Time-lapse Fluorescence Imaging of Arabidopsis Root Growth with Rapid Manipulation of The Root Environment Using The RootChip
Summary
This article provides a protocol for cultivation of Arabidopsis seedlings in the RootChip, a microfluidic imaging platform that combines automated control of growth conditions with microscopic root monitoring and FRET-based measurement of intracellular metabolite levels.
Abstract
The root functions as the physical anchor of the plant and is the organ responsible for uptake of water and mineral nutrients such as nitrogen, phosphorus, sulfate and trace elements that plants acquire from the soil. If we want to develop sustainable approaches to producing high crop yield, we need to better understand how the root develops, takes up a wide spectrum of nutrients, and interacts with symbiotic and pathogenic organisms. To accomplish these goals, we need to be able to explore roots in microscopic detail over time periods ranging from minutes to days.
We developed the RootChip, a polydimethylsiloxane (PDMS)- based microfluidic device, which allows us to grow and image roots from Arabidopsis seedlings while avoiding any physical stress to roots during preparation for imaging1 (Figure 1). The device contains a bifurcated channel structure featuring micromechanical valves to guide the fluid flow from solution inlets to each of the eight observation chambers2. This perfusion system allows the root microenvironment to be controlled and modified with precision and speed. The volume of the chambers is approximately 400 nl, thus requiring only minimal amounts of test solution.
Here we provide a detailed protocol for studying root biology on the RootChip using imaging-based approaches with real time resolution. Roots can be analyzed over several days using time lapse microscopy. Roots can be perfused with nutrient solutions or inhibitors, and up to eight seedlings can be analyzed in parallel. This system has the potential for a wide range of applications, including analysis of root growth in the presence or absence of chemicals, fluorescence-based analysis of gene expression, and the analysis of biosensors, e.g. FRET nanosensors3.
Protocol
Note: Perform all steps preparatory steps under sterile conditions.
1. Preparation of Plastic Cones for Seed Germination
- Fill a 10 cm Petri dish with growth medium containing 1% agar to a thickness of 5 mm. We use a half strength modified Hoagland medium4, but medium composition should be chosen to suit individual experimental requirements.
- While the medium is still liquid, use a multi-channel pipette to fill 10 μl pipette tips with 5 μl of medium from the Petri dish.
- Store the filled tips in the pipette tip box until the medium is solid, then cut to 4 mm long plastic cones and place upright into a Petri dish containing solid growth medium.
2. Seed Germination and Seedling Growth
- Surface sterilize seeds in 5% NaOCl for 5 min, wash three times with sterile water, then place a single seed on top of each of the medium-filled cones.
- Seal the dish with Micropore tape (3M) and store at 4 °C to synchronize germination.
- After three days, transfer the plates to a growth cabinet to start germination. Our growth conditions are 23 °C at a 16h high light/8h dark cycle (Light intensity: 100 μE m-2 s-1).
- Between 5 and 7 days after germination, the seedlings should be ready for transfer to the RootChip. At this time, root tips should be near the bottom outlets of the plastic cones. Check seedling health, root length and, if applicable, expression of a fluorescent marker under a dissecting microscope.
- Mark individual seedlings for transfer onto the chip. Select ten or so seedlings in case one is damaged during transfer.
3. Transfer of Seedlings onto the RootChip
- To sterilize the RootChip for long-term experiments, wrap the device in tissue paper, place in a glass Petri dish, and autoclave.
- Once the RootChip has cooled, cover it with liquid growth medium. The RootChip should be completely immersed, but the fluid level should be no more than 3 mm above the RootChip surface.
- With a 20 μl pipette, pull medium through the root inlet and chamber outlet to fill the observation chamber with medium.
- Plug plastic cones selected in step 2.5 into the RootChip inlets. The cones should fit snugly in the inlets. Since the RootChip is mounted on a thin layer of optical glass, do not apply too much pressure to the chip.
- Incubate the RootChip overnight in liquid medium. To prevent floating, place two glass slides onto the chip. Add a magnetic stir bar and close the dish.
- Transfer the assembly to a magnetic stirrer and gently agitate the medium.
- The RootChip’s inlets intersect the channels at a 30° angle to the device normal to facilitate root growth into the channels (Figure 1A). To further support growth in the desired direction, tilt the assembly slightly by placing a glass slide under the Petri dish on the side of the chip opposite of the outlets.
- To maintain the light/ dark cycle, illuminate the seedlings with a ring lamp (Light intensity: 100 μE m-2 s-1) connected to a timer.
4. Connecting the RootChip to the Carrier
- The following day, fill a sealable, pressurizable vial with liquid growth medium (Figure 1B).
- Invert the chip carrier and place it on a stable surface. Remove the RootChip from the liquid medium and insert it PDMS side down into the bottom aperture of the chip carrier. Orient the chip so that the side containing the control layer inlets is facing the side of the pressure line tubing connectors in the carrier side wall.
- Dry the cover glass on the bottom of the chip by gently blotting with tissue paper. Secure the RootChip to the carrier with tape and right the whole assembly.
- Tubing connectors are made by cutting flexible plastic microbore tubing (TYGON, 0.20″ ID x 0.060″ OD) into 5 cm long pieces and connecting them to stainless steel microbore tubes (New England Small Tube, 0.025″ OD x 0.013″ ID x 0.75″ long). Fill the tubing connectors with water using a syringe and plug each tubing connector into the corresponding control layer inlet on the chip. The water will later fill the control layer channels and be used to transmit the pressure to the micromechanical valves.
- Plug the opposite ends of the lines into the media/solution vial(s). Apply pressure to the solution vial with a syringe of air. The increased air pressure within the solution vial will force liquid into the lines.
5. Mounting the RootChip at the Microscope
- Place the carrier onto the microscope stage. To reduce the possibility of the assembly shifting over the course of the experiment due to vibrations in the room, the carrier should fit exactly into the notches of the stage insert.
- The chip valves and the flow of medium through the chip are controlled by air pressure. Two lines with regulators are branched off of a main pressure line – one is used to control the medium flow through the channels, and the other is connected to solenoid air valves that actuate the push-up valves of the control layer. The solenoid valves are operated from the computer via the USB valve controller (developed by Rafael Gómez-Sjöberg, Lawrence Berkeley National Lab). Close both pressure regulators before connecting the chip.
- Add a few ml of water to the reservoirs of the carrier to keep the humidity high within the assembly. This step should be repeated over course of longer experiments to keep the plants from drying out. Keep the volume low to minimize the amount of liquid that could be spilled onto the microscope. For long-term experiments, the outflow from the chip outlets can be guided into the reservoirs of the carrier by connecting the chip outlets to the reservoirs with microbore tubing (see step 4.4). Alternatively, the outflow that accumulates on the chip surface can be collected by pipetting.
- Prepare squared sheets of transparent plastic from sheet protectors (C-Line). Fix the transparent plastic to the carrier by double-sided tape to maintain high humidity in the assembly.
- Position the ring light over the chip and maintain the light/dark cycle. The ring light should be switched off prior to the start of any experiment that uses fluorescent markers, as the direct illumination will interfere with image collection.
6. Operating the RootChip using the LabView Interface
The RootChip controller interface for the LabView software platform can be downloaded from our website http://dpb.carnegiescience.edu/technology/rootchip.
- The valves on the chip are closed by applying pressure to the control layer, in this case by opening the solenoid air valves. The controller interface allows actuation of the valves by clicking the button below the valve number. Bright green indicates application of pressure and closing of a chip valve (Figure 2B). Activate all three solution-inlet valves in the controller interface before opening the pressure regulators. Note: The controller interface features a feedback loop, which allows monitoring of the system’s status. This feature may be activated by clicking on the “Readback” button in the controller interface.
- Open the pressure regulator for the control layer and initially set to 15 psi, then open the regulator for the flow layer and initially set to 5 psi. Depending on the desired flow rate, the pressures may be adjusted later.
- Open the inlet valve for the growth medium of choice to flush the chambers with medium.
- Check flow paths under the microscope. Typically, air is trapped in the flow channels and must be removed. Additionally, the channels of the control layer still contain air that must be forced out and replaced by the water from the tubing connectors (dead-end filling). Both tasks are achieved by flushing each of the eight chambers several times (5 psi) until all air is forced from the channels into the PDMS (“degassing”). Note: The controller interface can be programmed to automate experiments. Such routines may also be used to degas the chip.
7. Representative Results
The prime purpose of the RootChip is to combine an imaging platform and a perfusion system in a single device with a high level of integration. To demonstrate the manipulation of the microenvironment of roots we flushed the chambers with dark food coloring (1:4 dilution in hydroponic medium) and measured the exchange of fluid within the chambers. At the recommended pressure of 5 psi we measured a full exchange within 10 seconds at a calculated flow rate of approximately 1.5 μl/min (Figure 3).
We also observed root growth of seedlings, in this case grown in the dark and supplied with 10 mM Glucose as an external energy source (Figure 4). Depending on the growth conditions such as light and composition of the medium, plants can be observed in the RootChip for up to three days.
The RootChip has been used to monitor intracellular glucose and galactose levels in roots expressing genetically encoded nanosensors, based on Förster Resonance Energy Transfer (FRET)5-7. Roots in the chip were perfused with square pulses of glucose or galactose solution (Figure 5). The intracellular levels of sugars were monitored and are shown here expressed as a ratio of the intensity of the acceptor fluorophore Citrine to the intensity of the donor ECFP. The rise in ratio indicates accumulation of sugar.
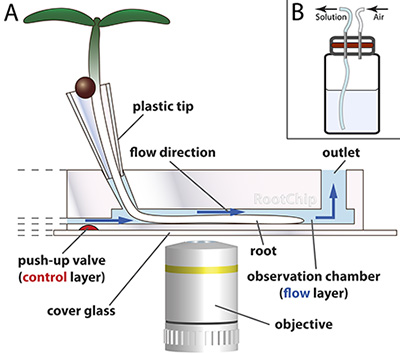
Figure 1. RootChip principle.
- The RootChip features eight observation chambers for the growth and imaging of roots. The seeds first germinate in plastic cones – fabricated from plastic pipette tips – which are filled with solid medium. The root tip grows through the medium and reaches the chamber where a continuous flow of liquid medium keeps the conditions in the chamber constant. Micromechanical valves (red) control the flow. The chip is mounted on an optical cover glass.
Drawing not to scale. (Adapted with permission from Grossmann et al., 2011 Plant Cell.) - Scheme of a pressurizable solution vial with diaphragm (red).
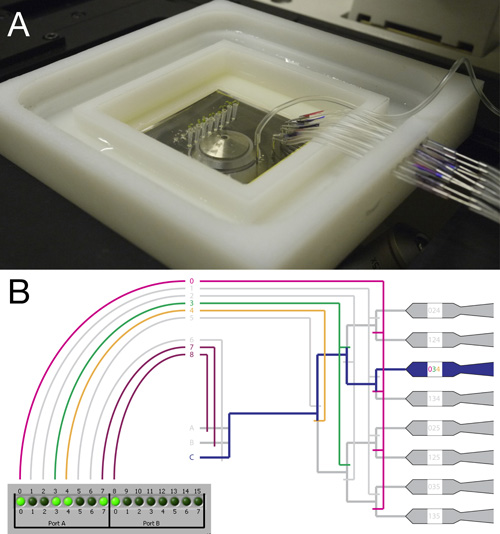
Figure 2. Connecting and mounting the RootChip.
- Top view of the fully connected RootChip and carrier mounted on an inverted microscope.
- Scheme illustrating the valving system and the controller interface. An example for a valve setting to guide the fluid flow to a single chamber is shown. While valves 4 through 8 act as single valves, valves 0 through 3 act in groups. With this system an individual chamber can be addressed by activating a combination of valves.
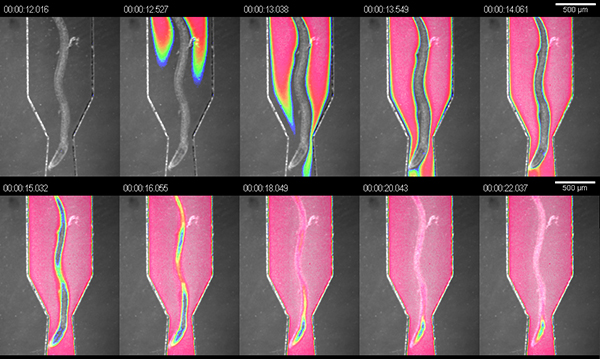
Figure 3. Exchange of solutions in the observation chambers. Visualization of the exchange of fluid in an observation chamber using dye solution. The image is an overlay of bright field and false-colored intensity of the dye signal.
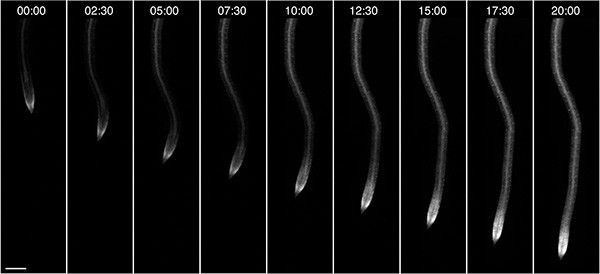
Figure 4. On-chip root growth. Observation of a single growing root expressing a fluorescent FRET nanosensor for glucose/galactose over the course of 20h. Time format: hh:mm; scale bar: 100 μm.
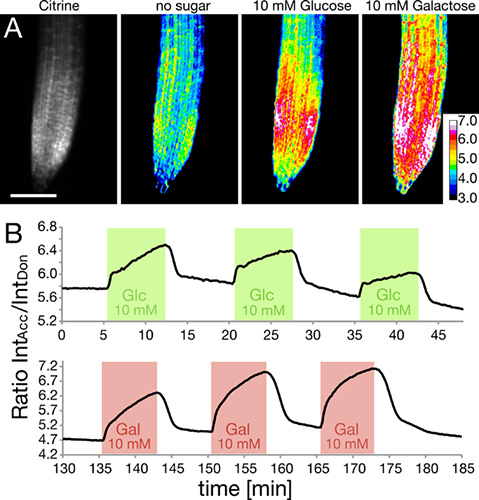
Figure 5. Measuring intracellular sugar levels using FRET nanosensors.
- The amount of sensor is shown as citrine intensity within the root tip (left). The response of the intracellular FRET nanosensor to the application of glucose or galactose solution is shown as ratiometric images. (Adapted with permission from Grossmann et al., 2011 Plant Cell.) Scale bar: 100 μm.
- Tracing FRET ratio changes as a response to three repeated square pulses of glucose (green) and galactose (red).
Discussion
The main advantages of the RootChip over conventional growth methods are the minimally invasive preparation for microscopy, the ability to reversibly and repeatedly alter the root environment, and the capacity for continuous observation of developmentally competent and physiologically healthy tissue over a period of several days. Previously, seedlings were grown vertically on gelled media and transferred to a perfusion system immediately before the experiment, which allowed only measuring single roots at a time8. Microfluidic tools have been used for Arabidopsis, but on a low integration level9 or without perfusion control10. The RootChip combines a high level of integration with the ability to automate experiments through precise flow guidance. Another advantage of this platform, characteristic of all microfluidic devices11, is that only minimal amounts of liquid are required to supply the root with the necessary nutrients, even for experiments spanning several days. The RootChip is currently designed as a single-use device, but since production costs of chips are low, the small amounts of consumed reagents makes the chip still very cost-effective.
There are a few critical steps that must be taken to guarantee the health of the seedlings:
The volume in the plastic cones is only 3-4 μl, which will begin to dry when exposed to air. Hence it is critical that the cones are transferred onto the chip quickly and humidity is kept high until the roots have reached the observation chambers, which will supply them with sufficient water. Steps 4.2 to 4.5 should be performed quickly and without interruption to prevent drying of the seedlings.
Steps 3.5 – 3.8 describe the incubation of the chip in liquid media during which the roots grow into the observation chambers. This step may be skipped by mounting the chip into the carrier immediately and starting constant perfusion with growth medium. However, we recommend soaking in growth medium overnight, as it has some advantages: 1) it creates a humid environment so the seedlings are less likely to become desiccated as they grow into the observation chamber; 2) the chip is soaked in liquid, so degassing (step 6.4) will be faster.
It is important to use media with low solute concentrations. More concentrated solutions may precipitate and clog the channels, especially if the chip is used over several days.
Once the device is connected to the air pressure line, flow of medium is controlled by changing hydraulic pressure in the valves. To guarantee proper closure of the micromechanical valves, it is important to choose a control pressure that is about three times higher than the flow pressure. The flow pressure should not exceed 15 psi as the fluid will be pushed out of the root inlets. Higher pressures may also cause delamination of the chip, which renders the chip unusable.
A limitation of the RootChip is that PDMS is porous and hydrophobic. While the material is practically inert to aqueous solutions, it may absorb organic compounds12. This can interfere with a rapid exchange of solutions as organic compounds may leak from the material even when the supply of this compound has been stopped at the inlet. Due to the porosity, using organic solvents may cause swelling of the PDMS12.
We continue to optimize the RootChip and extend its utility, for example with roots of crop plants. We believe that by improving access to the root for treatments and observation, microfluidic tools like the RootChip will open up new dimensions of root research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Philipp Denninger for help with video preparation and Bhavna Chaudhuri for providing plant lines expressing FRET sensors. This work was supported by grants from the National Science Foundation (MCB 1021677), the Department of Energy (DE-FG02-04ER15542) to W.B.F, the National Institutes of Health, and the Howard Hughes Medical Institute to S.R.Q. G.G. was supported by an EMBO long-term fellowship. M.M. was supported by the Alexander von Humboldt Foundation.
Materials
| Items | Source | Information |
| Chip carrier, software and other information. | Carnegie Institution – DPB | CAD and CNC files for carrier fabrication, controller software and further information are available for download from the website http://dpb.carnegiescience.edu/technology/rootchip Carriers can also be ordered from this website. |
| RootChip | Stanford Foundry | Mask designs and fabrication protocols are available upon request. Ready-to-use RootChips can be ordered from http://www.stanford.edu/group/foundry/ |
| Chip controller | -home built- | The automated valve controller system was originally developed by Rafael Gómez-Sjöberg , Lawrence Berkeley National Lab. A detailed instruction how to build your own actuated valve controller can be found at https://sites.google.com/a/lbl.gov/microfluidics-lab/valve-controllers |
References
- Grossmann, G. The RootChip: An Integrated Microfluidic Chip for Plant Science. Plant Cell. 23, 4234-4240 (2011).
- Unger, M. A., Chou, H. P., Thorsen, T., Scherer, A., Quake, S. R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 288, 113-116 (2000).
- Okumoto, S., Jones, A., Frommer, W. B. Quantitative Imaging with Fluorescent Biosensors: Advanced Tools for Spatiotemporal Analysis of Biodynamics in Cells. Annu. Rev. Plant Biol. , (2012).
- Loqué, D., Lalonde, S., Looger, L. L., von Wirén, N., Frommer, W. B. A cytosolic trans-activation domain essential for ammonium uptake. Nature. 446, 195-198 (2007).
- Okumoto, S. Imaging approach for monitoring cellular metabolites and ions using genetically encoded biosensors. Curr. Opin. Biotechnol. 21, 45-54 (2010).
- Fehr, M., Frommer, W. B., Lalonde, S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc. Natl. Acad. Sci. USA. 99, 9846-9851 (2002).
- Takanaga, H., Chaudhuri, B., Frommer, W. B. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim. Biophys. Acta. 1778, 1091-1099 (2008).
- Chaudhuri, B., Hörmann, F., Frommer, W. B. Dynamic imaging of glucose flux impedance using FRET sensors in wild-type Arabidopsis plants. J. Exp. Bot. 62, 2411-2417 (2011).
- Meier, M., Lucchetta, E. M., Ismagilov, R. F. Chemical stimulation of the Arabidopsis thaliana root using multi-laminar flow on a microfluidic chip. Lab Chip. 10, 2147-2153 (2010).
- Parashar, A., Pandey, S. Plant-in-chip: Microfluidic system for studying root growth and pathogenic interactions in Arabidopsis. Appl. Phys. Lett. 98, 263703-26 (2011).
- Whitesides, G. M. The origins and the future of microfluidics. Nature. 442, 368-373 (2006).
- Lee, J. N., Park, C., Whitesides, G. M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 75, 6544-6554 (2003).

