制备碳纳米管高频纳电子生物传感器的传感高离子强度的解决方案
Summary
我们描述了基于碳纳米管的高频生物传感器的设备制造和测量协议。高频感应技术减轻了根本的离子(德拜)的屏蔽效应,并允许碳纳米管传感器工作在高离子强度的解决方案,在传统的电子生物传感器失败。我们的技术提供了一个独特的平台,为护理点(POC)在生理条件下运行的电子生物传感器。
Abstract
独特的电子性质和高表面体积比单壁碳纳米管(SWNT)和半导体纳米线(NW)1-4让他们很好的候选人高灵敏度生物传感器。当一个带电的分子结合到这样的传感器表面,改变载流子浓度5中的传感器,其直流电导的变化。然而,在离子溶液中的带电表面也吸引从该溶液中的反离子,形成双电层(EDL)。此EDL有效屏幕关闭充电,在生理条件下〜100毫摩尔(MM),特性电荷屏蔽长度(德拜长度)小于一个纳米(nm)。因此,在高离子强度的解决方案,充电(DC)的检测是从根本上阻碍了6-8。
我们克服电荷屏蔽效应通过检测分子偶极子,而不是在高频收费,经营碳nanot的UBE场效应晶体管高频混频器9-11。在高频时,的AC驱动力可以不再克服溶液阻力和溶液中的离子,没有足够的时间形成的EDL。另外,混频技术使我们能够工作在频率足够高,以克服离子筛选,而检测到的感测信号在较低频率的11-12。此外,单壁碳纳米管的晶体管的高跨导,提供了一个内部的感测信号,避免了需要外部信号放大器的增益。
在这里,我们描述协议(一)制备单壁碳纳米管晶体管,(二)生物分子功能化碳纳米管13,(C)到设备设计和邮票聚二甲基硅氧烷(PDMS)微流控室14,(D)进行高频感应离子强度不同的解决方案11。
Introduction
当一个带电的分子结合到一个单壁碳纳米管或NW电子传感器,它可以捐赠/接受电子或作为本地静电门。在这两种情况下,可以改变结合的分子的电荷密度,在单壁碳纳米管或洒水通道,导致的传感器测得的直流电导的变化。 15-20分子种类繁多的已成功检测研究纳米传感器的直流特性,在这样的结合事件。即使负责检测的传感机理有很多优势,包括无标记检测21毫微摩尔灵敏度22,和电子读出能力15,它是有效的,只有在低离子强度的解决方案。在高离子强度的解决方案,直流检测离子筛选6-8阻碍。甲充电表面吸引,形成表面附近的双电层(EDL)从该溶液中的反离子。 EDL有效地筛选这些费用。作为T他的离子强度的溶液的增加,EDL的变窄和筛选的增加。该屏蔽效应的Debye屏蔽长度λD,其特征在于
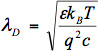
式中,ε是介质的介电常数,K B为玻尔兹曼常数,T是温度,q是电子电荷,c为电解质溶液的离子强度。对于一个典型的100mM的缓冲溶液中,λD是约1纳米的表面电势在几个纳米的距离完全筛选。其结果是,大多数基于单壁碳纳米管或纳米线的纳米电子传感器操作无论是在干燥状态下20或低离子强度的解决方案5,15,17,21-22(C〜1纳米- 10毫摩尔),其他样品需要进行脱盐步骤15,23。点医疗诊断设备需要在生理离子强度,来样加工能力有限的病人站点。因此,减轻离子筛查效果是POC纳米电子生物传感器的开发和实施的关键。
我们减轻离子屏蔽效应,基于单壁碳纳米管的纳米电子传感器在兆赫频率范围经营。该协议提供了在这里制造的单壁碳纳米管晶体管为基础的纳米电子传感平台和高频混合测量生物分子检测。单壁碳纳米管生长的化学气相沉积图案与Fe催化剂24的基板上。对于我们的单壁碳纳米管晶体管,我们将暂停顶栅25以上500纳米的碳纳米管,这有助于提高高频传感器响应,也可以让一个紧凑的MICR邻流体腔密封装置。单壁碳纳米管晶体管的运行为高频率混频器9-11为了克服背景离子屏蔽效应。在高频时,溶液中的离子移动,没有足够的时间来形成的EDL和生物分子偶极子波动仍然可以门的单壁碳纳米管生成一个混合的电流,这是我们的传感信号。混频的产生是由于碳纳米管FET的非线性伏安特性 。我们的检测技术不同于常规技术充电检测和阻抗谱26-27。首先,我们检测生物分子的偶极子的相关费用,而不是在高频率。其次,高跨导的单壁碳纳米管的晶体管提供了一个内部的感测信号的增益。这省却了在高频阻抗测量的情况下,无需进行外部放大。最近,其他团体也高BA解决生物分子检测的ckground浓度23,28。然而,这些方法更复杂,需要复杂的制造或受体分子化学工程小心。我们的高频率的单壁碳纳米管传感器采用一个简单的设计,并采用混合属性的碳纳米管晶体管的固有频率。我们有能力,以减轻离子屏蔽效应,因此看好一个新的生物传感平台进行实时的护理点检测,需要直接在生理条件下运作的生物传感器。
Protocol
Representative Results
Discussion
碳纳米管的生长,不仅取决于炉况,但也基板清洁度。必须仔细校准,一旦固定,它们都或多或少的稳定的最佳气体流速,温度和压力的增长。即使这些条件得到满足,我们发现增长取决于图案的催化剂,面积,金额的催化剂和基板清洁度。因此,我们注册成立了多个催化剂坑的大小占增长的变异。一小时的高温退火步骤有助于从基片上除去任何污染物,例如:PR残余物等, 图2示出?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
我们感谢在康奈尔大学教授保罗·麦克尤恩早期讨论。这项工作是支持的启动基金提供的密歇根大学和美国国家科学基金会可扩展的纳米制造计划(DMR-1120187)。这项工作在美国密歇根大学,由美国国家科学基金会资助的美国国家纳米技术基础设施网络的成员劳瑞加工设施。
Materials
| REAGENTS | |||
| Reagents which were provided within Lurie Nanofabrication Facility (University of Michigan) are marked as LNF in the catalogue column. Chemicals which require protective equipment (gloves, safety goggles, face mask, apron) and/or fume hood are denoted with PPE in comments section. | |||
| Silicon wafers (P-type, <100>, 500-550 μm thick) | Silicon Valley Microelectronics | ||
| SPR 220 3.0 | Dow (Rohm and Haas) | Megaposit SPR | PPE |
| AZ 300MIF | AZ Electronic Material Corporation | PPE | |
| Acetone | J T Baker | 9005-05 | PPE |
| Isopropanol (IPA) | J T Baker | 9079-05 | |
| Buffered Hydrofluoric Acid | Transene | PPE | |
| 1-Pyrene Butanoic Acid, succinimidyl ester | Molecular Probes | P130 | PPE |
| Biotin PEO Amine | Thermo Scientific | EZ- Link PEG2 Biotin, # 21346 | PPE |
| Streptavidin | Invitrogen | S 888 | PPE |
| Dimethylformamide | MP Biomedicals | 0219514791 | PPE |
| Polydimethylsiloxane Elastomer Base and Curing Agent | Dow Corning | Sylgard 184 Elastomer Kit | PPE |
| SU-8 2015 | Microchem | Y111064 | PPE |
| SU-8 Developer | Microchem | Y020100 | PPE |
| Silanizing agent | Sigma Aldrich | 452807 | PPE |
| Hydrogen | Purity Plus | LNF | |
| Ethylene | Purity Plus | LNF | |
| Argon | Purity Plus | LNF | |
| Phosphate Buffer Saline System | Sigma Aldrich | PBS1 | |
| EQUIPMENT | |||
| Equipment provided by Lurie Nanofabrication Facility (University of Michigan) is denoted as LNF in Catalogue column. | |||
| GCA 200 Autostepper | GCA | LNF | |
| Low Pressure Chemical Vapor Deposition Tool | Tempress | LNF | |
| e-beam Evaporator | Enerjet | LNF | |
| CNT growth Furnace | First Nano | Easy Tube 3000 (LNF) | |
| Photomasks | Nanofilm | LNF | |
| Petri dish (150mm) | LNF | ||
| Desiccator | Bel-Art | F420100000 | |
| Biopsy Punch | Ted Pella | 15071/78 | |
| Scalpel | Ted Pella | 548 | |
| Polyethylene Tubing PE-50 | VWR | 20903-414 | |
| Syringe Pump | New Era Pump Systems | NE-1000 | |
| Syringe | Fisher Scientific | BD Safety-Lok Syringes | |
| Syringe Needles | Fisher Scientific | 14-821-13A | |
| DAQ card | National Instruments | 779111-01 | |
| GPIB connector | National Instruments | 778032-51 | |
| Lock-in Amplifier | Stanford Research Systems | SR 830 | |
| Frequency Generator | HP Agilent | 8648B, 9kHz -2GHz | |
| Bias Tee | Picosecond | 5575A-104 | |
| Current Preamplifier | DL Instruments, LLC | DL 1211 | |
| BNC cables | Allied Electronics | 665-xxxx | |
| SMA cables | Sentro Tech Corp | SCF65141 |
References
- Dekker, C. Carbon nanotubes as molecular quantum wires. Phys. Today. 52, 22-28 (1999).
- McEuen, P. L., Fuhrer, M. S., Park, H. K. Single-walled carbon nanotube electronics. IEEE Transactions on Nanotechnology. 1, 78-85 (2002).
- Duan, X. F., Huang, Y., Cui, Y., Wang, J. F., Lieber, C. M. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature. 409, 66-69 (2001).
- Cui, Y., Zhong, Z. H., Wang, D. L., Wang, W. U., Lieber, C. M. High performance silicon nanowire field effect transistors. Nano Letters. 3, 149-152 (2003).
- Heller, I., Janssens, A. M., et al. Identifying the mechanism of biosensing with carbon nanotube transistors. Nano Letters. 8, 591-595 (2008).
- Stern, E., Wagner, R., et al. Importance of the debye screening length on nanowire field effect transistor sensors. Nano Letters. 7, 3405-3409 (2007).
- Zhang, G. J., Zhang, G., et al. DNA sensing by silicon nanowire: Charge layer distance dependence. Nano Letters. 8, 1066-1070 (2008).
- Sorgenfrei, S., Chiu, C. -. y., Johnston, M., Nuckolls, C., Shepard, K. L. Debye Screening in Single-Molecule Carbon Nanotube Field-Effect Sensors. Nano Letters. 11, 3739-3743 (2011).
- Appenzeller, J., Frank, D. J. Frequency dependent characterization of transport properties in carbon nanotube transistors. Applied Physics Letters. 84, 1771-1773 (2004).
- Rosenblatt, S., Lin, H., Sazonova, V., Tiwari, S., McEuen, P. L. Mixing at 50 GHz using a single-walled carbon nanotube transistor. Applied Physics Letters. 87, (2005).
- Kulkarni, G. S., Zhong, Z. H. Detection beyond the Debye Screening Length in a High-Frequency Nanoelectronic Biosensor. Nano Letters. 12, 719-723 (2012).
- Sazonova, V. . A Tunable Carbon Nanotube Resonator. , (2006).
- Chen, R. J., Zhang, Y. G., Wang, D. W., Dai, H. J. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. Journal of the American Chemical Society. 123, 3838-3839 (2001).
- Duffy, D. C., McDonald, J. C., Schueller, O. J. A., Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane. Analytical Chemistry. 70, 4974-4984 (1998).
- Zheng, G. F., Patolsky, F., Cui, Y., Wang, W. U., Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nature Biotechnology. 23, 1294-1301 (2005).
- Star, A., Han, T. R., Gabriel, J. C. P., Bradley, K., Gruner, G. Interaction of aromatic compounds with carbon nanotubes: Correlation to the Hammett parameter of the substituent and measured carbon nanotube FET response. Nano Letters. 3, 1421-1423 (2003).
- Besteman, K., Lee, J. O., Wiertz, F. G. M., Heering, H. A., Dekker, C. Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Letters. 3, 727-730 (2003).
- Snow, E. S., Perkins, F. K., Houser, E. J., Badescu, S. C., Reinecke, T. L. Chemical detection with a single-walled carbon nanotube capacitor. Science. 307, 1942-1945 (2005).
- Kong, J., Franklin, N. R., et al. Nanotube molecular wires as chemical sensors. Science. 287, 622-625 (2000).
- Star, A., Tu, E., et al. Label-free detection of DNA hybridization using carbon nanotube network field-effect transistors. Proceedings of the National Academy of Sciences of the United States of America. 103, 921-926 (2006).
- Patolsky, F., Zheng, G. F., Lieber, C. M. Nanowire-based biosensors. Analytical Chemistry. 78, 4260-4269 (2006).
- Stern, E., Klemic, J. F., et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature. 445, 519-522 (2007).
- Krivitsky, V., Hsiung, L. -. C., et al. Nanowires Forest-Based On-Chip Biomolecular Filtering, Separation and Preconcentration Devices: Nanowires Do it All. Nano Letters. 12, 4748-4756 (2012).
- Kong, J., Soh, H. T., Cassell, A. M., Quate, C. F., Dai, H. J. Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers. Nature. 395, 878-881 (1998).
- Liu, G., Velasco, J., Bao, W. Z., Lau, C. N. Fabrication of graphene p-n-p junctions with contactless top gates. Applied Physics Letters. 92, (2008).
- Katz, E., Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-Sensors, and enzyme biosensors. Electroanalysis. 15, 913-947 (2003).
- K’Owino, I. O., Sadik, O. A. Impedance spectroscopy: A powerful tool for rapid biomolecular screening and cell culture monitoring. Electroanalysis. 17, 2101-2113 (2005).
- Elnathan, R., Kwiat, M., et al. Biorecognition Layer Engineering: Overcoming Screening Limitations of Nanowire-Based FET Devices. Nano Letters. 12, 5245-5254 (2012).

