Micron-scale Resolution Optical Tomography of Entire Mouse Brains with Confocal Light Sheet Microscopy
Summary
In this article we describe the full experimental procedure to reconstruct, with high resolution, the fine brain anatomy of fluorescently labeled mouse brains. The described protocol includes sample preparation and clearing, specimen mounting for imaging, data post-processing and multi-scale visualization.
Abstract
Understanding the architecture of mammalian brain at single-cell resolution is one of the key issues of neuroscience. However, mapping neuronal soma and projections throughout the whole brain is still challenging for imaging and data management technologies. Indeed, macroscopic volumes need to be reconstructed with high resolution and contrast in a reasonable time, producing datasets in the TeraByte range. We recently demonstrated an optical method (confocal light sheet microscopy, CLSM) capable of obtaining micron-scale reconstruction of entire mouse brains labeled with enhanced green fluorescent protein (EGFP). Combining light sheet illumination and confocal detection, CLSM allows deep imaging inside macroscopic cleared specimens with high contrast and speed. Here we describe the complete experimental pipeline to obtain comprehensive and human-readable images of entire mouse brains labeled with fluorescent proteins. The clearing and the mounting procedures are described, together with the steps to perform an optical tomography on its whole volume by acquiring many parallel adjacent stacks. We showed the usage of open-source custom-made software tools enabling stitching of the multiple stacks and multi-resolution data navigation. Finally, we illustrated some example of brain maps: the cerebellum from an L7-GFP transgenic mouse, in which all Purkinje cells are selectively labeled, and the whole brain from a thy1-GFP-M mouse, characterized by a random sparse neuronal labeling.
Introduction
When compared to our understanding of the molecular and cellular mechanisms underlying brain function, our knowledge of fine neuroanatomy looks still in its infancy1. In fact, we still lack comprehensive maps of the position of neuronal somata or of long-range projections throughout the brain. Actually, many technological efforts are devoted to reconstruct the mouse brain with microscopic resolution. Optical methods based on serial sectioning of resin-embedded samples, as Knife-Edge Scanning Microscopy (KESM)2, Micro-Optical Sectioning Tomography (MOST)3 and fluorescence-MOST (fMOST)4, can provide sub-micron three-dimensional resolution imaging of entire mouse brains. However, the total time needed for the preparation and imaging of a single sample is of the order of several weeks, limiting the practical application of such methods. Another technique based on serial sectioning (but without resin embedding), Serial Two-Photon tomography (STP)5, allows high three-dimensional resolution imaging. However, this technique has been demonstrated in whole mouse brains only with a very sparse sampling, collecting 1 section every 50 μm 5, and to our knowledge STP have not produced yet a full-sampling reconstruction of a murine brain.
A cutting-edge optical method to reconstruct whole specimens in three dimensions at high speed is light sheet microscopy6. In this optical scheme the sample is illuminated with a thin sheet of light and the fluorescence emission is collected along an axis perpendicular to the illumination plane. In this way only in-focus fluorophores are excited and it is possible to achieve optical sectioning in wide field configuration, guaranteeing high frame rates. When coupled to clearing protocols based on the substitution of water with a refractive index matching liquid7, light sheet microscopy has been applied to macroscopic specimens, as whole mouse brains8. However, specimen-induced light scattering, which affects even cleared specimens, degrades the light sheet leading to the excitation of out-of-focus fluorophores which adds an overall blurring to the acquired images.
To selectively collect only in-focus photons, we recently coupled light sheet illumination to a confocal detection scheme9. In confocal light sheet microscopy (CLSM), out-of-focus and scattered photons are rejected by a linear spatial filter (slit) prior to detection (Figure 1). To achieve confocal operation in light sheet architecture, the illumination is produced by means of a scanning line, and a de-scanning system in the detection path creates a static image of the excitation scanning line at the location of the slit. A third scanning system reconstructs a two-dimensional image on the camera sensor. CLSM affords a 100% contrast enhancement in cleared mouse brains with respect to conventional light sheet microscopy, while maintaining a 10 Hz frame rate. With CLSM it is possible to reconstruct entire mouse brains with resolution of 2 μm in XY and 9 μm in Z, and with sufficient contrast to discern single fluorescently labeled neurons.
In this Article we describe the experimental pipeline to reconstruct a mouse brain with CLSM. Aldehyde-fixed mouse brains are dehydrated in a graded series of peroxide-free tetrahydrofuran and subsequently cleared in peroxide-free dibenzyl ether (Figure 2), following the protocol developed by Becker et al.10 The cleared specimen is then carefully mounted on a tipped plate and positioned in the imaging chamber of a custom-made confocal light sheet microscope. The sample can be directly mounted by plunging it on a tip of the plate (Figure 3a); alternatively it can be glued on a disc of cleared agarose gel (Figure 3b) or placed in the cavity of a beaker made of cleared agarose gel (Figure 3c). An optical tomography of the entire brain is then performed, as many parallel adjacent stacks are acquired to cover all the brain volume (Figure 4). A pre-tomography sampling, which produces a rough map of the specimen position and shape, guarantees that imaging is performed only in the volume occupied by the sample. The tomography stacks are subsequently stitched together with custom-made software and saved in a multi-resolution hierarchical scheme11. Mouse brains can eventually be explored with prototypal multi-resolution Google Maps-like visualization software. Both these software instruments are integrated as plugins in the open-source platform Vaa3D12.
We finally show representative images of mouse brains to demonstrate the capabilities of the described experimental pipeline in studying fine murine neuroanatomy.
Protocol
1. Tissue Preparation and Storage
- Fix mouse brain through standard transcardial perfusion13 with 20 ml 0.01 M phosphate buffered saline (PBS) solution, followed by 100 ml of paraformaldehyde 4% in 0.01 M PBS. Carefully adjust the pH of both solutions to 7.6: slightly lower values of the pH may quench the fluorescence of EGFP.
- Post-fix the excised brains overnight in paraformaldehyde 4% at 4 °C.
- Rinse two times (30 min each) in 0.01 M PBS and store in 0.01 M PBS at 4 °C.
2. Dehydration and Clearing
Note: the protocol for dehydration and clearing closely follows the one described by Becker et al.10
- To remove peroxides from the dehydration solvent (tetrahydrofuran, THF), which strongly quench XFP fluorescence, filter THF with basic activated aluminum oxide (activity grade Brockman I, about 250 g/L of THF) using a chromatography column. Avoid cracking of the column, which would reduce peroxide removal.
- Add stabilizer (butylated hydroxytoluene, 250 mg/L) to the final solution, even if the THF was already stabilized before filtration. Stabilizer is in fact retained by aluminum oxide: THF without stabilizer may develop large amounts of peroxides and can be explosive and dangerous.
- Dehydrate the whole mouse brain in a graded series of THF in pure water: 50%, 70%, 80%, 90%, 96%, 100%, 1 hr each, then 100% overnight. Place the vial with the sample on a rotating wheel. To avoid light exposure, wrap the vials with an aluminum foil.
- Filter the clearing solvent (dibenzyl ether, DBE) with aluminum oxide (activated basic, activity grade Brockman I, about 250 g/L of DBE) using a filtering funnel.
- Clear the sample in 100% DBE. Change the solution after 3 and 6 hr.
3. Specimen Mounting
- When the brain looks transparent by eye (typically 1 hr after the second DBE change), mount it on the tipped imaging plate by one of the following methods:
-
- Direct mounting – Gently plunge the sample on one of the tips (Figure 3a).
- Agarose disc – Plunge a disc made of 6% agarose gel on the tips. Gently fix the sample on the agarose disc with acrylic glue (Figure 3b). Wait about 1 min for curing.
- Agarose beaker – Plunge a beaker made of 3% agarose gel on the tips. Gently place the sample on the bottom of the beaker (Figure 3c).
Note 1: Mounting in agarose beaker should be preferred when it is important imaging the sample in its entirety; however, the agarose gel surrounding the specimen gives rise to additional light scattering which could be detrimental to image quality. If a small part of the sample could be excluded from imaging, it is better to mount the specimen on agarose disk or directly on the tip. The former method is best suited to keep the brain horizontal (excluding from imaging a ventral or dorsal portion of the brain), the latter to keep the brain vertical (excluding from imaging a part of olfactory bulbs or of spinal cord).
Note 2: the agarose gel should be prepared in water, then dehydrated with THF (50% and 100% 4 hr each) and cleared in DBE 100% for 4 hr or until it looks clearly transparent.
- After mounting the specimen following one of the previous methods, position the imaging plate inside the specimen chamber using tweezers. Fill the chamber with clearing solution.
4. Tomography Preparation
- Remove the slit to collect as much fluorescence as possible.
- Illuminate the sample with low laser power to prevent photobleaching.
- Move the sample in the three directions using the software-driven micropositioning system. Identify for each direction the minimum and maximum coordinates for which the sample is illuminated and is within the field of view of the camera (Figure 4a).
- Insert the above coordinates as parameters for the 'Pre-tomography' subroutine. Specify also the distance between adjacent stacks to be used in the tomography, and the pre-tomography sampling step in z direction.
- Start the pre-tomography, which collects the images in every stack with the z sampling specified at (4.4).
Note: The collected images are used by a software to prepare a rough map of specimen shape and position (Figure 4b), which allows restricting tomography acquisition only to the volume actually occupied by the sample, reducing imaging time and thus photobleaching.
5. Tomography Acquisition
- Move the sample outside of the light sheet using the micropositioning system.
- Increase laser power and stop the motion of all the scanning systems, in order to illuminate only a fixed line at the center of the field of view.
- Mount the slit and align it using autofluorescence signal from the clearing solution. You should clearly see the slit line at the center of the field of view.
- Re-activate the scanning system, reduce laser power and move the sample inside the light sheet using the micropositioning system. Note that the laser power used with the slit will be higher than that used without it, since the spatial filter blocks a non-negligible fraction of light.
- Adjust the amplitude and offset of the scanning and de-scanning systems with the control software, until the images look clear and bright.
- Run the tomography acquisition software, after setting the appropriate z step for the experiment. The volume will be imaged in many parallel, partially overlapping stacks (Figure 5a), and the images will be saved in a hierarchical folder structure.
6. Stitching and Visualization
- Complete the acquired volume with synthetic black images (i.e. images of the same type and dimensions of the collected ones-, but with zero intensity) using an automated software (Figure 4c). This step is mandatory in order for the stitching software to deal with a complete cubic volume.
- Launch Vaa3D software (freely downloadable from http://www.vaa3d.org/) with the plugins TeraStitcher and TeraManager installed.
- Load the TeraStitcher plugin. Select the directory containing the imaged volume, indicate the relative orientations of the axes (with respect to a reference right-handed coordinate system) and the voxel size.
- Launch the first part of the stitching. The software will calculate the relative displacement between couples of stacks, and find an overall optimal placement for all the stacks together (Figure 5b).
- Select to save the stitched volume in single-resolution or in multi-resolution format. The latter enables for multi-resolution visualization with the TeraManager plugin. In both cases, if the higher resolution image is larger than a few gigabytes, also select the multi-stack save modality and specify the size of individual substacks. This will enable efficient access to the stored data.
- Launch the second part of the stitching. The software will merge the aligned stacks, and save them in either single- or multi-stack mode (Figures 5c and 5d). At the end close the TeraStitcher plugin.
- Load the TeraManager plugin. Select the folder with the multi-stacked multi-resolution volume, and indicate voxel size and axes orientations. The volume will be loaded at the minimum resolution.
- To zoom to a higher resolution, select a landmark using the right-click modality of Vaa3D, then zoom in using mouse scroll. Alternatively you can directly select the ROI to zoom in with a right-click, or specify the coordinates of the volume of interest.
- To zoom back to lower resolution, simply use the mouse scroll.
Representative Results
The described protocol can be used to reconstruct with micron-scale resolution either entire mouse brains or excised parts, without the need for physical sectioning. As a representative result, in Figure 6 the whole cerebellum of an L7-GFP mouse (post natal day 10) is shown. In this animal all Purkinje neurons are labeled with EGFP. If we zoom in, the typical lamellar structure of the cerebellar cortex can be seen (Figure 7). Further zooming in allows clearly distinguishing each Purkinje cell soma (Figure 8). The described protocol can thus be used to screen neuronal spatial organization in various neurodevelopment studies.
As a second representative result, we present images from the unsectioned brain of an adult thy1-GFP-M mouse. In this transgenic animal EGFP is expressed in a random sparse neuronal subset. The right half of the brain is shown at in Figure 9. As we zoom-in further and further (Figures 10 and 11), neuronal processes become distinguishable. This result demonstrates that the described protocol allows micron-scale resolution imaging in entire adult mouse brains, opening the possibility of studying whole-brain anatomy at cellular resolution in mouse models of neurodegenerative disease.

Figure 1. Optical scheme of confocal light sheet microscopy (CLSM). (a) Top view of the apparatus, showing the excitation pathway. Laser emission from a 488 nm diode-pumped solid state (DPSS) laser, after expansion and collimation by a first telescope, enters an acousto-optic tunable filter (AOTF) which regulates beam intensity. Then, a second telescope further expands the beam. A galvo mirror vertically (along y) scans the beam, which is focused by a lens inside the specimen chamber. (b) Lateral view of the apparatus, showing the detection pathway. Light collected by the detection objective is vertically descanned by a galvo mirror and focused by a lens, producing a fixed image of the moving excitation line. At the position of the fixed image a linear spatial filter (slit) is placed. After the slit, a galvo mirror placed inside a 4f lens system reconstructs a 2-dimentional image on the chip of an electron-multiplying charge-coupled device (EM-CCD). A fluorescence bandpass filter remove all excitation stray light before detection. Representative rays of excitation, detection and bandpass-filtered detection light are depicted by blue, light blue and green rays, respectively. The focal lengths of all lenses actually used in the microscope are indicated. Click here to view larger figure.

Figure 2. Specimen clearing. Formaldehyde-fixed mouse brains, which are stored in PBS 0.1 M at 4 °C (a), are dehydrated in a graded series of tetrahydrofuran (THF, b) and subsequently cleared in 100% dibenzyl ether (DBE, c). The dehydration step causes also some tissue shrinkage.
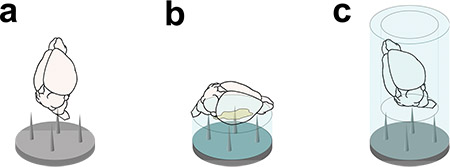
Figure 3. Specimen mounting for CLSM imaging. The cleared brain can be directly plunged on the tipped mounting plate (a), glued on a cleared agarose disc (b), or inserted in a cleared agarose beaker (c).
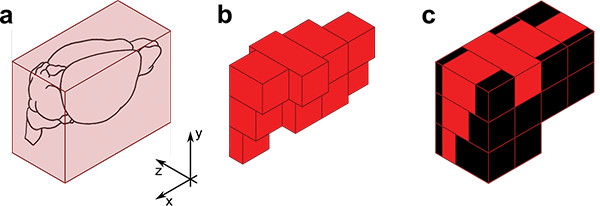
Figure 4. Tomography acquisition. The pre-tomography routine samples a parallelepiped containing the brain (a) to define the number and length of parallel adjacent stacks needed to image all the specimen (b). After tomography, black images are generated to complete the virtual acquired volume to a parallelepiped (c).

Figure 5. Volume stitching. The collected image stacks partially overlap between each other (a), allowing their precise alignment with the TeraStitcher plugin (b). The plugin then merges the aligned stacks in either a single-stacked (c) or multi-stacked (d) multi-resolution representation.

Figure 6. Whole cerebellum of a P10 L7-GFP mouse, in which all the Purkinje neurons are labeled with EGFP. Scale bar, 1 mm.

Figure 7. Zoom-in of a portion of the cerebellum shown in Figure 6, showing the typical lamellar structure of the cerebellar cortex. Scale bar, 500 μm.

Figure 8. Further zoom-in of a portion of the cerebellum shown in Figure 6. Purkinje cells soma can be clearly distinguished. Scale bar, 200 μm.

Figure 9. Right half of the brain from an adult thy1-GFP-M mouse, characterized by a sparse unspecific neuronal EGFP labeling. Scale bar, 1 mm.

Figure 10. Zoom-in of a portion of the half brain shown in Figure 9, showing part of the hippocampus and of cortex. Scale bar, 200 μm.

Figure 11. Further zoom-in of a portion of the half brain shown in Figure 9. Several neurites in the cortex can be clearly distinguished. Scale bar, 50 μm.
Discussion
CLSM coupled with chemical clearing represent a powerful tool to study the neuroanatomy of entire mouse brains labeled with fluorescent probes, either proteins or synthetic dyes. Since the light emitted outside the focal plane is blocked by a physical spatial filter, high-contrast imaging is possible also in thick specimens, while maintaining the high frame rates typical of light sheet architecture.
The main issue to deal with when using the described methods is the maximization of the contrast. In fact, the available signal is reduced both by chemical and optical factors. From the chemical point of view, the clearing procedures based on organic solvent may quench emission from fluorescent proteins and other fluorescent dyes7. Filtration of the solvent to remove peroxides, as described by Becker et al.10, allows maintaining a good level of fluorescence also in solvent-cleared tissues. Published water-based clearing methods14 do not reduce fluorescence efficiency, but result in poorer transparency when dealing with entire murine brains, at least with linear optical techniques.
On the other hand, at the moment there are no commercial objectives with long working distance corrected for the high refractive index (n ≈ 1.56) clearing solutions used. Thus, the refractive index mismatch between the medium in which the sample is immersed and the design one of the objective gives rise to strong spherical aberrations. Besides reducing the resolution of the microscope, such aberrations result in a drop of image intensity and contrast, since the light is spread on a wider area. Possible workarounds to this issue include the use of adaptive optics15 and/or static aspheric correctors.
Any improvement in image contrast and intensity readily affects also imaging speed, since a shorter integration time per image can be chosen. At the moment CLSM can afford an imaging speed of about 106 μm3/sec, with a camera exposure time of 100 msec 9. At this speed it takes from 1-3 days to image an entire mouse brain, depending on sample size. Although no significant degradation in image quality is observed throughout such long imaging session, mostly because of the intrinsic low photobleaching of light sheet illumination6, the long time needed to image a single mouse brain could in practice reduce the applicability of CLSM. A 10-fold increase in system efficiency, coupled with the use of a high-speed camera for detection, would reduce imaging time to several hours, allowing a routine use of the described protocol.
Despite all the abovementioned limitations, CLSM imaging coupled to chemical clearing of tissues permits to reconstruct entire mouse brains with micron-scale resolution in less than a week. Other optical methods for whole brain imaging afford higher resolution than CLSM, but at the price of longer preparation and/or imaging time2-4, or of a sparse z sampling5.
The methods described in this article can be used in combination with different strategies for fluorescent labeling: transgenic animals expressing fluorescent proteins in selected neuronal subsets, viral transfection, intraparenchymal dye injection, etc. In practice, all staining strategies preceding animal sacrifice are compatible with CLSM. In fact, post-mortem fluorescent labeling of entire mouse brains has not been demonstrated up to now; anyway, if such kind of staining would be possible in the near future, the number of specimens that could be reconstructed with CLSM will become much larger, potentially including human brain tissue.
In conclusion, confocal light sheet microscopy used in combination with tissue clearing and multi-resolution data management may allow researchers to reconstruct and analyze macroscopic specimens with resolution in the micron scale, with technological efforts that are well at the hand of most laboratories. Potential applications of CLSM are of course not limited to neuroscience, but spans all the research fields in which light sheet microscopy has already been used, as embryogenesis16,17 and anatomy of small animals18.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreements n. 228334 and 241526. This research project has been also supported by Human Frontier Science Program research grant (RGP0027/2009), and by the Italian Ministry for Education, University and Research in the framework of the Flagship Project Nanomax and by Italian Ministry of Health in the framework of the “Stem Cells Call for proposals”. This research has been carried out in the framework of the research activities of ICON foundation supported by “Ente Cassa di Risparmio di Firenze”.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| Phosphate Buffered Saline Tablets | Sigma-Aldrich | P4417_100TAB | |
| Paraformaldehyde | Agar Scientific | R1018 | |
| Peroxide-free Tetrahydrofuran | Sigma-Aldrich | 186562 | |
| Aluminum oxide activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Dibenzyl ether | Sigma-Aldrich | 108014 | |
| Butylated hydroxytoluene | Sigma-Aldrich | W218405 | |
| Agarose Type III-A, High EEO | Sigma-Aldrich | A9793 | |
| Acrylic glue | Bostik | D2498 | |
| Vaa3D software | open source | freely downloadable from http://www.vaa3d.org/ |
References
- Lichtman, J. W., Denk, W. The big and the small: challenges of imaging the brain’s circuits. Science. 334, 618-623 (2011).
- Mayerich, D., Abbott, L., McCormick, B. Knife-edge scanning microscopy for imaging and reconstruction of three-dimensional anatomical structures of the mouse brain. J. Microsc. 231, 134-143 (2008).
- Li, A., et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science. 330, 1404-1408 (2010).
- Gong, H., et al. Continuously tracing brain-wide long-distance axonal projections in mice at a one-micron voxel resolution. Neuroimage. 74, 87-98 (2013).
- Ragan, T., et al. Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat. Methods. 9, 255-258 (2012).
- Keller, P. J., Dodt, H. U. Light sheet microscopy of living or cleared specimens. Curr. Opin. Neurobiol. 22, 138-143 (2012).
- Erturk, A., et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 7, 1983-1995 (2012).
- Dodt, H. U., et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods. 4, 331-336 (2007).
- Silvestri, L., Bria, A., Sacconi, L., Iannello, G., Pavone, F. S. Confocal light sheet microscopy: micron-scale neuroanatomy of the entire mouse brain. Opt. Express. 20, 20582-20598 (2012).
- Becker, K., Jahrling, N., Saghafi, S., Weiler, R., Dodt, H. U. Chemical clearing and dehydration of GFP expressing mouse brains. PLoS One. 7, e33916 (2012).
- Bria, A., Iannello, G. TeraStitcher – A Tool for Fast Automatic 3D-Stitching of Teravoxel-Sized Microscopy Images. BMC Bioinformatics. 13, 316 (2012).
- Peng, H., Ruan, Z., Long, F., Simpson, J. H., Myers, E. W. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets. Nat. Biotechnol. 28, 348-353 (2010).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. (65), e3564 (2012).
- Hama, H., et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 14, 1481-1488 (2011).
- Booth, M. J., Neil, M. A. A., Wilson, T. Aberration correction for confocal imaging in refractive-index-mismatched media. J. Microsc. 192, 90-98 (1998).
- Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J., Stelzer, E. H. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 305, 1007-1009 (2004).
- Keller, P. J., Schmidt, A. D., Wittbrodt, J., Stelzer, E. H. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 322, 1065-1069 (2008).
- Jahrling, N., Becker, K., Schonbauer, C., Schnorrer, F., Dodt, H. U. Three-dimensional reconstruction and segmentation of intact Drosophila by ultramicroscopy. Front Syst. Neurosci. 4, 1 (2010).

