Isolation and Culture of Endothelial Cells from the Embryonic Forebrain
Summary
This video demonstrates an easy and reliable strategy for preparation of pure cultures of endothelial cells from the embryonic forebrain within 10-12 days and will be useful for research focused on many aspects of cerebral angiogenesis.
Abstract
Embryonic brain endothelial cells can serve as an important tool in the study of angiogenesis and neurovascular development and interactions. The two vascular networks of the embryonic forebrain, pial and periventricular, are spatially distinctive and have different origins and growth patterns. Endothelial cells from the pial and periventricular vascular networks have unique gene expression profiles and functions. Here we present a step-by-step protocol for isolation, culture, and verification of pure populations of endothelial cells from the periventricular vascular network (PVECs) of the embryonic forebrain (telencephalon). In this approach, telencephalon devoid of pial membrane obtained from embryonic day 15 mice is minced, digested with collagenase/dispase, and dispersed mechanically into a single cell suspension. PVECs are purified from cell suspension using positive selection with anti-CD-31/PECAM-1 antibody conjugated to MicroBeads using a strong magnetic separation method. Purified cells are cultured on collagen 1 coated culture dishes in endothelial cell culture medium until they become confluent and further subcultured. PVECs obtained with this protocol exhibit cobblestone and spindle shaped phenotypes, as visualized by phase-contrast light microscopy and fluorescence microscopy. Purity of PVEC cultures was established with endothelial cell markers. In our hands, this method reliably and consistently yields pure populations of PVECs. This protocol will benefit studies aimed at gaining mechanistic insights into forebrain angiogenesis, understanding PVEC interactions, and cross-talks with neuronal cell types and holds tremendous potential for therapeutic angiogenesis.
Introduction
Angiogenesis, neurogenesis and neuronal migration are critical events in central nervous system (CNS) development, repair and regeneration. Several elegant studies have shown that endothelial cells stimulate neuronal proliferation and vice versa through release of soluble factors and by direct contact. We found it curious that in majority of these studies1-3, while neuronal progenitors/neural stem cells are isolated from the embryonic brain, they are cocultured with endothelial cells from the adult brain, other adult tissue sources, or with endothelial cell lines. This might in part be due to the technical difficulties associated with isolating and culturing pure populations of endothelial cells from the embryonic brain. However, angiogenesis, neurogenesis, and neuronal migration are concurrent events occurring in orders of magnitude more robust in the embryonic brain than in the normal adult brain. The periventricular vascular network of the embryonic forebrain (telencephalon) originates from a vessel located within the basal ganglia primordium and develops in the form of an orderly gradient from ventral to dorsal telencephalon by embryonic day 11 (E11)4,5. This plexus of vessels of the periventricular vascular network are distinct from pial vessels based on origins, anatomical location, growth patterns, and developmental regulation4,5. The direction of propagation of the periventricular angiogenesis gradient matches the telencephalic transverse neurogenetic gradient. Within the telencephalon, the periventricular angiogenesis gradient and the gradient of GABA neurons migrating tangentially overlaps spatially as well6. With respect to timing, the angiogenesis gradient is in advance of the neurogenetic gradient and GABA neuron gradient by about a day. Thus, periventricular endothelial cells are spatially and temporally well positioned to provide critical cues to support telencephalic neurogenesis and neuronal migration4,6. Therefore, use of embryonic periventricular endothelial cells in coculture experiments with neuronal progenitors and/or neurons would provide a more favorable model for studying neurovascular interactions and developing novel avenues for treatment of neurodegenerative disease or ischemic/traumatic brain injury.
We emphasize the importance of removing the pial membrane, not only to limit epithelial cell contamination but also to separate pial endothelial cells which are molecularly and functionally distinct from endothelial cells of the periventricular vascular network4,6 (termed PVECs to distinguish from pial ECs). Here, we describe the method that we routinely use in our laboratory to obtain a rich and pure yield of PVECs. These endothelial cells are prepared from embryonic forebrains isolated from a single timed-pregnant mouse. They can be expanded, subcultured, and frozen down successfully for future use.
Protocol
1. Preparation of Reagents and Solutions
- Coating of 35 mm culture dish: Collagen Type 1 solution is supplied as an aqueous solution in 20 mM acetic acid (~100 mg protein/vial). Dilute an appropriate volume of collagen solution to a working concentration of 0.01% using sterile tissue culture grade water. Coat dishes with 1 ml of collagen solution for 3-4 hr at room temperature (RT) or 37 °C, or overnight at 2-8 °C. Remove excess solution from the coated dish and allow it to dry overnight. Rinse the dish with tissue culture grade water or PBS before adding media. Coated dishes can be stored at 4 °C for one month.
- Prepare complete Dulbecco's Modified Eagle Medium (DMEM). Supplement the DMEM (500 ml) with 10% FBS and antibiotic-antimycotic solution (1x concentration; 1 ml/100 ml). Complete DMEM can be stored at 4 °C for two months.
- Prepare an aliquot (50 ml) of DMEM with DNase I (0.001 mg/ml), (referred to as DMEM-DNase I).
- Prepare another aliquot (10 ml) of DMEM with collagenase and dispase (1 mg/ml) (referred to as DMEM-collagenase/dispase).
- Prepare Endothelial Cell Culture Medium (ECCM, 500 ml), supplemented with EGF (5 μg), ECGS (100 mg) and 5 μl of antibiotic-antimycotic solution. ECCM can be stored at 4 °C for two months.
- Prewarm all media before use.
- Prepare Purification buffer: PBS, pH 7.2 with 0.5% BSA and 2 mM EDTA. Keep buffer cold (2-8 °C).
2. Removal of Embryos and Dissection of Telencephalon
All experiments using laboratory animals are approved by the animal care and use committees of McLean Hospital and conform to NIH guidelines for the care and use of laboratory animals.
- Use timed pregnant CD1 mice (embryonic day 15). The day of vaginal plug discovery is considered embryonic day 0 (E0). CD1 dams generally produce large litters, so 10-12 embryos are expected from a pregnant dam.
- For hysterotomy, anesthetize pregnant mice with an intraperitoneal injection of Ketamine (100 mg/kg)/Xylazine (5 mg/kg). For mice approximately 20-30 g, deliver 0.3 ml of a 10 ml/kg Ketamine/Xylazine anesthetic solution and ensure that pain and distress is minimal during intraperitoneal injections of the anesthetic. Use hand restraint for the injections.
- Check deep anesthesia by toe pinch. Remove embryos from deeply anesthetized mice. Decapitate each embryo immediately upon removal from the mother. Place the embryonic heads in sterile Petri dishes in ice cold PBS.
- Follow hysterectomy by immediate euthanasia with anesthetic overdose (130 mg/kg pentobarbital, i.p.), a method for euthanasia, which is consistent with the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition.
- Under a stereo microscope, remove the brain from the head with fine microtip scissors and fine forceps. Note: A high quality dissection is essential to successfully remove the pial membrane from the embryonic brain. This is achieved by practice. Fine forceps and microtip scissors are also key tools for getting a good dissection.
- Use the forceps to get a firm grip on the brain and the microtip scissors to peel away the pial membrane. Remove the mesencephalon and metencephalon and isolate the telencephalon. Transfer pial-free telencephalon from all embryos to a 35 mm culture dish with 2 ml PBS in ice.
3. Cell Isolation
- Mince the telencephalon into 1-2 mm fragments with a scalpel blade and collect tissue in a 15 ml Falcon tube containing 2 ml of complete DMEM.
- Dissociate tissue gently but thoroughly with a 1 ml pipette until clumps disappear and a milky suspension is achieved.
- Centrifuge dissociated cells at 800-1,000 x g for 5 min at RT.
- Carefully remove the supernatant and resuspend pellet in DMEM-DNase I. Gently dissociate the cells again using 1 ml pipette and centrifuge at 800-1,000 x g for 5 min at RT.
- Resuspend the pellet in warm complete DMEM. Filter cells through a sterile 70 μm Nylon mesh. Collect filtered cells and keep in ice.
- Collect the cell-portions retained on the mesh and digest further with DMEM-collagenase/dispase (2 ml) for 1-2 min at RT. Wash cells 3x in DMEM-DNase I through centrifugation at 800-1,000 x g for 5 min at RT. Pool these cells with the filtered cells collected in step 3.5. Wash total cells once with complete DMEM. Proceed to determine cell number and magnetic labeling steps. Note: It is important to obtain a single-cell suspension before magnetic labeling.
4. Magnetic Labeling
- This protocol shows the magnetic labeling of 107 cells. For cell numbers lower than 107, use the same reagent volume and for cell numbers greater than 107, scale up all reagent volumes and total volumes accordingly (e.g. for 2 x 107 total cells, use twice the volume of all indicated reagents). Keep all the reagents and cells in ice.
- Determine cell number with a hemocytometer.
- Centrifuge cell suspension at 300 x g for 10 min at RT. Aspirate supernatant completely.
- Add 90 μl/107 cells of purification buffer to the cell pellet followed by 10 μl of CD31 MicroBeads. The Microbeads provided by manufacturer are conjugated to monoclonal anti-mouse CD31 antibody.
- Mix well and incubate for 15 min in the refrigerator. Note: Higher temperatures and/or longer incubation time during magnetic labeling may result in nonspecific binding.
- Wash cells by adding 1-2 ml/107 cells of purification buffer and centrifuge at 300 x g for 10 min. Remove supernatant and resuspend cells in 500 μl of purification buffer. Proceed to magnetic separation or purification step.
5. Purification
- Place MS column in the magnetic field of MACS Separator.
- Rinse column with 500 μl of purification buffer.
- Apply cell suspension onto the column. Load appropriate number of cells to MS column as higher cell number may clog the column. Caution: Each MS column can accommodate a maximum number of 2 x 107 cells, for a higher cell number, use more columns.
- Collect and discard flow-through containing unlabeled cells.
- Wash column with 500 μl of purification buffer three times. During wash steps, make sure that the column reservoir is empty before adding subsequent purification buffer aliquots.
- Remove column from the MACS separator and place it on a 15 ml Falcon tube.
- A plunger is supplied along with the MS column. Pipette 1 ml of purification buffer onto the MS column and immediately flush out the magnetically labeled cells by firmly pushing the plunger into the column.
- Plate cells on a 35 mm culture dish precoated with collagen Type 1 and grow cells in ECCM in an incubator set to 37 °C with 95% O2 and 5% CO2.
- Change the medium every four days. Use PVECs for experiments or subculture after 10-12 days when the dish is confluent.
6. Subculture of PV ECS
- Remove the ECCM and rinse the monolayer of PVECS with 1-2 ml of sterile PBS.
- Gently add 1.5 ml of 0.25% Trypsin-EDTA solution to cover the cell monolayer.
- Return the dish to the incubator. Within 5-7 min, cells will detach from the surface of the dish.
- After the cells have detached, immediately add an equal volume of trypsin inhibitor and triturate several times.
- Transfer the cells to a sterile 15 ml Falcon tube. Centrifuge the tube at 500 x g for 5 min.
- Remove the supernatant and resuspend the pellet in 1 ml of prewarmed ECCM.
- Seed the cells for expanding the culture, imaging or other assays.
Representative Results
The phenotypic characterization of PVECs from day 1-12 is shown by phase-contrast light microscopy (Figure 2). The cells attached to the dish on day 1 show morphology characteristic of cell division (Figure 2A). Between 5-8 days, PVECs transition from cobble stone to spindle shaped morphology typical for endothelial cells and more akin to its in vivo state (Figures 2B and 2C). By day 12 the PVEC culture achieves full confluence (Figure 2D). PVECs can be subcultured easily to expand the colony (Figures 2E-G). Purity of endothelial cell cultures was established with endothelial cell markers – Isolectin B4, CD-31/PECAM-1,Von Willebrand Factor (vWF)and VE-Cadherin and was determined to be a hundred percent after subculture (Figures 2H-K).
High magnification images of PVECs prepared from CD1 embryos as well as Tie2-GFP embryos4 (whose endothelial cells express GFP) are shown (Figure 3). In addition to the common cobblestone and spindle shaped morphologies (Figures 3A-C), polygonal morphologies with slender processes were also observed in isolectin B4-labeled and Tie2-GFP+ve PVECs (Figures 3D and 3E). In some of our collagen coated culture dishes (in the absence of Matrigel), PVECs formed lattice patterns resembling the unique characteristic of the in vivo periventricular vascular network (Figure 3F). This reflects the high angiogenic potential of PVECs. An angiogenesis assay was performed in which PVECs (2 x 104 cells) were added to culture dishes coated with Matrigel matrix. PVECs showed robust tube formation within 18 hr (Figure 3G). These results provide strong evidence that this system can be used as an in vitro endothelial cell model. PVECs isolated from CD1 embryos can be rapidly labeled with Qdot nanocrystals (Figure 3H) that deliver intense stable fluorescence into the cytoplasm of live cells and can be used for long-term studies of live PVECs, including migration, motility, morphology, cell-function assays as well as in vivo experiments. PVECs can be transfected with cell tracers like Cell Light Plasma Membrane-RFP, BacMam 2.0 (Figure 3I) as per manufacturer's instructions for clear visualization of cell morphology in live cell imaging experiments.
We cultured PVECs not only in ECCM, but also in rat brain endothelial cell growth medium (RBECGM) and DMEM F12 medium. Both phase contrast (Figures 4A, 4C, and 4E) and isolectin B4 labeled images (Figures 4B, 4D, and 4F) of PVECs cultured in ECCM (Figures 4A and 4B), RBECGM (Figures 4C and 4D) and DMEM F12 (Figures 4E and 4F) are shown. Interestingly, differences in PVEC morphology and growth rate became striking. While PVECs grown in ECCM showed spindle shaped morphology (Figures 4A and 4B) and was confluent by day 12, PVECs grown in RBECGM showed very elongated morphology (Figures 4C and 4D) and was confluent by day 20. On the other hand, PVECs cultured in DMEM F12 media showed only flat and polygonal morphology (Figures 4E and 4F) with no spindle formation, very rapid growth and was confluent by Day 4.
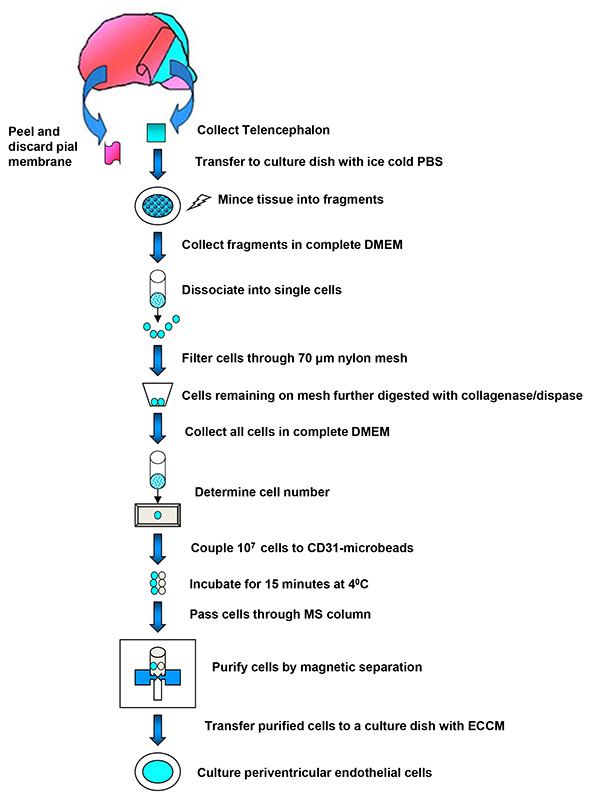
Figure 1. Schematic of PVEC isolation. The protocol used for isolation and purification of PVECs from the embryonic telencephalon is summarized into a schema. Click here to view larger image.
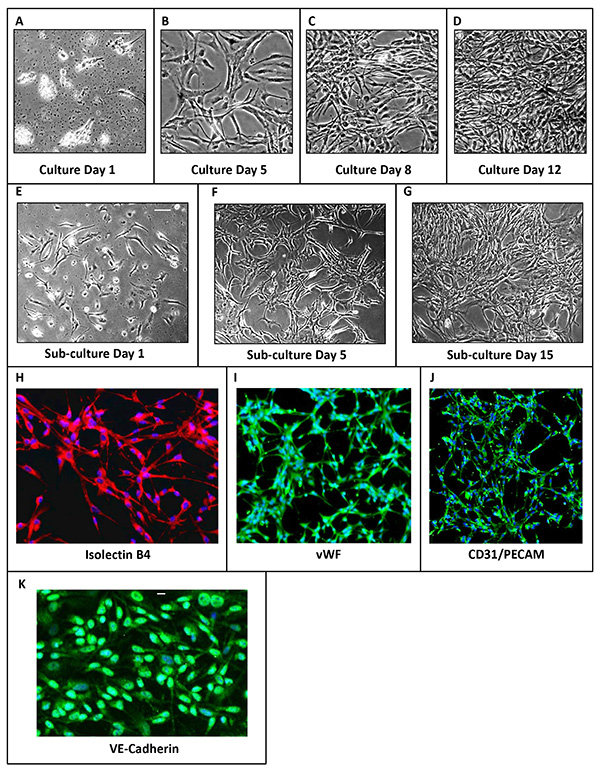
Figure 2. Phenotypic characterization of PVECs. (A-G) Phase contrast images of PVEC culture at different time points after plating. (A) PVEC culture on day 1. Cells have attached and show dividing morphologies. (B) PVEC culture on day 5 and (C) on day 8 transitioning from cobblestone to spindle shape morphology. (D) PVEC culture attained confluence on day 12. (E) PVECs after subculture on day 1, (F) day 5 (G) and day 15. (H-J) Immunolabeling of PVECs using endothelial cell markers: Isolectin B4 (H), Von Willebrand Factor (I), CD31/PECAM (J) and VE-Cadherin (K). Scale bars: A, 100 µm (applies B-J), K, 50 µm. Click here to view larger image.
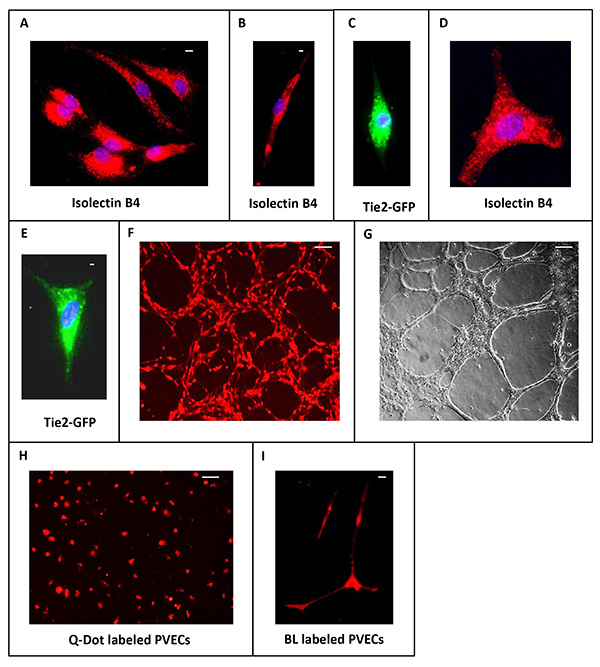
Figure 3. Morphologies of PVECs. (A-E) High magnification images of isolectin B4-labeled and Tie2-GFP+ve PVECs showing cobblestone and spindle shaped morphology (A-C) as well as polygonal morphology with slender long extensions (D, E). (F) PVECs show high angiogenic potential in culture dish even in the absence of Matrigel. (G) PVECs show robust tube formation in an angiogenesis assay on Matrigel. (H) PVECs labeled with Qdot nanocrystals. (I) PVECs transfected with Cell Light Plasma Membrane-RFP, BacMam 2.0. Scale bars: A, 50 µm, B, 15 µm (applies C-E), F, 100 µm (applies G,H), I, 50 µm. Click here to view larger image.
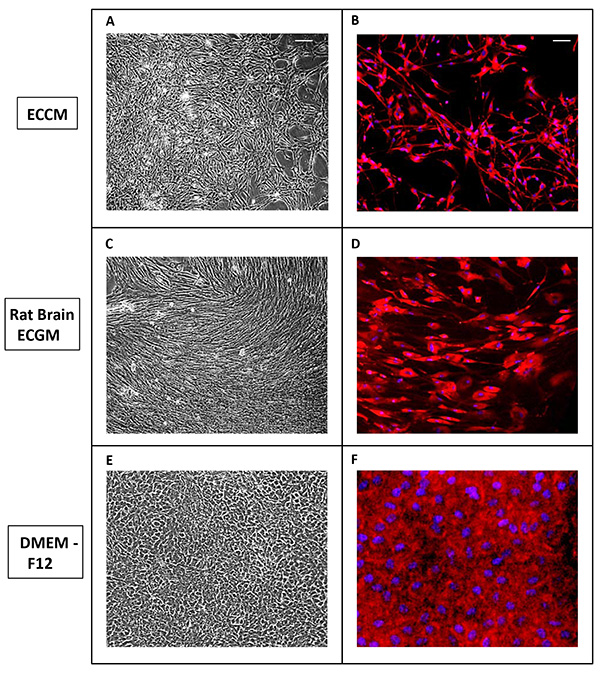
Figure 4. PVECs grown in different culture media show variant morphology. (A-F) Phase contrast (A, C, E) and isolectin B4 labeled images (B, D, F) of PVECs cultured in ECCM (A, B), RBECGM (C, D) and DMEM F12 (E, F). Scale bars: A, 100 µm (applies B-F). Click here to view larger image.
Discussion
PVEC's are more physiologically relevant than adult brain endothelial cells and ECs from other tissue sources for studies focusing on neurovascular interactions and also have therapeutic potential. For PVEC preparation, it is critical beginning with dissection to work fast to achieve a good viability since dead cells may bind nonspecifically to CD31 MicroBeads. In addition, if single-cell suspension is not achieved prior to the magnetic labeling step, this will result in troubleshooting since cell clumps will clog the column. An advantage of this method is that there is no need for incubation with trypsin to detach cells from magnetic beads, a time-consuming and often difficult step used in other magnetic bead-based separation methods for EC preparations4,7. In addition, this isolation technique does not require large quantities of embryonic brain tissue. PVEC's can be consistently prepared with the use of a single timed pregnant mouse in approximately 12 days, subcultured, and used in a wide variety of in vitro and in vivo experiments.
The purity of PVECs after isolation is over 90% but the purity of PVECs after subculture is 100%. In addition to endothelial cells, PECAM-1 is found on the surface of monocytes/macrophages. Microglia, the macrophage population of the CNS is however very low in the embryonic mouse brain when compared to postnatal or adult brain8. Monocytes/microglia need medium supplemented with 10% fetal calf serum and specific growth factors to grow; they will die out eventually in ECCM and will not last after subculture of PVECs. When 100% pure PVECs are needed immediately after isolation (for example, to test gene expression), we use fluorescence activated cell sorting (FACS) method. PVECs are then isolated from Tie2GFP embryos (in which only endothelial cells express GFP) and stringently sorted by dual FACS analysis. The purity of sorted endothelial cells are ensured by collection of cells that are double positive for GFP and CD31/PECAM-16.
Cultures are also closely observed in the first week of isolation for contaminating cell types like pericytes. Since pericyte cells are irregularly shaped and will never form a confluent monolayer unlike endothelial cells which consist of spindle shaped or polygonal, contacted cells that grow as colonies, they are easy to recognize if present. If pericyte contamination is detected, a lower concentration of trypsin (0.05%) followed by a quick trypsinization procedure (2-3 min) is used for subculture of PVECs. Pericytes require an extended trypsinization time (7-10 min) and will remain attached. In addition, the plating efficiency of trypsinized pericytes (if any) is very poor. These steps eliminate pericyte contamination and ensure that subcultured PVECs are pure.
The ECCM medium is a low serum medium containing hydrocortisone, heparin and 2% fetal bovine serum and is supplemented with Epidermal Growth Factor (EGF) and Endothelial Cell Growth Supplement (ECGS). PVECs grown in ECCM showed spindle shaped morphology consistently. The rat brain ECGM contains 10% fetal bovine serum, growth factors, and VEGF. PVECs grown in this media have very elongated morphologies and take longer to attain confluency. The DMEM-F12 medium is supplemented with 10% fetal bovine serum, GlutaMAX and endothelial cell growth supplement and it induces more proliferation than other media. Although PVECs grown in this media showed very rapid growth and attained confluency in 4 days, their morphologies were flat or polygonal only. We have grown PVECs in ECCM for up to three passages without loss of phenotype and function. Therefore, ECCM is the medium that we prefer for PVEC culture.
PVECs cocultured with neuronal precursors by this protocol are likely to stimulate neurogenesis and neuronal migration to far-greater extents than endothelial cells from adult brain or from other sources and may be valuable for aiding recovery of neurological function in stroke, traumatic brain injury or neurodegeneration. Neuronal precursors transplanted in the adult brain have often been reported to stall and fail to migrate into regions that require new neurons. This problem is accounted to the absence of substrates that facilitate neuronal migration like radial glial guides9. Vasculature is increasingly being appreciated as substrates for neuronal migration6,10,11. PVECs may offer a solution for these stalled neuronal precursors at transplantation sites. Coimplantation of PVECs and neurons into damaged brain regions may facilitate guided migration of neurons by endothelial cells and help achieve functional recovery. The close association of endothelial cells with migrating neurons also makes them uniquely suited to deliver growth factors and morphogens directly and selectively into migrating neurons.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award and National Institutes of Health grant R01NS073635 to AV.
Materials
| DNase I | Sigma | D-4527 | |
| Collagen, Type 1 solution from rat tail | Sigma | C3867 | |
| DPBS | Quality Biologicals | 114057-131 | |
| EDTA | Fisher Scientific | M4055 | |
| BSA | Sigma | A2058 | |
| MS column | Miltenyi Biotech | 130-042-201 | |
| CD31 microbeads | Miltenyi Biotech | 130-097-418 | |
| MACS separator | Miltenyi Biotech | 130-042-102 | |
| MACS multi stand | Miltenyi Biotech | 130-042-303 | |
| Cell strainer 70 µm | BD Bioscience | 352350 | |
| Antibiotic and antimycotic solution | Sigma | A5955 | |
| FBS | Sigma | F4135 | |
| Collagenase/Dispase | Roche | 10269638001 | |
| DMEM | Lonza | 12-604F | |
| 35 mm culture dish | BD Bioscience | 353001 | |
| 15 ml falcon tube | BD Bioscience | 352097 | |
| 50 ml falcon tube | BD Bioscience | 352098 | |
| ECCM kit | BD Bioscience | 355054 | Kit Includes Endothelial cell growth supplement, EGF and Soybean Trypsin Inhibitor |
| Endothelial Cell Growth Supplement (ECGS) | BD Bioscience | 354006 | |
| RBECGM | Cell applications | R819-500 | |
| DMEM F12 | Life Technologies | 10565-018 | |
| glutamax | Life Technologies | 305050-061 | |
| tissue culture grade water | Life Technologies | 15230162 | |
| 0.25% Trypsin | Life Technologies | 15050 | |
| Soyabean trypsin inhibitor | BD Bioscience | 5425 | |
| Matrigel | BD Bioscience | 354234 | |
| Qtracker 655 Cell Labeling Kit | Life Technologies | Q25021 | |
| CellLight Plasma Membrane-RFP, BacMam 2.0 | Life Technologies | C10608 | |
| Biotinylated Isolectin B4 antibody | Sigma | L2140 | |
| Anti-Von Willebrand factor | Sigma | F3520 | |
| Anti-CD31/PECAM-1 | BD Pharmingen | 550274 | |
| Vectashield Hardset Mounting media with DAPI | Vector Laboratories | H-1500 | |
| Ketamine | Butler Schein Animal Health Supply | 44028 | |
| Xylazine | Lloyd Laboratories | 1009 | |
| Stereomicroscope | Motic | SMZ-168 | |
| hemocytometer | Fisher Scientific | 267110 | |
| inverted microscope | Olympus CK-40 | CK-40 | |
| Flouroscent microscope | Olympus FSX-100 | FSX-100 | |
| Fine forceps | Roboz surgical instrument | 7 inox | |
| Fine microtip scissors | Roboz surgical instrument | RS5611 |
References
- Shen, Q., S, G., et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 304, 1338-1340 (2004).
- Milner, R. A novel three-dimensional system to study interactions between endothelial cells and neural cells of the developing central nervous system. BMC Neurosci. 8, 3 (2007).
- Rauch, M. F., Michaud, M., Xu, H., Madri, J. A., Lavik, E. B. Co-culture of primary neural progenitor and endothelial cells in a macroporous gel promotes stable vascular networks in vivo. 19, 1469-1485 (2008).
- Vasudevan, A., Long, J. E., Crandall, J. E., Rubenstein, J. L., Bhide, P. G. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 11, 429-439 (2008).
- Vasudevan, A., Bhide, P. G. Angiogenesis in the embryonic CNS: a new twist on an old tale. Cell Adh. Migr. 2, 167-169 (2008).
- Won, C. K., et al. Autonomous vascular networks synchronize GABA neuron migration in the embryonic forebrain. Nat. Commun. 4, 2149-2162 (2013).
- Dong, Q. G., et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler. Thromb. Vasc. Biol. 17, 1599-1604 (1997).
- Erblich, B., Zhu, L., Etgen, A. M., Dobrenis, K., Pollard, J. W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 6, (2011).
- Wichterle, H., Garcia-Verdugo, J. M., Herrera, D. G., Alvarez-Buylla, A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. 2, 461-466 (1999).
- Snapyan, M., et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 29, 4172-4188 (2009).
- Whitman, M. C., Fan, W., Rela, L., Rodriguez-Gil, D. J., Greer, C. A. Blood vessels form a migratory scaffold in the rostral migratory stream. J. Comp. Neurol. 516, 94-104 (2009).

