Microwave-assisted Intramolecular Dehydrogenative Diels-Alder Reactions for the Synthesis of Functionalized Naphthalenes/Solvatochromic Dyes
Summary
Microwave-assisted intramolecular dehydrogenative Diels-Alder (DA) reactions provide concise access to functionalized cyclopenta[b]naphthalene building blocks. The utility of this methodology is demonstrated by one-step conversion of the dehydrogenative DA cycloadducts into novel solvatochromic fluorescent dyes via Buchwald-Hartwig palladium-catalyzed cross-coupling reactions.
Abstract
Functionalized naphthalenes have applications in a variety of research fields ranging from the synthesis of natural or biologically active molecules to the preparation of new organic dyes. Although numerous strategies have been reported to access naphthalene scaffolds, many procedures still present limitations in terms of incorporating functionality, which in turn narrows the range of available substrates. The development of versatile methods for direct access to substituted naphthalenes is therefore highly desirable.
The Diels-Alder (DA) cycloaddition reaction is a powerful and attractive method for the formation of saturated and unsaturated ring systems from readily available starting materials. A new microwave-assisted intramolecular dehydrogenative DA reaction of styrenyl derivatives described herein generates a variety of functionalized cyclopenta[b]naphthalenes that could not be prepared using existing synthetic methods. When compared to conventional heating, microwave irradiation accelerates reaction rates, enhances yields, and limits the formation of undesired byproducts.
The utility of this protocol is further demonstrated by the conversion of a DA cycloadduct into a novel solvatochromic fluorescent dye via a Buchwald-Hartwig palladium-catalyzed cross-coupling reaction. Fluorescence spectroscopy, as an informative and sensitive analytical technique, plays a key role in research fields including environmental science, medicine, pharmacology, and cellular biology. Access to a variety of new organic fluorophores provided by the microwave-assisted dehydrogenative DA reaction allows for further advancement in these fields.
Introduction
Small molecule design and synthesis is critical to the development of a range of scientific fields that includes pharmaceuticals, pesticides, organic dyes, and many more 1. The Diels-Alder (DA) and dehydro-Diels-Alder (DDA) reactions are especially powerful tools in the synthesis of small cyclic and aromatic compounds 2-4. Additionally, thermal dehydrogenative DA reactions of styrene dienes with alkyne dienophiles provide a potentially beneficial route to the synthesis of aromatic compounds by initially forming cycloadducts that can further aromatize under oxidative conditions 5. By employing a thermal intramolecular dehydrogenative DA reaction of styrene dienes with alkynes, the problems typically associated with utilizing styrene as a diene, such as undesired [2 + 2] cycloaddition 5,6 and polymerization reactions 7 and poor regioselectivity, are alleviated and naphthalene compounds can be generated.
The thermal intramolecular dehydrogenative DA reaction of styrenes with alkynes is not without considerable issues. First, most reactions suffer from low yields, long reaction times, and high reaction temperatures 8-11. Additionally, many reactions do not promote exclusive formation of the naphthalene product; both naphthalene and dihydronaphthalene are produced, often as inseparable mixtures by column chromatography 11,12. The tethers of the precursor styrene-ynes are also restricted to include heteroatoms and/or carbonyl moieties. Only one example is reported for an all carbon-containing tether, requiring conditions of 250 °C neat for 48 hr in order to obtain naphthalene formation 10.
In addition to limited variety within the tethers of the starting materials, one of the most severe constraints of this methodology is the lack of functionality tolerated under the conventional thermal conditions. The alkyne terminus of the starting material is either unsubstituted or appended with a phenyl or trimethylsilyl (TMS) moiety 8-13. In one instance, an ester at the alkyne terminus is shown to undergo the dehydrogenative DA reaction, but this results in a mixture of naphthalene and dihydronaphthalene products 11. A later proposal suggests that a TMS group appended to the alkyne terminus is necessary to achieve exclusive naphthalene formation in high yields 10. The deficiency of diverse functionality reported for thermal dehydrogenative DA reactions severely limits the potential of this reaction toward the assembly of unique naphthalene structures.
The desire for variation in naphthalene structures stems from their function as small molecule building blocks in several scientific fields, especially organic fluorescent dyes 14,15. The excellent spatial resolution and response-times of small organic dyes for monitoring real-time events 16 has led to the development of hundreds of commercially available fluorescent compounds. Many of these dyes are naphthalenes with discrete photophysical and chemical properties 15. Choosing fluorescent dyes with specific properties to monitor individual functions is challenging, which leads to an increasing need for new classes of fluorophores with more diverse photophysical properties. To this end, a thermal intramolecular dehydrogenative DA reaction of styrenes with alkynes that allows for diversification of a unique naphthalene scaffold would be potentially beneficial with application to developing new naphthalene-containing fluorescent dyes.
As an alternative to conventional heating, microwave-assisted chemistry is advantageous because it offers more uniform heating of the chemical sample, which leads to higher chemical yields, faster reaction rates, milder reaction conditions, and often different selectivity of products 17. Employing microwave-assisted versus conventional heating conditions for the intramolecular dehydrogenative DA reaction of styrenes serves to eliminate many of the problems associated with this methodology by reducing reaction time from days to minutes, increasing previously poor yields, lowering reaction temperatures, and offering more selective formation of the desired naphthalene product. Microwave-assisted reaction conditions may also be more likely to facilitate incorporation of a greater variety of functionality into the naphthalene products that was previously unattainable. Only one prior example has been reported utilizing microwave-assisted conditions for the dehydrogenative DA reaction in which a 90% yield of both naphthalene and dihydronaphthalene was obtained in as little as 15 min at 170 °C 12.
Herein is reported a microwave-assisted intramolecular dehydrogenative DA reaction of styrenyl derivatives that leads to the exclusive formation of functionalized and diverse naphthalene products in as little as 30 min and in high to quantitative yields 18. The utility of this protocol is further demonstrated by the one-step conversion of a naphthalene product into a novel solvatochromic fluorescent dye with photophysical properties which rival that of the popular commercially available dye Prodan 19.
Protocol
1. Microwave-assisted Dehydrogenative DA Reaction
- Add the para-chloro-styrene derivative (0.045 g, 0.18 mmol) and 1,2-dichloroethane (3 ml) to a 2-5 ml microwave irradiation vial equipped with a stir bar to create a 0.060 M solution. This concentration is used because higher concentrations lead to the formation of undesired products.
- Cap the microwave irradiation vial and place it in the microwave synthesizer cavity.
- Irradiate the solution at 180 °C for 200 min with stirring and with fixed hold time on. The hold time is how long irradiation will occur at the designated temperature. The reaction mixture will turn golden in color. Longer reaction times are not detrimental to the yield of the reaction.
- Confirm the reaction is complete by thin layer chromatography (TLC) employing 5% ethyl acetate/hexane as the eluent. Visualize the TLC plate with UV light and potassium permanganate stain. The Rf of the reactant and product are 0.2 and 0.25, respectively.
- Transfer the reaction to a scintillation vial using 1 ml of 1,2-dichloroethane to rinse the microwave reaction vial. This results in approximately 3 ml of solution in the scintillation vial.
- Concentrate the contents of the scintillation vial under reduced pressure at 40 °C using a rotary evaporator (10-30 mmHg). Evaporation of the solvent will require 5-10 min, and 45 mg of a crude brown oil will be obtained. The crude oil is stable and can be stored indefinitely without decomposition.
- Purify the crude oil by filtration through a pipette of silica gel with 5% ethyl acetate/hexanes as eluent to acquire 41 mg of naphthalene as a white solid.
- Confirm the identity of the product by 1H nuclear magnetic resonance (NMR) spectroscopy using deuterated chloroform (CDCl3) as solvent. For a 300 MHz NMR spectrometer, the 1H NMR spectrum of the naphthalene is as follows: 7.80 (d, J = 1.8 Hz, 1H), 7.72 (d, J = 9.0 Hz, 1H), 7.70 (s, 1H), 7.38 (dd, J = 1.8, 9.0 Hz, 1H), 3.07 (t, J = 7.1 Hz, 4H), 2.66 (s, 3H), 2.18 (p, J = 7.1 Hz, 2H) ppm.
2. Buchwald-Hartwig Palladium-catalyzed Cross-coupling Reaction
- Add RuPhos palladacycle (3 mg, 0.004 mmol) to an oven-dried 0.5-2 ml Biotage microwave irradiation vial equipped with a stir bar and cap the vial.
- Evacuate and refill the vial with nitrogen three times by piercing the septum of the cap with a small gauge needle. Once purging of the vial is complete, remove the needle. The microwave irradiation vial will act as a sealed tube during the reaction, and the best results are obtained when minimal air is present in the reaction vessel.
- Through the septum, add lithium bis(trimethylsilyl)amide (0.32 ml of a 1.0 M solution in THF, 0.32 mmol) via syringe with stirring. The solution will turn red.
- After stirring for 2-10 min, add naphthalene (0.038 g, 0.16 mmol) in 0.3 ml anhydrous tetrahydrofuran (THF) via syringe. Additional THF (up to 0.2 ml) can be used to fully dissolve the naphthalene.
- After 2-10 min of stirring, add dimethylamine (0.12 ml of a 2.0 M solution in THF, 0.24 mmol) via syringe and lower the reaction vessel into a preheated 85 °C oil bath.
- Heat the reaction mixture for 3 hr at 85 °C, or until the reaction is complete by TLC. The reaction mixture will be dark brown in color. For TLC, utilize 20% ethyl acetate/hexanes as the eluent, and visualize the resulting plate with UV light and potassium permanganate stain. The Rf of the reactant and product are 0.5 and 0.4, respectively.
- Cool the reaction to room temperature, remove the vial cap, and quench the reaction with saturated aqueous ammonium chloride solution (10 ml).
- Using a 60 ml separatory funnel, separate the aqueous layer from the organic layer. Extract the aqueous layer three times with ethyl acetate (12 ml).
- Combine the organic layers in the separatory funnel and wash once with brine (15 ml).
- Dry the combined organic layers over sodium sulfate for 10 min, and then remove the sodium sulfate by gravity filtration.
- Using a rotary evaporator, concentrate the resulting solution under reduced pressure at 30 °C (10-30 mmHg). Evaporation of the solvent will require 5-10 min, and a crude brown oil will be obtained.
- Purify the crude product by silica gel column chromatography with a 1.5 cm chromatography column and 5% ethyl acetate/hexanes as eluent. The dye will be obtained as 27 mg of a yellow solid.
- Confirm the identity of the product by 1H NMR spectroscopy using CDCl3 as solvent. For a 400 MHz NMR spectrometer, the 1H NMR spectrum for the dye is as follow: 7.64 (d, J = 9.0 Hz, 1H), 7.56 (s, 1H), 7.11 (dd, J = 2.5, 9.0 Hz, 1H), 6.87 (d, J =2.5 Hz, 1H), 3.02 (s, 6H), 3.02 – 2.87 (m, 4H), 2.65 (s, 3H), 2.12 (p, J = 7.3 Hz, 2H) ppm.
3. Preparation of Dye Solution for Photophysical Studies
- Transfer 1 mg of the dye into a clean, dry 10 ml volumetric flask and dilute to volume with dichloromethane (DCM) to obtain a 0.4 x 10-3 M stock solution of the dye.
- Transfer 253 μl of the stock solution to a second 10 ml volumetric flask and dilute to volume with DCM to prepare a 1 x 10-5 M solution of the dye. This solution will be used to collect both the UV-Vis and the fluorescence data for the dye.
4. UV-Visible Absorption Spectroscopy
- Fill two quartz spectrophotometer cells with DCM. These are the blank samples. Place them into the UV-Vis spectrophotometer cavity. Never touch the optical surfaces of the cell. Handle the cells at the top of the side plates that do not face the optical axis.
- Set the instrumental parameters to a slit width of 2 and an acquisition rate of 480 nm/min. Choose a name for the sample and select an acquisition range from 600 to 200 nm.
- Collect the background spectrum, remove the sample cell from the instrument, empty it, and rinse with several portions of the 1 x 10-5 M dye solution before filling. Avoid overfilling the cell. Before inserting the sample cell back into the holder, carefully wipe the cell windows with a clean lens tissue.
- Collect the absorption spectrum of the sample. The absorption maximum is observed at 377 nm.
- Carefully clean the quartz spectrophotometer cells with water, acetone, and ethanol before running UV-Vis absorption analyses on other samples.
- Use Excel or Origin software to plot and analyze the collected data.
5. Fluorescence Emission Spectroscopy
- Fill a quartz fluorometer cell with the 1 x 10-5 M dye solution and place it into the spectrofluorometer. Avoid skin contact with the optical surfaces of the cell.
- Set the instrumental parameters: excitation wavelength at 334 nm, slit width of 2, acquisition rate of 0.1 nm/sec, acquisition range from 390 to 750 nm. A 390 nm cut-on filter is needed to remove scattered light from the emission source.
- Collect the fluorescence emission spectrum of the sample. The fluorescence emission maximum is observed at 510 nm.
- Clean the quartz fluorometer cell with water, acetone, and ethanol before running fluorescence analyses on other samples.
- Use Excel or Origin software to plot and analyze the collected data.
Representative Results
Microwave irradiation (MWI) of styrenyl derivatives at 180 °C results in complete cyclopenta[b]naphthalene formation in as little as 30 min and in high to quantitative yields (Figure 1) 18. No dihydronaphthalene byproduct is observed, and by 1H NMR spectroscopy the products appear pure without the need for additional purification after irradiation (Figure 2). Various changes to the naphthalene framework are well tolerated utilizing these thermal conditions, including variations to the tether and the substitution pattern of the naphthalene ring, incorporation of an array of electron-withdrawing group, and also altering the location of the electron-withdrawing group to create fused cyclopentanone products.
The synthesis of fluorophores follows a two-step protocol of a microwave-assisted dehydrogenative DA reaction followed by a Buchwald-Hartwig palladium-catalyzed cross-coupling reaction. A representative example of fluorophore synthesis is portrayed in Figure 3. A para-chloro-substituted styrene is cyclized under the aforementioned conditions, and then subjected to palladium-catalyzed cross-coupling conditions with RuPhos palladacycle, LHMDS, and dimethylamine to produce the desired fluorescent dye.
The photophysical properties of the dye are studied in various solvents of differing polarity 19. For both UV-Vis absorption spectroscopy and fluorescence emission measurements, 1 x 10-5 M solutions of the fluorescent compound in 10 mm quartz cells are analyzed employing an excitation wavelength of 334 nm for fluorescence analyses and a 2 nm slit width. Either Excel or Origin software can be used to normalize and plot the collected data, as well as to calculate the absorption and emission maxima of the samples 20. As shown in Figures 4 and 5, the fluorescent dye exhibits remarkable solvatochromic behavior, with a bathochromic shift from cyclohexane to ethanol of 112 nm. These results are also improved from those reported for a commonly used, commercially available solvatochromic dye Prodan.
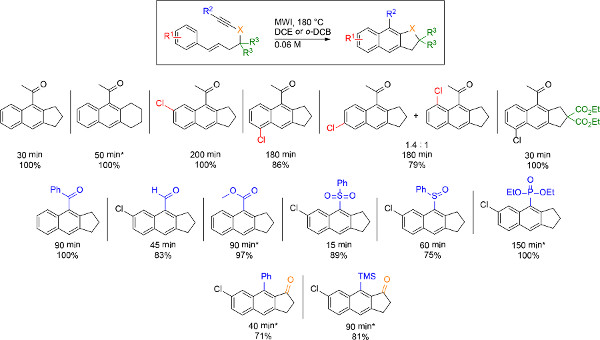
Figure 1. Scope of the microwave-assisted dehydrogenative DA reaction. MWI of solutions of styrenyl precursors in 1,2-dichloroethane or o-dichlorobenzene at 180 °C afforded naphthalene compounds with variations in tether and naphthalene substitution pattern (top row), electron-withdrawing substituent (second row), and location of the electron-withdrawing group (third row). Reaction times and yield are located beneath each structure, and asterisks denote reactions that were performed at higher temperature (225 °C or greater) to reduce reaction time 18. Click here to view larger figure.
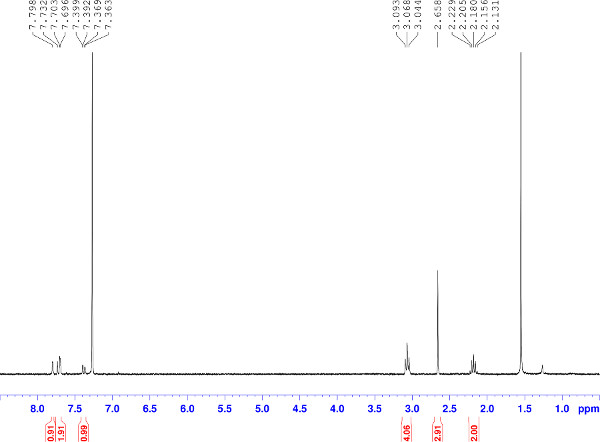
Figure 2. 1H NMR spectrum of naphthalene product. A 1H NMR spectrum of the naphthalene product in CDCl3 shows that the crude product requires no further purification and that there is no contamination with dihydronaphthalene byproduct. Click here to view larger figure.
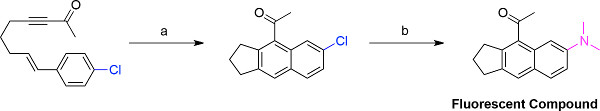
Figure 3. Synthetic strategy to generate solvatochromic fluorescent dyes. The following reaction conditions were employed to produce the representative fluorescent compound: a) MWI, 180 °C, DCE (0.060 M), 200 min, 100% yield; b) Dimethylamine (1.5 equiv), LHMDS (2 equiv), RuPhos palladacycle (2.5 mol %), THF, N2, 3 hr, 85 °C, 70% yield 18.

Figure 4. Solvatochromism of the representative dye. From left to right: fluorescent dye solubilized in toluene, 1,4-dioxane, DCM, and dimethyl sulfoxide (DMSO), and observed under longwave UV light 19.
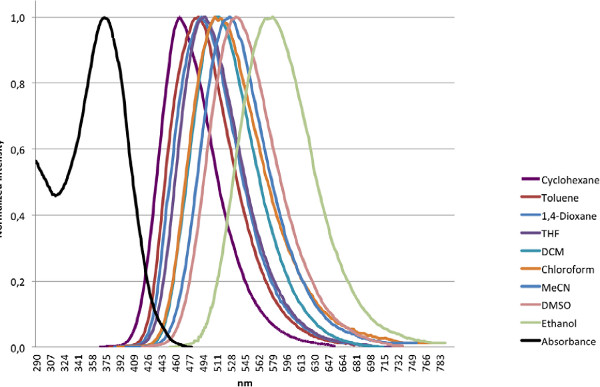
Figure 5. Photophysical properties of the representative fluorescent dye. Normalized absorption (dash) and fluorescence (solid) spectra of the new fluorescent dye in cyclohexane, toluene, 1,4-dioxane, THF, DCM, chloroform, acetonitrile (MeCN), DMSO, and ethanol. The absorption spectrum was recorded in DCM, and fluorescence data was collected by analyzing 1 x 10-5 M solutions of the fluorescent dyes in 10 mm quartz cells. The excitation wavelength for fluorescence analysis was 334 nm 19. Click here to view larger figure.
Discussion
Microwave-Assisted Dehydrogenative DA Reaction
The intramolecular dehydrogenative DA reaction of styrenyl precursors by microwave irradiation (MWI) produces diverse naphthalene structures in high yields of 71-100% and short reaction times, requiring as little as 30 min (Figure 1) 18. The most difficult aspect of performing the dehydrogenative DA reaction is solvent selection, which is often complicated because a variety of solvent characteristics need to be considered to ensure optimal heating. First and foremost, a successful reaction must be possible in a solvent that is compatible with microwave conditions. Factors such as boiling point, microwave absorption, polarity, and quantity of solvent in the microwave vial all affect heating and reaction outcome. Both 1,2-dichloroethane (DCE) and o-dichlorobenzene (DCB) are suitable MWI solvents for the dehydrogenative DA reaction, but occasionally DCE has difficulty reaching 180 °C by MWI. This problem is solved by adding more solvent, recapping the MWI vial, or performing the reaction in DCB, which is a better microwave absorber than DCE and has a higher boiling point. Reaction progress is monitored by TLC, especially if the scale of the reaction is increased as this may lengthen the time of the reaction. While the majority of substrates reported do undergo cyclization at 180 °C, heating at 225 °C in DCB serves to significantly reduce reaction time for examples involving reaction times greater than 200 min at 180 °C. Only one example concerning a naphthalene with a fused cyclohexane ring requires a temperature of 300 °C to complete the reaction, establishing this as the first reported example of a cyclohexane-fused naphthalene scaffold generated via a dehydrogenative DA reaction. These results vary greatly from previous works where naphthalenes were produced under conventional heating conditions, but required extended heating and lower yields of the naphthalene products were obtained 8,9. Similarly, when the cyclization depicted in Figure 3 is conducted in a 180 °C oil bath, the reaction requires 2 days to complete in a 61% yield. This is a drastic difference from the 200 min and quantitative yield observed utilizing MWI conditions 18.
In addition to expediting the reaction and increasing yield, the microwave-assisted dehydrogenative DA reaction incorporates a vast amount of functionality not previously tolerated under conventional heating conditions. Chlorine substitution at various positions of the styrene allows for formation of unique scaffolds of naphthalene products (Figure 1). Additionally, most conventional heating examples only include substitution of non-electron-withdrawing moieties at the terminus of the alkyne of the starting material 8-13. Only examples including substitution of a TMS group at the alkyne resulted in exclusive naphthalene formation in high yields 10,13. Figure 1 shows an array of electron-withdrawing functionality that can be incorporated by utilizing the microwave-assisted dehydrogenative DA reaction, which includes ketones, aldehydes, esters, sulfones, sulfoxides, and phosphonates. While the reaction occurs readily with electron-withdrawing substitution at the alkyne terminus, unsubstituted alkyne or TMS substituted alkyne precursors fail to undergo cyclization.
Finally, the microwave-assisted dehydrogenative DA reaction not only increases the scope of achievable cyclopenta[b]naphthalene compounds (Figure 1), but produces these naphthalenes without contamination with undesired dihydronaphthalene. Alterations to the styrene-yne tether by introducing a propargyl ketone or a diester moiety are also possible to afford different frameworks of naphthalene without byproduct formation. Previous examples of the dehydrogenative DA reaction of styrenes are limited to starting materials that include heteroatoms and/or carbonyls in the styrene-yne tether 8-13. Only one example reported a cyclopenta[b]naphthalene formed from a styrene-yne containing an all carbon tether 10. While styrene-ynes containing all carbon tethers give exclusive formation of the naphthalene via the microwave-assisted reaction, one limitation to this methodology is that incorporation of heteroatoms, such as nitrogen or oxygen atoms, into the tether produce mixtures of products.
Buchwald-Hartwig Palladium-catalyzed Cross-coupling Reaction
The Buchwald-Hartwig palladium-catalyzed cross-coupling reaction is a one-step transformation of the naphthalenes produced by the microwave-assisted dehydrogenative DA reaction into novel fluorescent dyes. While purification of the naphthalenes generated from the DA reaction is not necessary for a successful palladium-catalyzed cross-coupling reaction, it does increase the yield of the cross-coupling. Simple filtration through a plug of silica gel is substantial to purify the DA adduct, and only results in a 5-10% reduction in yield for the microwave-assisted reaction. Coupling of this naphthalene with an amine utilizing RuPhos palladacycle results in a 70% yield of the cross-coupling product (Figure 3) 19. The best results for the cross-coupling are obtained when fresh reagents, such as LHMDS and dimethylamine are employed, and when care is taken to maintain an inert reaction environment.
UV-Vis Absorption and Fluorescence Emission Spectroscopy
The photophysical properties of the dye were studied using 1 x 10-5 M solutions for both UV-Vis absorption and fluorescence emission spectroscopy. In doing so, absorbance values between 0.01 and 0.1 were obtained at a wavelength of 334 nm, as well as good signal to noise ratios. Concentration of samples should be chosen to give absorbance values in the range of 0.01 to 0.1.
The intensity of the sample’s fluorescence is diminished by a wide variety of factors including the quality of the solvents employed and the presence of oxygen in the sample being analyzed. To overcome this issue, spectroscopic grade solvents can be used to prepare the dye solutions. In addition, the best results are usually obtained by degassing the sample with an inert gas, such as nitrogen or argon, prior to collecting spectroscopic data.
The photophysical properties of the new dye can be compared with commercially available fluorescent dye Prodan to show enhanced solvatochromism of the dye over Prodan. For instance, the bathochromic shift of the fluorescence emission maxima from toluene to ethanol is 112 nm for the dye versus only 69 nm for Prodan. Moreover, the new fluorophore exhibits a Stokes shift of 133 nm and fluorescence emission maximum of 510 nm in dichloromethane, a dramatic increase from the 85 nm Stokes shift and 440 nm emission maximum of Prodan 19. Red-shifted emissions are particularly important for biological applications where the natural fluorescence of biomolecules can limit the detection of fluorophores emitting and absorbing at shorter wavelengths. These results confirm the applicability of this protocol to the synthesis of valuable fluorescent dyes.
Applications and Conclusions
Implementing the microwave-assisted dehydrogenative DA reaction in the synthesis of novel solvatochromic fluorescent dyes is only one application of this versatile methodology. In addition to investigating the solvatochromism of the synthetic dyes, this reaction can be utilized to synthesize a variety of fluorescent compounds with interesting photophysical properties by allowing for unique functionalization of napthalene structures. Also, the quick and facile synthesis of naphthalenes via this microwave-assisted methodology would provide an expedient route to the synthesis of highly functionalized naphthalene-containing natural products.
In conclusion, the methodology described herein provides concise access to a variety of functionalized naphthalenes, as well as to a new solvatochromic dye. The aforementioned advantages and versatility of the microwave-assisted dehydrogenative DA reaction allow for further applications to the expanding field of organic fluorescent dyes, as well as potentially to the synthesis of natural and biological molecules.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the National Science Foundation (CHE0910597) and the National Institutes of Health (P50-GM067982) for supporting this work. We are grateful to professor Michael Trakselis (University of Pittsburgh) for helpful discussions regarding fluorescence measurements. We acknowledge Kristy Gogick and Robin Sloan (University of Pittsburgh) for their assistance in collecting fluorescence data.
Materials
| Reagent/Material | |||
| 1,2-Dichloroethane, ACS reagent ≥99.0% | Sigma-Aldrich | 319929 | |
| SiliaPlate G TLC – glass-backed, 250 μm | Silicycle | TLG-R10011B-323 | |
| Ethyl acetate, certified ACS ≥99.5% | Fisher Scientific | E14520 | |
| Hexanes, certified ACS ≥98.5% | Fisher Scientific | H29220 | |
| Silica gel, standard grade | Sorbent Technologies | 30930M | 60 A, 40-63 μM (230 x 400 mesh) |
| RuPhos palladacycle | Strem | 46-0266 | |
| Nitrogen gas | Matheson TRIGAS | NI304 | Nitrogen 304cf, industrial |
| Lithium bis(trimethylsilyl) amide solution | Sigma-Aldrich | 225770 | 1.0 M solution in THF |
| Tetrahydrofuran anhydrous ≥99.9% | Sigma-Aldrich | 401757 | Inhibitor-free |
| Dimethylamine solution | Sigma-Aldrich | 391956 | 2.0 M solution in THF |
| Ammonium chloride | Fisher Scientific | A661-500 | |
| Sodium sulfate, anhydrous (granular) | Fisher Scientific | S421-500 | |
| Chromatography column | Chemglass | CG-1188-04 | ½ in ID x 18in E.L. |
| Cyclohexane, ≥99.0% | Fisher Scientific | C556-1 | |
| Toluene anhydrous, 99.8% | Sigma-Aldrich | 24451 | |
| 1,4-Dioxane anhydrous, 99.8% | Sigma-Aldrich | 296309 | |
| Tetrahydrofuran anhydrous, ≥99.9% | Sigma-Aldrich | 186562 | 250 ppm BHT as inhibitor |
| Dichloromethane | Sigma-Aldrich | 650463 | Chromasolv Plus |
| Chloroform, ≥99.8% | Fisher Scientific | C298-1 | |
| Acetonitrile anhydrous, 99.8% | Sigma-Aldrich | 271004 | |
| Dimethyl sulfoxide, ≥99.9% | Fisher Scientific | D128 | |
| Ethyl alcohol | Pharmco-AAPER | 11ACS200 | Absolute |
| Equipment | |||
| Microwave Synthesizer | Biotage | Biotage Initiator Exp | |
| Microwave Vial | Biotage | 352016 | 0.5 – 2 ml |
| Microwave Vial | Biotage | 351521 | 2 – 5 ml |
| Microwave Vial Cap | Biotage | 352298 | |
| Microwave Synthesizer | Anton Paar | Monowave 300 | |
| Microwave Vial G4 | Anton Paar | 99135 | |
| Microwave Vial Cap | Anton Paar | 88882 | |
| NMR Spectrometer | Bruker | Avance | 300 or 400 MHz |
| UV-Visible Spectrometer | PerkinElmer | Lamda 9 | |
| Spectrophotometer cell | Starna Cells | 29B-Q-10 | Spectrosil quartz, path length 10 mm, semi-micro, black wall |
| Spectrofluorometer | HORIBA Jobin Yvon | FluoroMax-3 S4 | |
| Fluorometer cell | Starna Cells | 29F-Q-10 | Spectrosil quartz, path length 10 mm, semi-micro |
References
- Wender, P. A., Miller, B. L. Synthesis at the molecular frontier. Nature. 460, 197-201 (2009).
- Takao, K. -. i., Munakata, R., Tadano, K. -. i. Recent Advances in Natural Product Synthesis by Using Intramolecular Diels-Alder Reactions. Chem. Rev. 105 (12), 4779-4807 (2005).
- Winkler, J. D. Tandem Diels-Alder Cycloadditions in Organic Synthesis. Chem. Rev. 96 (1), 167-176 (1996).
- Wessig, P., Müller, G. The Dehydro-Diels-Alder Reaction. Chem. Rev. 108 (6), 2051-2063 (2008).
- Wagner-Jauregg, T. Thermische und photochemische Additionen von Dienophilen an Arene sowie deren Vinyloge und Hetero-Analoge; II. Synthesis. (10), 769-798 (1980).
- Ohno, H., et al. A Highly Regio- and Stereoselective Formation of Bicyclo[4.2.0]oct-5-ene Derivatives through Thermal Intramolecular [2 + 2] Cycloaddition of Allenes. J. Org. Chem. 72 (12), 4378-4389 (2007).
- Stille, J. K., Chung, D. C. Reaction of Vinylidene Cyanide with Styrene. Structure of the Cycloadduct and Copolymer. Macromolecules. 8 (1), 83-85 (1975).
- Klemm, L. H., Klemm, R. A., Santhanam, P. S., White, D. V. Intramolecular Diels-Alder reactions. VI. Synthesis of 3-hydroxymethyl-2-naphthoic acid lactones. J. Org. Chem. 36 (15), 2169-2172 (1971).
- Klemm, L. H., McGuire, T. M., Gopinath, K. W. Intramolecular Diels-Alder reactions. 10. Synthesis and cyclizations of some N-(cinnamyl and phenylpropargyl)cinnamamides and phenylpropiolamides. J. Org. Chem. 41 (15), 2571-2579 (1976).
- Ozawa, T., Kurahashi, T., Matsubara, S. Dehydrogenative Diels-Alder Reaction. Org. Lett. 13 (19), 5390-5393 (2011).
- Chackalamannil, S., et al. A facile Diels-Alder route to dihydronaphthofuranones. Tetrahedron Lett. 41 (21), 4043-4047 (2000).
- Ruijter, E., et al. Synthesis of Polycyclic Alkaloid-Type Compounds by an N-Acyliminium -Pictet-Spengler/Diels-Alder Sequence. Synlett. 2010, 2485-2489 (2010).
- Toyota, M., Terashima, S. A novel synthesis of the basic carbon framework of fredericamycin A. Promising routes for the spiro chiral center construction of the CD-ring system. Tetrahedron Lett. 30 (7), 829-832 (1989).
- de Koning, C. B., Rousseau, A. L., van Otterlo, W. A. L. Modern methods for the synthesis of substituted naphthalenes. Tetrahedron. 59 (1), 7-36 (2003).
- Johnson, I., Spence, M. T. Z. . The Molecular Probes Handbook, A Guide to Fluorescent Probes and Labeling Technologies. , 1051 (2010).
- Fernández-Suárez, M., Ting, A. Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell. Biol. 9 (12), 929-943 (2008).
- Kappe, O. C., Dallinger, D., Murphree, S. . Practical Microwave Synthesis for Organic Chemists. , (2009).
- Kocsis, L. S., Benedetti, E., Brummond, K. M. A Thermal Dehydrogenative Diels-Alder Reaction of Styrenes for the Concise Synthesis of Functionalized Naphthalenes. Org. Lett. 14 (17), 4430-4433 (2012).
- Benedetti, E., Kocsis, L. S., Brummond, K. M. Synthesis and Photophysical Properties of a Series of Cyclopenta[b]naphthalene Solvatochromic Fluorophores. J. Am. Chem. Soc. 134 (30), 12418-12421 (2012).
- OriginLab Corporation. . Origin 8 User Guide. , (2007).

