lowe, G., Gold, G. H. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J. Physiol. 442, 147-168 (1991).
- Reisert, J., Matthews, H. R. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J. Gen. Physiol. 112, 529-535 (1998).
- Reisert, J., Matthews, H. R. Adaptation of the odour-induced response in frog olfactory receptor cells. J. Physiol. 519, 801-813 (1999).
- Matthews, H. R. A compact modular flow heater for the superfusion of mammalian cells. J. Physiol. 518P, 13 (1999).
- Reisert, J., Matthews, H. R. Simultaneous recording of receptor current and intraciliary Ca2+ concentration in salamander olfactory receptor cells. J. Physiol. 535, 637-645 (2001).

