Fluorescence Microscopy Methods for Determining the Viability of Bacteria in Association with Mammalian Cells
Summary
Central to the field of bacterial pathogenesis is the ability to define if and how microbes survive after exposure to eukaryotic cells. This article outlines protocols for the use of fluorescent dyes that reveal the viability of individual bacteria inside and associated with host cells.
Abstract
Central to the field of bacterial pathogenesis is the ability to define if and how microbes survive after exposure to eukaryotic cells. Current protocols to address these questions include colony count assays, gentamicin protection assays, and electron microscopy. Colony count and gentamicin protection assays only assess the viability of the entire bacterial population and are unable to determine individual bacterial viability. Electron microscopy can be used to determine the viability of individual bacteria and provide information regarding their localization in host cells. However, bacteria often display a range of electron densities, making assessment of viability difficult. This article outlines protocols for the use of fluorescent dyes that reveal the viability of individual bacteria inside and associated with host cells. These assays were developed originally to assess survival of Neisseria gonorrhoeae in primary human neutrophils, but should be applicable to any bacterium-host cell interaction. These protocols combine membrane-permeable fluorescent dyes (SYTO9 and 4',6-diamidino-2-phenylindole [DAPI]), which stain all bacteria, with membrane-impermeable fluorescent dyes (propidium iodide and SYTOX Green), which are only accessible to nonviable bacteria. Prior to eukaryotic cell permeabilization, an antibody or fluorescent reagent is added to identify extracellular bacteria. Thus these assays discriminate the viability of bacteria adherent to and inside eukaryotic cells. A protocol is also provided for using the viability dyes in combination with fluorescent antibodies to eukaryotic cell markers, in order to determine the subcellular localization of individual bacteria. The bacterial viability dyes discussed in this article are a sensitive complement and/or alternative to traditional microbiology techniques to evaluate the viability of individual bacteria and provide information regarding where bacteria survive in host cells.
Introduction
There is a dynamic interaction and co-evolution between bacteria and the hosts in which they reside. Bacteria have evolved adherence organelles, secretion systems, and/or the ability to produce toxins that enable their productive infection of host phagocytic and non-phagocytic cells. The bacteria must also contend with recognition and antimicrobial activities of the host immune system. The host immune system is comprised of innate and adaptive components including physical and chemical barriers, immune cells, the complement system, and other components of humoral immunity. While many bacteria are susceptible to killing and clearance by the multilayered host immune response, some pathogenic and opportunistic bacteria have evolved mechanisms to infect a variety of host cells and subvert clearance by the host immune response 1. Neisseria gonorrhoeae is one example of a bacterial pathogen that is highly adapted to persist in its human host. N. gonorrhoeae readily colonizes the luminal surfaces of mucosal epithelial cells of the urogenital tract, pharynx, conjunctiva, and rectum. Colonization triggers the abundant recruitment of neutrophils at mucosal sites. Neutrophils are professional phagocytes that possess a variety of antimicrobial processes to kill microorganisms; however, N. gonorrhoeae is capable of surviving in the presence of neutrophils 2-5. Understanding how bacterial pathogens such as N. gonorrhoeae subvert, suppress, and hijack the immune response to ultimately survive in normally hostile host environments is crucial to the development of new therapies for combating infectious diseases.
Experimental protocols often used to investigate bacterial survival in host cells include colony count assays, gentamicin protection assays, and electron microscopy. In colony count assays, a population of infected cells is lysed (for instance, with a detergent to which the bacteria are resistant) to liberate the bacteria. The lysates are diluted and plated on agar-based media, and colony-forming units in the lysates are enumerated for each time point and/or experimental condition. This approach reports the viability of the entire bacterial population but is not capable of differentiating between intracellular and extracellular survival. A variation on the colony count assay, the gentamicin protection assay, specifically measures intracellular bacterial survival, based on the inability of the antibiotic gentamicin to cross the eukaryotic plasma membrane 6. However, this assay is dependent on the bacteria being susceptible to killing by gentamicin (or another antibiotic that is similarly eukaryotic membrane-impermeant) and the inability of the antibiotic to have access to internal bacteria. Therefore, a gentamicin protection assay may not be effective for examination of all bacterial species or when examining bacterial survival in highly pinocytic cells such as neutrophils. Neither of these approaches reveals the subcellular localization or other behavior of individual bacteria (e.g. if the bacteria form aggregates or microcolonies that behave differently from individual bacterial cells). Another frequently used approach to examine the viability of individual external and internal bacteria is thin-section transmission electron microscopy (TEM). This approach is advantageous in that it can provide information regarding the location of the bacteria in host cells (e.g. phagosome, cytoplasm, autophagosome), which can be further investigated by immunoelectron microscopy with gold-coupled antibodies against subcellular markers. However, electron microscopy is not especially sensitive at assessing bacterial viability. When embedded sections are stained with uranyl acetate, lead citrate, or other electron-dense reagents and imaged by electron microscopy, electron-dense bacteria are considered viable and electron-lucent nonviable 7,8. However, this assumption overestimates bacterial viability, since only those dead bacteria with severely disrupted membranes and devoid of cytoplasm appear electron-lucent. In addition, some bacterial species may display a range of electron densities depending on their stage of growth, making it difficult to determine viability.
As an alternative or in addition to these widely used methods, here we provide protocols and rationale for the use of fluorescent dyes that indicate bacterial viability to assess the survival of bacteria attached to and internalized by host cells. To identify extracellular bacteria, infected cells are first exposed to a fluorescent reagent, such as a lectin or bacteria-specific antibody. The infected cells are then permeabilized and exposed to DNA-specific dyes that are differentially accessible to bacteria with intact vs. degraded membranes, as a surrogate for bacterial viability. In the first protocol, the membrane permeable dye SYTO9 identifies the total bacterial population, while propidium iodide is only accessible to those bacteria that have compromised membranes and are thus considered nonviable. Propidium iodide and SYTO9 have been used to evaluate bacterial viability in biofilms, discriminate pathogenic from nonpathogenic bacteria, and enumerate viable water-borne bacteria 9-12. In the second protocol, 4',6'-diamidino-2-phenylindole (DAPI) identifies total bacteria, while SYTOX Green is only accessible to the nonviable population. These viability dye pairs can be combined with immunofluorescence to determine each bacterium's location in relation to a protein of interest, for instance to define bacterial subcellular localization. The use of these assays provides key insight into the interactions that result in bacterial killing or survival during infection of host cells. The protocols outlined in this article were used to assess the viability of N. gonorrhoeae that is attached to and inside primary human neutrophils, including in different populations of neutrophil phagosomes 5,13,14. However, these protocols can be applied to assess viability of gram-positive and gram-negative bacteria in professional phagocytes, non-professional phagocytes, and protozoa 15-24.
Protocol
1. Assessing Bacterial Viability with Propidium Iodide and SYTO9
- Infect cells that are adherent to 12 mm diameter circular glass coverslips in 24-well plates with bacteria of interest. Do NOT fix the cells with aldehydes or organic solvents.
- Rinse cells once gently in 0.1 M 3-(N-morpholino)propanesulfonic acid (MOPS), pH 7.2, containing 1 mM MgCl2 (MOPS/MgCl2).
- Incubate cells for 10 min in the dark at room temperature with Alexa Fluor 647-coupled antibody or lectin that binds to the bacterial species of interest, in MOPS/MgCl2, to detect external bacteria.
NOTE: Conduct controls with live and dead bacteria, in the absence of host cells, to show that the lectin or antibody of interest binds all bacteria regardless of viability (Figure 1).
- Rinse cells two times with MOPS/MgCl2.
- Aspirate media from cells and add 0.5 ml Live/Dead Staining Solution. Live/Dead Staining Solution is 5 μM SYTO9, 30 μM propidium iodide, and 0.1% saponin (final concentrations) in MOPS/MgCl2.
- Incubate cells for 15 min at room temperature in the dark.
- Rinse cells two times in MOPS/MgCl2.
- Invert coverslips face down onto glass slides and seal with clear nail polish. Do not use mounting media.
- Acquire images within 30 min, using a fluorescence microscope with filter sets compatible with green, red, and far-red image acquisition.
NOTE: Both conventional fluorescence microscopy and confocal laser scanning microscopy can be used. After 30 min the fluorescent dyes begin to leak from the bacteria and the data acquired are no longer accurate. Images shown in this article were acquired on a Nikon Eclipse E800 upright fluorescence microscope with Hamamatsu Orca-ER digital camera using Openlab software. Fluorescence of Alexa Fluor 647 was detected using a filter with excitation wavelength of 590 – 650 nm and an emission filter of 663 – 735 nm, and is false-colored blue. Fluorescence from propidium iodide was detected using a filter with excitation wavelength of 540 – 580 nm and an emission filter of 600 – 660 nm. Fluorescence from SYTO9 was detected using a filter with excitation wavelength of 465 – 495 nm and an emission filter of 515 – 555 nm.
- This protocol will result in images where external nonviable bacteria appear blue + red, internal nonviable bacteria appear red only, external viable bacteria appear blue + green, and internal viable bacteria appear green only. Count by eye the numbers of bacteria that are external nonviable, internal nonviable, external viable, and internal viable.
- Calculate the percent of external viable bacteria by dividing the number of external viable bacteria by the total number of external bacteria (viable plus nonviable). Calculate the percent of internal viable bacteria by dividing the number of internal viable bacteria by the total number of internal bacteria (viable plus nonviable) (Figure 4).
- There are two essential controls to perform with this protocol. First, validate that all nonviable bacteria are propidium iodide-positive and all live bacteria are SYTO9-positive. A mid-logarithmic culture of bacteria (in the absence of any host cells) should be >95% SYTO9-positive. Shown in Figure 2 is a mid-logarithmic culture of N. gonorrhoeae (at 108 colony forming units per ml) incubated with Live/Dead Staining Solution. Second, validate that both propidium iodide and SYTO9 can enter permeabilized, infected host cells.
- To generate a population of dead bacteria, collect 2 x 108 bacterial colony forming units in a 1.5 ml microfuge tube, add 70% isopropanol and sit for 10 min. Pellet the bacteria in a microfuge and wash the bacteria twice in PBS to remove residual isopropanol. Then follow protocol steps 1.5 – 1.7. Add 5 μl of bacterial suspension to a glass slide and overlay with a coverslip. Image samples as in protocol step 1.9. Under these conditions, 100% of the population should be propidium iodide-positive (Figure 2). In some bacterial species, propidium iodide may not completely overwhelm SYTO9 staining, and nonviable bacteria may appear yellow or orange.
- For phagocytic cells like neutrophils, expose the cells to isopropanol-killed bacteria and ensure that 100% of the intracellular bacteria are propidium iodide-positive (Figure 3). For nonphagocytic cells that may not internalize dead bacteria, it may be sufficient to treat infected cells with sodium azide or other cell-permeant antimicrobial agents prior to adding Live/Dead Staining Solution.
2. Assessing Bacterial Viability with SYTOX Green and DAPI
- Label bacteria of interest with 10 μg/ml DAPI in Morse's Defined Medium 25 for 20 min at room temperature in the dark.
- Infect cells that are adherent to 12 mm diameter circular glass coverslips in 24-well plates with DAPI-labeled bacteria. Do NOT fix the cells with aldehydes or organic solvents.
- Rinse cells once with MOPS/MgCl2.
- Incubate cells for 10 min at room temperature in the dark with Alexa Fluor 647-coupled antibody or lectin that binds to the bacterial species of interest, in MOPS/MgCl2, to detect external bacteria. See note in protocol step 1.3 for suggested controls.
- Aspirate media from cells and add 0.5 ml 0.4 μM SYTOX Green in MOPS/MgCl2.
- Incubate cells for 5 min at room temperature in the dark.
- Rinse cells two times in MOPS/MgCl2.
- Wash cells one time in MOPS/MgCl2 for 5 min.
- Acquire images within 30 min with a fluorescence microscope. See protocol step 1.9 for description of microscope, digital camera, and acquisition software.
NOTE: Fluorescence from Alexa Fluor 647 was detected using a filter with excitation wavelength of 590 – 650 nm and an emission filter of 663 – 735 nm, and is false-colored red. Fluorescence from SYTOX Green was detected using a filter with excitation wavelength of 465 – 495 nm and an emission filter of 515 – 555 nm. Fluorescence from DAPI was detected using a filter with excitation wavelength of 355 – 375 nm and barrier filter of 400 nm.
- This protocol will result in images where external nonviable bacteria appear red + green, internal nonviable bacteria appear green + blue, external viable bacteria appear red + blue, and internal viable bacteria appear blue only (Figure 4). Quantify the percent of external and internal bacteria as described in protocol step 1.11.
- The controls described in protocol step 1.12 should be performed with the DAPI/SYTOX Green dye combination (Figures 2 and 3).
3. Assessing Bacterial Viability Alongside Subcellular Localization
- Label bacteria of interest with 10 μg/ml DAPI in Morse's Defined Medium for 20 min at room temperature in the dark.
- Infect cells that are adherent to 12 mm diameter circular glass coverslips in 24-well plates with DAPI-labeled bacteria. Do NOT fix the cells with aldehydes or organic solvents.
- Rinse cells once with MOPS/MgCl2.
- Incubate 10 min at room temperature in the dark with Alexa Fluor 647-coupled antibody or lectin that binds to the bacterial species of interest, in MOPS/MgCl2, to detect external bacteria. See note in protocol step 1.3 for suggested controls.
- Aspirate media from cells and rinse cells two times in MOPS/ MgCl2.
- Incubate cells with Alexa Fluor 555-coupled antibody against subcellular marker of interest for 20 min, in MOPS/MgCl2 containing 0.2% saponin.
- Rinse cells two times in MOPS/MgCl2.
- Wash cells one time in MOPS/MgCl2 for 5 min.
- Aspirate media from cells and add 0.4 μM SYTOX Green in MOPS/MgCl2.
- Incubate cells 5 min at room temperature in the dark.
- Rinse cells two times in MOPS/MgCl2.
- Wash cells one time in MOPS/ MgCl2 for 5 min.
- Acquire images of slides within 30 min on fluorescence microscope. See protocol step 1.9 for description of microscope, digital camera, and acquisition software.
NOTE: Fluorescence from Alexa Fluor 647 was detected using a filter with excitation wavelength of 590 – 650 nm and emission filter of 663 – 735 nm, and is false-colored purple. Fluorescence from Alexa Fluor 555 was detected using a filter with excitation wavelength of 540 – 580 nm and an emission filter of 600 – 660 nm. Fluorescence from SYTOX Green was detected using a filter with excitation wavelength of 465 – 495 nm and an emission filter of 515 – 555 nm. Fluorescence from DAPI was detected using a filter with excitation wavelength of 355 – 375 nm and barrier filter of 400 nm.
- This protocol will result in images where external nonviable bacteria appear purple + green, internal nonviable bacteria appear green + blue, external viable bacteria appear purple + blue, internal viable bacteria appear blue only, and subcellular protein appears red (Figure 4). Quantify the perecent of external and internal viable bacteria as described in protocol steps 1.11.
- The controls described in protocol step 1.12 should be performed with the DAPI/SYTOX Green dye combination (Figures 2 and 3).
- In addition to counting viable and nonviable bacteria, classify each bacteria as either positive or negative for colocalization with the subcellular marker of interest.
- Calculate the percent of viable bacteria colocalized with the subcellular marker by dividing the number of colocalized viable bacteria by the total number of viable bacteria. Calculate the percent of nonviable bacteria colocalized with the subcellular marker by dividing the number of nonviable colocalized bacteria by the total number of nonviable bacteria.
Representative Results
The protocols outlined were used to examine survival of N. gonorrhoeae after exposure to primary human neutrophils 5,26. Neutrophils were infected with N. gonorrhoeae and processed with protocol 1, using the green-fluorescent viability dye SYTO9 and the red-fluorescent propidium iodide (Figure 4A). The dyes were added in the presence of saponin, which sequesters cholesterol to preferentially permeabilize host cell plasma membranes, not N. gonorrhoeae membranes. Other detergents may need to be tested if the membranes of the bacteria of interest contain high amounts of cholesterol. All N. gonorrhoeae stain with SYTO9, but only bacteria with compromised membranes stain with propidium iodide. The propidium iodide overcomes the SYTO9 fluorescence, so live bacteria appear green and dead bacteria appear red 27. In some bacterial species, propidium iodide may not completely overcome SYTO9 staining, resulting in dead bacteria appearing yellow or orange. The ratio of propidium iodide to SYTO 9 may need to be optimized for different bacterial species. Prior to permeabilizing the cells and adding SYTO9 and propidium iodide, an Alexa Fluor 647-coupled soybean lectin was added to the infected cells to detect extracellular N. gonorrhoeae, and the far-red fluorescence signal was false-colored blue. Thus, in this protocol external viable bacteria appear turquoise (blue + green) and external nonviable appear magenta (blue + red). Internal viable bacteria appear green only (arrow), and internal nonviable bacteria appear red only (arrowhead). It is important to note that in infected cells, the eukaryotic cell nucleus will be stained by SYTO9 and propidium iodide ("N", Figure 4A). The external viable, external nonviable, internal viable, and internal nonviable bacteria were quantified from fluorescence images to yield the percent of internalized bacteria and the percent viable bacteria inside and outside host cells.
Figure 4B is a representative image of N. gonorrhoeae-infected neutrophils processed with protocol 2, using the viability dyes SYTOX Green and DAPI. This protocol avoids the use of propidium iodide, which fluoresces in both red and ultraviolet channels on the fluorescence microscope. All N. gonorrhoeae stain with DAPI (blue), but only bacteria with compromised membranes stain with SYTOX Green. As described above, before permeabilizing the cells, an Alexa Fluor 647-coupled soybean lectin was added to the infected cells to detect extracellular N. gonorrhoeae, whose fluorescence in this case is false-colored red. Therefore, external viable bacteria appear magenta (red + blue) and external nonviable bacteria appear yellow (red + green/blue). Internal viable bacteria appear blue only (arrows), and internal nonviable bacteria appear green/blue (arrowheads). Depending on the relative intensity of DAPI vs. SYTOX Green fluorescence, nonviable bacteria may appear turquoise. Similar to cells processed with propidium iodide and SYTO9, the neutrophil nucleus is stained by SYTOX Green and DAPI in Figure 4B (indicated by "N"). Exposure of infected cells to SYTOX Green and DAPI reveals the percent internalization and viability of bacteria in host cells, quantified as described above for propidium iodide and SYTO9. Results from N. gonorrhoeae-infected neutrophils processed using SYTOX Green and DAPI are comparable to those obtained with propidium iodide and SYTO9 26.
As depicted in Figure 4C, protocol 3 is an adaptation of protocol 2, in which the bacterial viability dyes were combined with immunofluorescence for the primary or azurophilic neutrophil granule protein CD63. This experiment was conducted in order to define the composition of phagosome compartments inside neutrophils that contain viable or nonviable N. gonorrhoeae 26. This protocol requires a microscope for four-color fluorescence capability to detect viable bacteria, nonviable bacteria, extracellular bacteria, and subcellular marker. Since the viability dyes are not compatible with aldehyde fixation (our unpublished observations), we performed immunofluorescence without fixation, using an antibody directly coupled to a fluorophore to minimize the time needed to detect the antigen inside cells. This antibody is coupled to the red fluorophore Alexa Fluor 555. As in protocol 2, viable and nonviable bacteria were distinguished using DAPI and SYTOX Green, respectively, and external bacteria were stained using Alexa 647-coupled soybean lectin. External bacteria fluoresce far-red and are false-colored purple. External viable bacteria appear purple + blue and external nonviable bacteria appear purple + green/blue. Internal viable bacteria appear blue (arrow) and internal nonviable bacteria appear green (arrowhead). CD63-positive granules and phagosomes appear red. The neutrophil nucleus is stained with the DNA specific dyes ("N"). When analyzing these images, each individual bacterium was classified as external vs. internal, viable vs. nonviable, and positive or negative for a ring of CD63 staining. N. gonorrhoeae was considered to reside in a neutrophil CD63-positive phagosome if there is ≥50% of a ring of CD63 fluorescence surrounding the bacteria, and in a CD63-negative phagosome if there is <50% of a ring of CD63 fluorescence surrounding the bacteria. The nonviable intracellular bacterium indicated by the arrowhead in Figure 4C has a ring of staining for CD63 surrounding the bacteria. This ring indicates primary granules are enriched at this phagosome. The viable intracellular bacterium, indicated by the arrow, lacks staining for CD63, indicating primary granules are not enriched at its phagosome. We used this technique to show a correlation between the viability of N. gonorrhoeae and residence in a primary granule-negative phagosome 26. Because this assay tracks both viability and bacterial localization using a protein of interest, it is possible to determine if nonviable and viable bacteria reside in the same or different locations. This information provides important initial insight into mechanisms that contribute to bacterial survival in host cells.
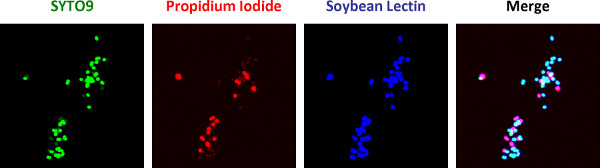
Figure 1. A mix of viable and nonviable N. gonorrhoeae was exposed to the Alexa Fluor 647-coupled soybean lectin, propidium iodide, and SYTO9 according to protocol 1, in the absence of host cells. All bacteria are accessible to the lectin and fluoresce blue. Nonviable bacteria appear blue + red and viable bacteria appear blue + green.
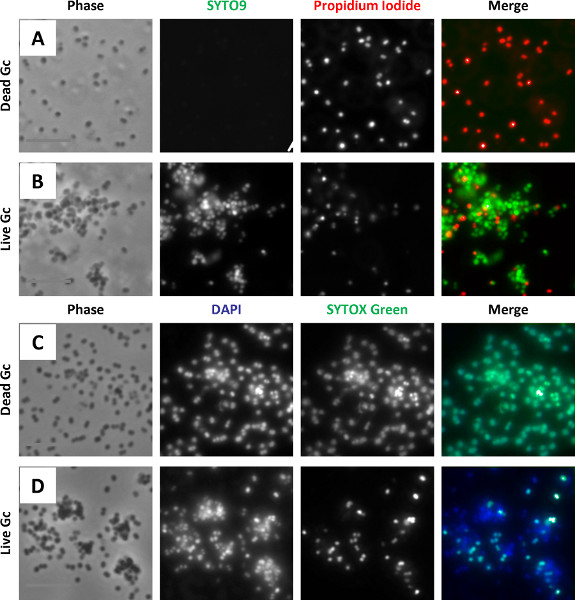
Figure 2. (A-B) Mid-logarithmic phase N. gonorrhoeae was exposed to propidium iodide and SYTO9 as in protocol 1, with (A) or without (B) isopropanol treatment to kill the bacteria. Nonviable bacteria are accessible to propidium iodide and appear red. Viable bacteria are stained with SYTO9 and appear green. (C-D) Mid-logarithmic phase N. gonorrhoeae was exposed to DAPI and SYTOX Green as in protocol 2, with (C) or without (D) isopropanol treatment to kill the bacteria. Nonviable bacteria are accessible to DAPI and SYTOX Green and appear blue + green. Viable bacteria are stained with DAPI only and appear blue only.
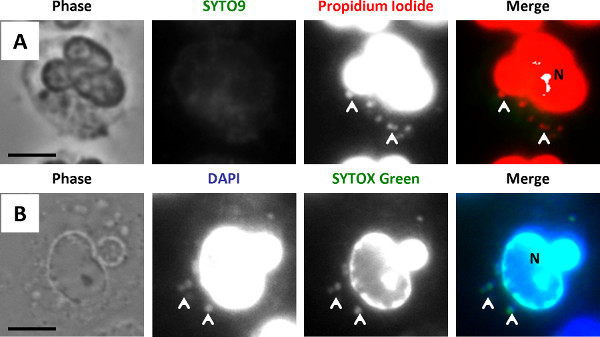
Figure 3. (A) Neutrophils were allowed to bind and internalize isopropanol-killed N. gonorrhoeae, then exposed to propidium iodide and SYTO9 according to protocol 1. Nonviable bacteria are stained with propidium iodide and appear red. As all bacteria are nonviable, no SYTO9 positive, green bacteria are detected. (B) Neutrophils were allowed to bind and internalize isopropanol-killed N. gonorrhoeae, then exposed to DAPI and SYTOX Green according to protocol 2. Nonviable bacteria are stained with DAPI and SYTOX Green and appear blue + green. As all bacteria are nonviable, no DAPI only, blue only bacteria are detected. N labels the neutrophil nucleus. Scale bar = 5 μm. Arrowheads indicate some of the nonviable bacteria.
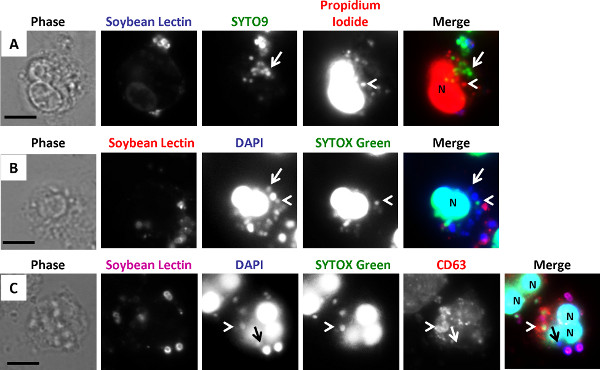
Figure 4. (A) N. gonorrhoeae-infected human neutrophils were exposed to Alexa Fluor 647-coupled soybean lectin (false-colored blue), then permeabilized with saponin in the presence of the viability dyes propidium iodide (red), to label dead bacteria, and SYTO9 (green), to counterstain live bacteria. External nonviable bacteria appear blue + red, internal nonviable bacteria appear red only, external viable bacteria appear blue + green, and internal viable bacteria appear green only. (B) N. gonorrhoeae was labeled with DAPI (blue), then presented to neutrophils. Infected cells were then exposed to Alexa Fluor 647-coupled soybean lectin (false-colored red), then permeabilized with saponin in the presence of SYTOX Green (green) to label dead bacteria. External nonviable bacteria appear red + green, internal nonviable bacteria appear green + blue, external viable bacteria appear red + blue, and internal viable bacteria appear blue only. (C) N. gonorrhoeae-infected neutrophils were processed as in B, except an Alexa Fluor 555-coupled antibody against the neutrophil primary granule protein CD63 (red) was added at the time of permeabilization. The Alexa Fluor 647-soybean lectin staining was false-colored purple. External nonviable bacteria appear purple + green, internal nonviable bacteria appear green only, external viable bacteria appear purple + blue, internal viable bacteria appear blue only, and CD63 staining appears red. The inset shows the CD63 fluorescence of the area of the image indicated with the white square. In A-C, N labels the neutrophil nucleus. Scale bar = 5 μm. Arrowheads indicate nonviable bacteria and arrows indicate viable bacteria.
Discussion
Presented here are two protocols that use DNA binding and viability dyes in conjunction with a fluorescent lectin to identify live and dead bacteria attached to and inside human cells. Since both protocols effectively discriminate live from dead bacteria, the choice of which protocol to use depends upon the goal of the experiment. The first protocol uses propidium iodide to detect nonviable bacteria and SYTO9 to detect intact bacteria. Shown in Figure 4A is a representative image of protocol 1 applied to human neutrophils infected with N. gonorrhoeae. These dyes were combined with a lectin, soybean agglutinin, to recognize extracellular N. gonorrhoeae, but a fluorescently coupled bacteria-specific antibody could be used as an alternative. Protocol 1 readily reveals the viability of individual bacteria, since exclusion of SYTO9 by propidium iodide makes nonviable bacteria fluoresce red and viable bacteria fluoresce green. However, this protocol cannot be combined with immunofluorescence for host proteins, due to the fluorescence of propidium iodide in two of the four channels available for conventional fluorescence microscopy. Therefore, protocol 2 was developed, which uses SYTOX Green to identify nonviable bacteria and DAPI to detect the total bacterial population. An example of N. gonorrhoeae associated with and inside human neutrophils and processed using SYTOX Green and DAPI is shown in Figure 4B and an example in combination with immunofluorescence is shown in Figure 4C. Besides the availability of two additional fluorescence channels, this protocol is advantageous for use by individuals with red-green colorblindness. However, nonviable bacteria can fluoresce green or blue-green, depending on the relative intensity of DAPI and SYTOX Green staining and on the image acquisition parameters. One drawback to the use of DAPI is that it can leak from N. gonorrhoeae during the course of infection, although this may not be an issue for other bacteria such as Staphylococcus aureus (our unpublished observations). To counteract this issue, infected cells were labeled with DAPI along with SYTOX Green after infection, but the intensity of the nuclear DAPI staining overpowered the fluorescence of N. gonorrhoeae (our unpublished observations). In addition to these two protocols, other commercially available dyes could be used to reveal bacterial viability. For instance, carboxyfluorescein succinimidyl ester (CFSE) could label the total bacterial population, and SYTOX Orange or Blue could identify nonviable bacteria. Since the relative efficacy of these procedures may vary with the bacterium and infected cell type, it is important that researchers compare different viability dye combinations in their infection systems.
Currently, we manually examine each image obtained with these protocols to quantify percent internalization, percent viability intracellularly and extracellularly, and colocalization with subcellular markers. While automated computer-based methods can theoretically be used to quantify these parameters, they may be complicated by any non-uniform staining of the bacteria with viability dyes and the strong fluorescence signal of the cell nucleus. Also, the definition of bacterial colocalization with a subcellular marker may need to be optimized for the bacteria, cell type, and marker of interest.
One limitation of these protocols is the assumption that permeability to propidium iodide or SYTOX Green is a true measure of bacterial death. The molecular weight of propidium iodide and SYTOX Green is 668.4 Da and 600 Da, respectively. These dyes are considered membrane-impermeant and should not access the bacterial cytoplasm unless the bacteria have unrepaired membrane damage. However, the possibility remains that the bacteria enter a state of stasis or even repair their membrane damage and continue to grow at later times. A second limitation is that the protocols do not allow the viability of individual bacteria to be tracked over time. Life Technologies recommends that assessments of bacterial viability with propidium iodide and SYTO9 be performed within 30 min after exposure to the dyes, since the bacteria will lose viability the longer they are mounted and imaged by fluorescence microscopy. Infected cells labeled with the DAPI/SYTOX Green dye combination must also be imaged rapidly after mounting (our unpublished observations). Third, the protocols are not compatible with commonly used fixation techniques. Methanol or acetone fixation will permeabilize and kill the bacteria associated with host cells, and aldehyde-based fixatives permeabilize N. gonorrhoeae inside human neutrophils to propidium iodide and SYTOX Green (unpublished observations). Thus using these protocols requires immediate access to a fluorescence microscope and the ability to complete the protocols in one day. Finally, each of the dyes used in these protocols bind double stranded nucleic acids and bind host nuclear DNA as well as the bacterial DNA (Figure 1). Because fluorescence of the nuclear DNA is significantly brighter than fluorescence of the bacterial DNA, the viability of bacteria that are in proximity to the eukaryotic nucleus cannot be assessed. Confocal laser scanning microscopy and prelabeling bacteria with DAPI help reduce the nuclear fluorescence signal. Fluorescence of nuclear DNA will remain a complication of these protocols until non-DNA-binding bacterial viability dyes are developed.
Despite these limitations, the protocols in this article provide a sensitive way to assess the viability of individual bacteria attached to and inside eukaryotic cells. Colony count or gentamicin-protection assays have been the backbone of microbial pathogenesis research. However, they provide an assessment of bacterial survival across a potentially heterogeneous population. In contrast, the viability dye protocols described here allow the integrity of individual bacteria to be examined. Therefore, researchers can gather data regarding the fates of extracellular vs. intracellular bacteria, bacteria in different phagosomal types, or bacteria in a phagosome vs. the cytoplasm. Thin section transmission electron microscopy has often been used to examine the viability of individual bacteria. While bacteria that appear electron-lucent or no longer intact by TEM are clearly nonviable, it is more difficult to assess whether bacteria that retain some electron density in their cytoplasm are actually intact. Additionally, it can take many hours of electron microscope time to acquire enough micrographs to analyze, while many low-power fields can be acquired from cells exposed to fluorescence-based viability dyes, even in the short time afforded for image acquisition. While the provided protocols were optimized with N. gonorrhoeae and primary human neutrophils, they can be adapted to assess the viability of many species of gram-negative and gram-positive bacteria in a variety of cell types, including all phagocytic and nonphagocytic eukaryotic cells 5,15-24,26. Bacterial viability dyes provide a powerful experimental tool to examine mechanisms of bacterial pathogenesis in eukaryotic cells and the ability of eukaryotic cells to control bacterial infection. In the future, these protocols are likely to become even more useful with better tools for automated image acquisition and processing, along with the development of bacterial viability dyes that are compatible with aldehyde fixation and specific for prokaryotes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Asya Smirnov and Laura Gonyar for critical reading of the manuscript. This work was supported by grants NIH R00 TW008042 and R01 AI097312 to A.K.C. M.B.J. was supported in part by NIH T32 AI007046.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| 21 G 3/4 butterfly needles for blood collection | Becton Dickinson | 367251 | |
| Blood collection tubes with Sodium Heparin 10 ml | Becton Dickinson | 366480 | |
| Sterile water for irrigation | Baxter | 07-09-64-070 | |
| Dextran 500 | Sigma | 31392 | |
| Sodium Chloride | Fisher Scientific | S641 | |
| Dextrose | Ricca Chemical Company | RDCD0200 | |
| Dulbecco's PBS no Ca2+ or Mg2+ | Thermo Scientific | SH3002802 | |
| Ficoll solution | GE Healthcare | 17-1440-03 | |
| Acetic Acid | Fisher Scientific | BP2401 | |
| 12 mm circular glass coverslips | Fisher Scientific | 12-545-80 12CIR-1 | |
| 24-well plates | Corning Incorporated | 3524 | |
| Pooled Human Serum | Sigma | S7023 | |
| RPMI | Mediatech | 15-040-CV | |
| Fetal Bovine Serum | Thermo Scientific | SH3007103 | |
| Human interleukin-8 | R&D Systems | 208-IL/CF | |
| MOPS | Sigma | M3183 | |
| MgCl2 | Fisher Scientific | BP214 | |
| Propidium Iodide | Life Technologies | L7007 or L7012 | |
| SYTO9 | Life Technologies | L7007 or L7012 | |
| Saponin | Fluka Analytical | 47036 | |
| Alexa Fluor 647-coupled soybean lectin | Life Technologies | L-32463 | |
| DAPI | Sigma | D8417 | |
| SYTOX Green | Life Technologies | S7020 | |
| Mouse anti-CD63 | Developmental Studies Hybridoma Bank | H5C6 | |
| Alexa Fluor 555 Antibody Labeling Kit | Life Technologies | A20187 | |
| Hemacytometer Bright Line | Hausser Scientific | 1492 | |
| Forceps | EMS | 78320 | |
| Sorvall Legend RT + Centrifuge | Thermo Scientific | 75004377 |
References
- Woolard, M. D., Frelinger, J. A. Outsmarting the host: bacteria modulating the immune response. Immunol. Res. 41, 188-202 (2008).
- Johnson, M. B., Criss, A. K. Resistance of Neisseria gonorrhoeae to neutrophils. Front Microbiol. 2, 77 (2011).
- Simons, M. P., Nauseef, W. M., Apicella, M. A. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect. Immun. 73, 1971-1977 (2005).
- Seib, K. L., et al. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect. Immun. 73, 5269-5272 (2005).
- Criss, A. K., Katz, B. Z., Seifert, H. S. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 11, 1074-1087 (2009).
- Edwards, A. M., Massey, R. C. Invasion of human cells by a bacterial pathogen. J. Vis. Exp. (49), e2693 (2011).
- Bozzola, J. J. Conventional specimen preparation techniques for transmission electron microscopy of cultured cells. Methods Mol. Biol. 369, 1-18 (2007).
- Dorward, D. W. Ultrastructural analysis of bacteria-host cell interactions. Methods Mol. Biol. 431, 173-187 (2008).
- Yang, L., et al. Rapid, absolute, and simultaneous quantification of specific pathogenic strain and total bacterial cells using an ultrasensitive dual-color flow cytometer. Anal. Chem. 82, 1109-1116 (2010).
- Mueller, R. S., et al. Vibrio cholerae strains possess multiple strategies for abiotic and biotic surface colonization. J. Bacteriol. 189, 5348-5360 (2007).
- Shen, C., et al. Enhanced inactivation of Salmonella and Pseudomonas biofilms on stainless steel by use of T-128, a fresh-produce washing aid, in chlorinated wash solutions. Appl. Environ. Microbiol. 78, 6789-6798 (2012).
- Hoefel, D., Grooby, W. L., Monis, P. T., Andrews, S., Saint, C. P. Enumeration of water-borne bacteria using viability assays and flow cytometry: a comparison to culture-based techniques. J Microbiol Methods. 55, 585-597 (2003).
- Oh, H., Siano, B., Diamond, S. Neutrophil isolation protocol. J. Vis. Exp.. (17), e745 (2008).
- Allen, L. A. Immunofluorescence and confocal microscopy of neutrophils. Methods Mol Biol. 412, 273-287 (2007).
- Kaplan, E. L., Chhatwal, G. S., Rohde, M. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin. Infect. Dis. 43, 1398-1406 (2006).
- Chow, O. A., et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 8, 445-454 (2010).
- Thulin, P., et al. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 3, e53 (2006).
- Kubica, M., et al. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One. 3, (2008).
- Swords, W. E., et al. Mycobacterium xenopi multiplies within human macrophages and enhances HIV replication in vitro. Microb. Pathog. 40, 41-47 (2006).
- Tamilselvam, B., Almeida, R. A., Dunlap, J. R., Oliver, S. P. Streptococcus uberis internalizes and persists in bovine mammary epithelial cells. Microb. Pathog. 40, 279-285 (2006).
- Martinez, A. N., et al. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. J. Clin. Microbiol. 47, 2124-2130 (2009).
- Botha, M., Botes, M., Loos, B., Smith, C., Dicks, L. M. Lactobacillus equigenerosi strain Le1 invades equine epithelial cells. Appl. Environ. Microbiol. 78, 4248-4255 (2012).
- Allen, L. A., Schlesinger, L. S., Kang, B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191, 115-128 (2000).
- Smith, C. D., Berk, S. G., Brandl, M. T., Riley, L. W. Survival characteristics of diarrheagenic Escherichia coli pathotypes and Helicobacter pylori during passage through the free-living ciliate, Tetrahymena sp. FEMS Microbiol. Ecol. 82, 574-583 (2012).
- Morse, S. A., Bartenstein, L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can. J. Microbiol. 26, 13-20 (1980).
- Johnson, M. B., Criss, A. K. Neisseria gonorrhoeae phagosomes delay fusion with primary granules to enhance bacterial survival inside human neutrophils. Cell Microbiol. , (2013).
- Stocks, S. M. Mechanism and use of the commercially available viability stain. BacLight. Cytometry A. 61, 189-195 (2004).

