An Optimized Procedure for Fluorescence-activated Cell Sorting (FACS) Isolation of Autonomic Neural Progenitors from Visceral Organs of Fetal Mice
Summary
An optimized procedure to purify neural crest-derived neuronal progenitors from fetal mouse tissues is described. This method takes advantage of expression from fluorescent reporter alleles to isolate discrete populations by fluorescence-activated cell sorting (FACS). The technique can be applied to isolate neuronal subpopulations throughout development or from adult tissues.
Abstract
During development neural crest (NC)-derived neuronal progenitors migrate away from the neural tube to form autonomic ganglia in visceral organs like the intestine and lower urinary tract. Both during development and in mature tissues these cells are often widely dispersed throughout tissues so that isolation of discrete populations using methods like laser capture micro-dissection is difficult. They can however be directly visualized by expression of fluorescent reporters driven from regulatory regions of neuron-specific genes like Tyrosine hydroxylase (TH). We describe a method optimized for high yields of viable TH+ neuronal progenitors from fetal mouse visceral tissues, including intestine and lower urogenital tract (LUT), based on dissociation and fluorescence-activated cell sorting (FACS).
The Th gene encodes the rate-limiting enzyme for production of catecholamines. Enteric neuronal progenitors begin to express TH during their migration in the fetal intestine1 and TH is also present in a subset of adult pelvic ganglia neurons2-4 . The first appearance of this lineage and the distribution of these neurons in other aspects of the LUT, and their isolation has not been described. Neuronal progenitors expressing TH can be readily visualized by expression of EGFP in mice carrying the transgene construct Tg(Th-EGFP)DJ76Gsat/Mmnc1. We imaged expression of this transgene in fetal mice to document the distribution of TH+ cells in the developing LUT at 15.5 days post coitus (dpc), designating the morning of plug detection as 0.5 dpc, and observed that a subset of neuronal progenitors in the coalescing pelvic ganglia express EGFP.
To isolate LUT TH+ neuronal progenitors, we optimized methods that were initially used to purify neural crest stem cells from fetal mouse intestine2-6. Prior efforts to isolate NC-derived populations relied upon digestion with a cocktail of collagenase and trypsin to obtain cell suspensions for flow cytometry. In our hands these methods produced cell suspensions from the LUT with relatively low viability. Given the already low incidence of neuronal progenitors in fetal LUT tissues, we set out to optimize dissociation methods such that cell survival in the final dissociates would be increased. We determined that gentle dissociation in Accumax (Innovative Cell Technologies, Inc), manual filtering, and flow sorting at low pressures allowed us to achieve consistently greater survival (>70% of total cells) with subsequent yields of neuronal progenitors sufficient for downstream analysis. The method we describe can be broadly applied to isolate a variety of neuronal populations from either fetal or adult murine tissues.
Protocol
1. Preparation of Media (All steps done in tissue culture hood)
- Combine the following: 44 ml L-15 Medium, 0.5 ml 100X Penicillin/Streptomycin (P/S), 0.5 ml 100 mg/ml Bovine Serum Albumin (BSA), 0.5 ml 1M HEPES, 5 ml Tissue Culture Grade Water. Be sure to mix BSA and P/S thoroughly before adding. This volume should be adequate for dissociation of up to five different tissues types. This media will be used in Step 3.4 to prepare Quenching solutions for use after enzymatic dissociation of tissue.
- Filter Media though 0.22 μm Polyethersulfone (PES) filter.
- Prepare 1X Hank’s Balanced Salt Solution (HBSS) and 1X Phosphate Buffered Saline (PBS) from 10X Stocks using tissue culture grade water. Filter through 0.22 μm PES filter. Large volumes of these reagents can be prepared ahead of time and stored at 4 °C, aliquoting smaller volumes in a tissue culture hood as needed.
- Fill multiple 15 ml conical tubes with 1X HBSS, (number of tubes to equal the number of tissues you plan on sub-dissecting) keeping tubes on ice.
2. Dissection
- Euthanize timed pregnant mouse in accordance with institutional animal care and use committee approved protocols and transfer uterus into a 60 or 100 mm Petri dish containing ice cold 1X PBS.
- Remove embryos from uterus and euthanize by decapitation in ice-cold 1X PBS. Screen individually under fluorescence illumination, dividing into positive transgenic and wild-type (WT) nontransgenic pools. Keep embryos in ice-cold 1X PBS throughout dissection.
- Under a dissecting microscope, sub-dissect the urogenital tract. Hold the embryo in place at the forelimb level with fine forceps. Remove the viscera from the liver down to the genital tubercle by inserting forceps at the level of the diaphragm then briskly pulling the internal organs down and away from the dorsal body wall.
- Further sub-dissect the tissue of interest away from the surrounding tissue (Figure 1). Place each individually sub-dissected tissue type into a 15 ml conical tube containing ice cold 1X HBSS. Pool each tissue type together according to embryo phenotype (i.e. all GFP+ samples of fetal intestine are pooled in a single 15 ml conical tube).
- In parallel, dissect comparable tissues from wild-type embryos to use for compensation controls at flow cytometry.
3. Dissociation of Subdissected Tissues
- Pellet sub-dissected tissue by centrifugation at 210 relative centrifugal force (rcf), 4 °C for 5 min. Following centrifugation, aspirate off as much HBSS as possible.
- Resuspend the tissue pellet in Accumax (Innovative Cell Technologies, Inc), being sure to change pipette tips between each sample to avoid cross-contamination of tissue types. The amount of Accumax added can be scaled for larger or smaller amounts of tissue. Typically for 1-5 fetal intestine samples, 1 ml of Accumax would be used but larger tissue pools will require a greater volume to achieve dissociation.
- Place tubes into 37 °C water bath for 20-45 min depending on stage of the tissue being isolated (e.g. 13.5 dpc intestine 20 min, 15.5 dpc intestine 35 min). Halfway though the dissociation time, manually break up the tissue by knocking the tube against the side of the water bath (or any solid surface) and by “flicking” the tube (as one would shake down an old -fashioned mercury thermometer). At the end of the dissociation time repeat the shake down procedure multiple times to further dissociate the sample. For more fragile samples, reduce the vigorousness of the shake down and instead use pipette trituration during Step 3.5 to achieve an appropriate level of dissociation. Typical dissociation times for 14.5 dpc and 15.5 dpc LUT are 35 and 45 min, respectively.
- While tissue is incubating in Accumax, make up Quenching solutions, termed Quench and Quench 1:5. Quench is made by adding 45 μl 5 mg/ml DNase I to 6 ml L-15 Media. Quench 1:5 is made by adding 45 μl 5 mg/ml DNase I to 30 ml L-15 Media.
- At end of dissociation, move 15 ml tubes onto ice and immediately add 1 ml Quench to each tube. Triturate each sample up and down until tissue is almost completely dissociated (Figure 2). There will still be some small chunks of tissue present in the solution. Achieving a completely homogenous sample is neither easily achievable nor desirable as it can result in poor cell viability.
- Keep sample on ice as much as possible for the remainder of the protocol. Be sure to use a fresh pipette tip when pipetting up Quench or Quench 1:5 to avoid cross-contamination of samples.
4. Filtering Cell Suspension
- Using forceps that have been soaked in 70% ethanol, place a 3 cm square of 38 μm nylon mesh membrane (Sefar America) over the mouth of a new 15 ml conical tube. Filter cells through mesh by pipetting into center of membrane using narrow bore tips. If the membrane becomes saturated while filtering, remove it, dry the mouth of the tube with a kimwipe, and use a new piece of nylon mesh to filter the remainder of cell suspension. Once all cells have been filtered, use 1 ml Quench 1:5 to rinse out the tube and filter any remaining cells.
- When filtering is complete, or when replacing a clogged membrane, use forceps to roll up the sides of the mesh and wipe the membrane across top of tube to remove any hanging droplets of cells.
- Pellet cell suspension by centrifugation at 210 rcf, 4 °C for 5 min. Aspirate off supernatant and resuspend pellet in 1 ml Quench 1:5.
- Filter cell suspension through nylon mesh into 5 ml polystyrene tubes. Wash out 15 ml tube with 1 ml Quench 1:5 and filter to capture any remaining cells.
5. Preparing Samples for FACS
- If using the same tissue for sorting and for compensation controls, transfer 1/10th to 1/20th of the sample volume to a new 5 ml polystyrene tube to use for compensation controls. Divide the wild-type tissue sample into two portions. One portion will be unstained wild-type (WT Only) while the second half will have 7-Aminoactinomycin D (7-AAD, a fluorescent intercalator excluded from live cells used as a “viability stain”) added to it (7-AAD only). These individual controls are needed to allow for compensation of any spectral overlap between emission of discrete fluorophores at the flow sorter. For instance, for a single fluorescent reporter like EGFP, necessary controls would include: EGFP only, unstained WT cells, and a tube stained with 7-AAD only (Figure 3).
- Fill all 5 ml tubes containing cell suspension with Quench 1:5 and centrifuge at 210 rcf, 4 °C for 5 min. Aspirate supernatant, leaving approximately 200 μl of liquid in tube.
- Dilute 7-AAD 1:1000 in Quench 1:5. Add 7-AAD staining dilution to 7-AAD only compensation control and samples to be sorted. Do not add 7-AAD to the EGFP only or WT only compensation controls. Volume of 7-AAD to be added will vary based on amount of starting material and size of cell pellet obtained. Once 7-AAD has been added to appropriate tubes, samples are ready to be sorted.
Tissue Sample Pool Size Volume 7-AAD to be added to tube* 15.5 dpc Intestine 1-5 200 μl 15.5 dpc LUT 1-5 150 μl
Table 1. Amount of 7-AAD added to different tissue dissociates * The final total amount in each tube will vary by an amount ranging from 50-100 μl since the aspiration of media is an approximate process and performed to avoid the pellet. Final volumes are not measured so as to minimize handling of cells in solution. - Prepare collection tubes to capture cells for RNA isolation by adding 0.75 ml TRIzol-LS to 1.5 ml microcentrifuge tubes.
- If collecting cells for in vitro culture, rather than RNA isolation, sort cells directly into self-renewal media in 6 well tissue culture plates, coated with fibronectin and filled with media as previously described.2,6,7
6. Flow Cytometry
- At the flow cytometer, evaluate the compensation controls first, being sure to back flush between each sample to avoid contamination. Use the profiles of the compensation samples to set voltages/ gates for sorting. Note that if positive cells in the tissue desired are present in limiting numbers, a distinct tissue can be used to establish compensation settings as long as the fluorescence intensity and cell size between samples are comparable.
- Set compensation parameters and gates to avoid dead cells that take up 7-AAD dye and collect cells exhibiting high intensity of GFP fluorescence relative to unstained controls (Figure 4).
- Sort maximally 25,000 cells into each microcentrifuge tube. Sorting should be performed at the lowest possible pressure with a wide bore nozzle and low flow rate (e.g., 17psi, 100 μm nozzle, 3,000 events/sec) to preserve neuronal progenitor viability. For sorts to isolate EGFP+ cells we perform our isolations on a BD Biosciences FACSAriaII using a 488nm 20mW laser to excite the EGFP reporter.
- If samples contain high cell concentrations, it is advisable to dilute the cell suspension further using 1:1000 7-AAD stain to achieve higher capture efficiencies when sorting.
- Vortex each tube of cells captured in TRIzol-LS immediately after sorting.
7. Representative Results
Tissue dissociation to produce a cell suspensions for flow sorting is a delicate balance between adequate enzymatic digestion and avoiding over-digestion that can result in low cell viabilities. An example of desired level of tissue dissociation is shown in Figure 2. In appropriately digested tissue before manual trituration pieces of sub-dissected organs are still clearly evident (Figure 2b, 2f). In tissues that are enzymatically treated for too long a period of time or at too high a concentration of enzyme, the resulting suspension lacks any residual large pieces of tissue (Figure 2d, 2h).
Appropriate dissociation and manual filtering produce sort profiles at flow cytometry that typically exhibit greater than 90% viable cells and show high levels of EGFP expression (Figure 4). Cell populations obtained by this method illustrate good viability and can be captured for subsequent culture or analysis of gene expression by gating for capture of EGFP+ neuronal progenitors.
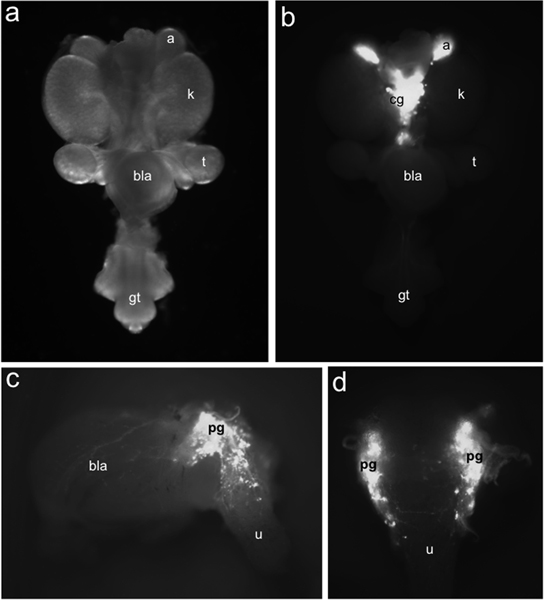
Figure 1. Distribution of TH-EGFP+ neuronal progenitors in fetal mouse LUT. Whole-mount urogenital tract at 15.5 dpc viewed ventrally under bright field illumination (a) compared to distribution of EGFP+ cells labeled by TH-EGFP transgene expression identified under fluorescence illumination (b). TH-EGFP expression is present in adrenals (a) and medially located celiac ganglia (cg). Lateral (c) view of 15.5 dpc TH-EGFP sub-dissected bladder exhibits fluorescence from transgene expression in pelvic ganglia (pg), the bladder wall (bla) and urethra (u). In dorsal view (d) EGFP+ cells are evident in the anterior dorsal urethra. Other labels: kidneys (k), testis (t), bladder (bla) and genital tubercle (gt).
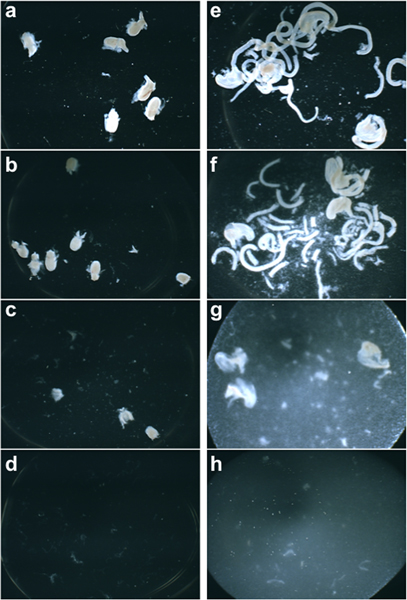
Figure 2. Brightfield images of 15 dpc fetal LUT (a) and intestine (e), respectively, imaged halfway through the dissociation incubation period, at the end of the dissociation incubation before disruption (b, f), after manual disruption (c, g), and in a sample that has been overly dissociated (d, h).
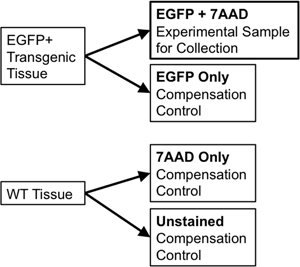
Figure 3. Schematic Diagram illustrates compensation controls needed to establish rigorous FACS gating parameters.
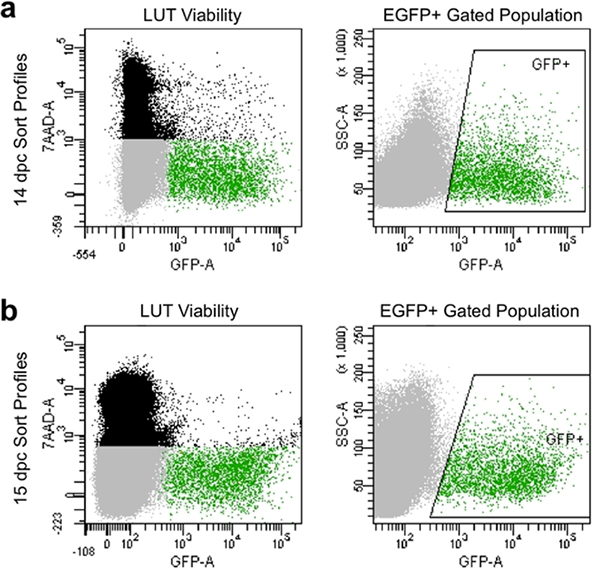
Figure 4. Representative image of flow sort profiles at 14.5 dpc (a) and 15.5 dpc (b). Black population is comprised of single cells based on forward and side scatter that are dead and labeled by 7-AAD fluorescence. Gray population is comprised of singlet cells based on forward and side scatter that have excluded 7-AAD and are thus viable. Green gated population is indicated by boxed “GFP+” area and is comprised of single cells that have excluded 7-AAD (viable) and exhibit EGFP fluorescence.
Discussion
Mouse reporter lines expressing fluorescent reporters are becoming widely available through multiple efforts in the murine genetics community1,8,9. As a result the dissociation method illustrated here can be widely applied for isolation of discrete neuronal subtypes based on neurotransmitter or receptor expression patterns from either fetal or adult tissues. While we have optimized this method based on expression of a fluorescent transgene reporter, it can also be applied to samples expressing cell surface receptors labeled by live cell immuno-labeling methods3-5,10.
In our hands we observed several factors that affected the percentage of cells that survived the dissociation procedure. These include elapsed time from initial dissection of fetal tissue to isolation of cells by flow cytometry, gentle handling of cell suspensions both during filtering and at the flow sorter, as well as the enzyme type used in dissociation. The survival of both enteric and LUT progenitors benefited from rapid dissection/dissociation and use of gentle conditions during cell sorting (17psi, 100 micron nozzle, 3,000 events/sec). We found that prior to isolation at the flow sorter, the total population of material observed by the instrument (all events including debris and multiplets) generally exhibited about 80% survival for cell dissociates generated from either fetal intestine or LUT. To exclude debris and clumps of cells, initial gates for forward scatter (FSC) and side scatter (SSC) were imposed on this total population. Then the total population is further refined to a “daughter” population by voltage pulse geometry gating for width and height measurement of FSC and SSC to exclude doublets. Within this refined “daughter” population non-viable cells that are 7-AAD+ were excluded so that only viable cells exhibiting the fluorescent EGFP reporter were collected. We observed that the frequency of survival for neuronal progenitors in this daughter population was relatively low when dissociation was performed in a blend of trypsin+collagenase that is routinely used in many cases for this purpose (0.5% Trypsin, Gibco; 10 mg/ml Collagenase IV, Worthington)2,5-7. To obtain higher percentages of surviving neuronal progenitors from LUT tissue isolates, we tested alternate enzyme treatments for dissociation of tissue to derive cell suspensions for flow sorting (Table 2). Neuronal progenitors exhibited improved viability upon dissociation in dispase (5 mg/ml) a gentle dissociation method that is often used for isolation of neural tubes or other neural progenitor populations11. However, the greatest viability observed was obtained with Accumax, a proprietary blend of protease, collagenolytic activity and DNase of unknown proportions, (Table 2). Dissociation in Accumax yielded the best survival for both enteric neuronal progenitors and LUT neuronal progenitors with modest differences in survival between the two sources. Our results suggest that distinct neuronal populations may differ in their sensitivity to dissociation conditions. Thus, investigators seeking to isolate neuronal progenitors or mature neurons should evaluate dissociation conditions to identify those that produce the greatest cell survival and yield for distinct tissue sources. The method we describe serves as a starting point for such procedures in the peripheral nervous system.
| Enzyme in dissociation solution | % Survival Enteric NP | % Survival LUT NP |
| Tryp + Collag | 29.5 +/- 8.2%, n=11 | 46.2 +/- 8.6%, n=4 |
| Dispase | 35.1 +/- 9.8%, n=10 | 46.3 +/- 6.6%, n=9 |
| Accumax | 66.5 +/- 7.8%, n=8 | 58.1 +/- 5.2%, n=10 |
Table 2. Viability of neuronal progenitors (NP) in different dissociation conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Catherine Alford for suggestions on cell dissociation methods and Kevin Weller, David Flaherty and Brittany Matlock for support in the Flow Cytometry Shared Resource at Vanderbilt University Medical Center and Melissa A. Musser for artistic assistance with illustrations. We thank Drs. Jack Mosher and Sean Morrison for advice on implementing isolation of neuronal progenitors. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (P30 DK058404). This work was supported by funding from US National Institutes of Health grants DK064251, DK086594, and DK070219.
Materials
| Reagent Name | Vendor | Catalog number | Comments |
| Accumax | Sigma(mfr: Innovative Cell Technologies) | A7089-100ML | Store frozen in 1 ml aliquots |
| DNase I | Sigma | D-4527 | Stored frozen at -20 °C 5 mg/ml in 1xHBSS, (Used in Quench, Quench 1:5) |
| 10X PBS pH 7.4 | Gibco | 70011-044 | Make up to 1x with tissue culture grade water then sterile filter |
| 10X HBSS w/o Ca or Mg | Gibco | 14185-052 | Make up to 1x with tissue culture grade water then sterile filter |
| Leibovitz’s L-15 medium | Gibco | 21083027 | |
| Penicillin /Streptomycin 100X | Gibco | 15140-133 | Store aliquoted at -20 °C |
| BSA | Sigma | A3912-100G | Store aliquoted at -20 °C, 100 mg/ml in water |
| Biowhittaker 1M HEPES in 0.85% NaCl | Lonza | 17-737E | |
| 38 μm NITEX Nylon Mesh Membrane | Sefar America | 3-38/22 | Cut into ~3 cm squares. UV treat overnight to sterilize in the tissue culture hood. |
| 7-AAD | Invitrogen | A1310 | 1 mg/ml |
| TRIzol LS | Invitrogen | 10296-028 | |
| 5 ml polystyrene tubes | Falcon | 352058 | |
| 15 ml conical tubes | Corning | 430790 | |
| Fine Dissecting Forceps | Fine Science Instruments | 11251-30 | Dumont#5 forcep, Dumoxel, standard tip 0.1×0.06mm |
| Dissecting Spoon | Fine Science Instruments | 10370-18 |
References
- Gong, S. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 425, 917-925 (2003).
- Morrison, S. J., White, P. M., Zock, C., Anderson, D. J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 96, 737-749 (1999).
- Kruger, G. M. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 35, 657-669 (2002).
- Bixby, S., Kruger, G. M., Mosher, J. T., Joseph, N. M., Morrison, S. J. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 35, 643-656 (2002).
- Walters, L. C., Cantrell, V. A., Weller, K. P., Mosher, J. T., Southard-Smith, E. M. Genetic background impacts developmental potential of enteric neural crest-derived progenitors in the Sox10Dom model of Hirschsprung disease. Human Molecular Genetics. , (2010).
- Corpening, J. C. Isolation and live imaging of enteric progenitors based on Sox10-Histone2BVenus transgene expression. Genesis. 49, 599-618 (2011).
- Morrison, S. J. . Isolation of fetal rat neural crest stem cells (NCSC) from gut and sciatic nerve. , (2012).
- Skarnes, W. C. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 474, 337-342 (2011).
- Harding, S. D. The GUDMAP database–an online resource for genitourinary research. Development. 138, 2845-2853 (2011).
- Joseph, N. M. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 121, 3398-3411 (2011).
- Newgreen, D. F., Murphy, M. Neural crest cell outgrowth cultures and the analysis of cell migration. Methods Mol. Biol. 137, 201-211 (2000).

