Large-scale Zebrafish Embryonic Heart Dissection for Transcriptional Analysis
Summary
To analyse cardiac gene expression profiles during zebrafish heart development, total RNA has to be extracted from isolated hearts. Here, we present a protocol for collecting functional/beating hearts by rapid manual dissection from zebrafish embryos to obtain cardiac-specific mRNA.
Abstract
The zebrafish embryonic heart is composed of only a few hundred cells, representing only a small fraction of the entire embryo. Therefore, to prevent the cardiac transcriptome from being masked by the global embryonic transcriptome, it is necessary to collect sufficient numbers of hearts for further analyses. Furthermore, as zebrafish cardiac development proceeds rapidly, heart collection and RNA extraction methods need to be quick in order to ensure homogeneity of the samples. Here, we present a rapid manual dissection protocol for collecting functional/beating hearts from zebrafish embryos. This is an essential prerequisite for subsequent cardiac-specific RNA extraction to determine cardiac-specific gene expression levels by transcriptome analyses, such as quantitative real-time polymerase chain reaction (RT-qPCR). The method is based on differential adhesive properties of the zebrafish embryonic heart compared with other tissues; this allows for the rapid physical separation of cardiac from extracardiac tissue by a combination of fluidic shear force disruption, stepwise filtration and manual collection of transgenic fluorescently labeled hearts.
Introduction
Zebrafish (Danio rerio) is widely used in developmental biology to study organogenesis in vivo due to its fast, transparent and extrauterine embryonic development, combined with small size and the availability of transgenic reporter lines with tissue-specific expression of fluorescent proteins. This small vertebrate is particularly well suited to study heart development because oxygenation of the early zebrafish embryo does not rely on heart beat and blood flow; these features have allowed the characterization of large numbers of cardiovascular mutants1,2 and the zebrafish is now a widely recognized model organism to study heart diseases3.
To study gene expression during embryonic development, transcripts are commonly analyzed by whole-mount in situ hybridization (WISH)4, RT-qPCR5, microarrays6, or next generation sequencing (RNA-Seq)7. While WISH allows a spatial-temporal analysis of gene expression within the entire embryo, transcript levels are usually assessed by RT-qPCR, microarrays or RNA-Seq approaches. However, these methods require tissue enrichment for specific gene expression profiling.
Since the zebrafish embryonic heart represents a small fraction of the entire embryo, transcriptome studies during cardiac development require a protocol for dissection and enrichment of hearts. In addition, to obtain physiologically relevant data, it is important to maintain the cardiac tissue fully functional until RNA extraction. Here, we describe a protocol for rapidly isolating physiologically normal and beating hearts from hundreds of zebrafish embryos to efficiently obtain high quality RNA samples for further analyses. The present method is based on the protocol reported by Burns and MacRae, 20068. For enrichment of cardiac tissue, both methods use a transgenic myocardial reporter line and take advantage of the differential adhesion properties of zebrafish embryonic hearts versus other tissues. Briefly, by pipetting many embryos up and down through a narrow pipette tip, hearts are simultaneously released from embryonic bodies and subsequently separated from embryonic debris in two rapid filtration steps; fluorescently labelled hearts are then manually sorted from remaining debris and collected for further processing.
Protocol
This protocol follows the animal care guidelines of the German and Berlin state law; zebrafish handling was monitored by the local authority for animal protection (LaGeSo, Berlin-Brandenburg).
1. Obtaining Zebrafish Embryos for Heart Extraction
- Cross cardiac reporter zebrafish such as the Tg(myl7:EGFP)twu34 transgenic line9 in order to obtain embryos with heart-specific GFP expression.
- Maintain the embryos in egg water at 28.5 °C until the desired embryonic stage10,11. Ensure that the collected embryo population is homogeneous both genetically and by developmental stage to limit variations in gene expression.
- Dechorionate the embryos manually.
2. Dissecting Zebrafish Embryonic Hearts
NOTE: Keep all the solutions on ice. If possible, do teamwork: one person dissects the hearts from the embryos while another person sorts the hearts under the fluorescence stereomicroscope. This allows the processing of several hundreds of embryos in a few hours.
- Transfer about 100 embryos into a 1.5 ml centrifugation tube. Anesthetize the embryos with tricaine (0.16 mg/ml in E3 medium). Proceed once the embryos are clearly anesthetized (they do not swim and sediment to the bottom of the tube, but their hearts are still beating).
- Remove the tricaine/E3 solution and wash the embryos once with 1 ml L-15/10% FBS medium and maintain on ice.
NOTE: The L-15/10% FBS medium is important to keep organs and cells alive during the entire dissection procedure until the hearts have been transferred into RNAlater or Trizol. - Add 1 ml L-15/10% FBS medium to the embryos and pipette up and down 5-8 times with a Round Gel Loading Tip until the yolk is completely disrupted. Pipette the embryos drop-wise onto the surface of the solution, so that the drop will burst and the embryos are gently disrupted.
NOTE: How vigorously and how often the embryos have to be pipetted up and down depends on the developmental stage of the embryos, i.e. more pipetting is needed at late stages (e.g. at 56 hpf). - For the first batch of dissected embryos, assess the integrity of the embryos and the proportion of dissected hearts under a stereomicroscope, since some hearts may remain attached to the embryos if too gently pipetted. Adjust the manner of pipetting for the next rounds of dissection accordingly.
NOTE: Use low retention pipette tips and microcentrifuge tubes to minimize hearts sticking to the plastic. - Apply the sample onto a 100 μm filter placed on a 50 ml centrifugation tube; rinse the 1.5 ml tube with 1 ml L-15/10% FBS medium and then apply this to the filter in order to minimize loss of the sample (some hearts might be sticking to the walls of the tube). Wash the filter twice with 1 ml L-15/10% FBS. In this step the hearts pass through the filter and are collected in the 50 ml tube.
- Apply the flow-through onto a 30 μm filter placed onto a 15 ml centrifugation tube and rinse the filter once with 1 ml L-15/10% FBS medium. In this second filtration step, the hearts are retained in the filter and smaller debris is washed off.
- Turn the filter upside down and flush the hearts out of the filter into a 1% agarose-coated petri dish by applying three consecutive washes with 1 ml L-15/10% FBS.
- Manually separate GFP-positive hearts from the nonfluorescent embryonic debris (e.g. eye lenses) with a pair of forceps under a fluorescence stereomicroscope and concentrate them in the center of the dish. Collect them according to step 3.
NOTE: If the embryos are older than 24 hpf, the hearts should be beating, indicating that the tissues are still physiologically normal.
3. Isolating mRNA from Dissected Hearts
- Collect the hearts in the smallest possible volume (e.g. 10 µl) and pipette into a 1.5 ml tube containing 0.75 ml RNAlater (on ice). Verify that no hearts remain in the pipette tip by viewing it under a fluorescence stereomicroscope.
- Alternatively, if a chemical hood is available in the immediate vicinity of the fluorescence microscope, transfer the collected hearts into Trizol (CAUTION) under the chemical hood. This circumvents the possible loss of hearts sticking in the pipette tip and also during centrifugation of the hearts (steps 3.4, 3.5 and 3.7). Pool the hearts from several rounds of isolation into the same tube with Trizol and go directly to step 3.8; the final volume should not exceed 10% of the original Trizol volume.
NOTE: Read the Trizol Material Safety Data Sheet (MSDS) before use. Handle Trizol reagent under a hood and wear recommended Personal Protective Equipment. Avoid contact with skin, eyes and clothing. Remove all sources of ignition. - Pool the hearts from several rounds of isolation into the same tube with RNAlater (on ice), as the efficiency of RNA isolation improves with the amount of tissue collected. If the total sample volume exceeds 10% of the original RNAlater volume, use an additional tube with 0.75 ml RNAlater for storage (on ice).
- Centrifuge the samples at 15,700 x g for 20 min at 4 °C to sediment the hearts.
- Under a fluorescence stereomicroscope, collect carefully as much supernatant as possible into a 1% agarose-coated petri dish containing 3 ml L-15/10% FBS medium, but do not disturb the sedimented hearts in the bottom of the centrifugation tube. Since up to 15% of the hearts can remain in the supernatant due to the high viscosity of RNAlater, retrieve them as described in step 3.7.
- Under a chemical hood, add 0.5 ml Trizol to the tube containing the sedimented hearts and keep on ice.
- Under the fluorescent microscope, transfer the hearts (from step 3.5) from the 1% agarose-coated dish containing the RNAlater/L-15/10% FBS solution into another 1% agarose-coated dish with 3 ml L-15/ 10% FBS to dilute out the RNAlater. Collect the hearts in the center of the petri dish and transfer them in a small volume into the tube of sedimented hearts in Trizol under a chemical hood.
- Vortex the 1.5 ml tube containing the hearts in Trizol to disrupt the hearts and incubate for 5 min at room temperature.
- Under a chemical hood, add 100 µl chloroform (CAUTION), mix thoroughly and transfer the Trizol/chloroform solution into a 1.5 ml pre-spun PLG tube (centrifuge 30 sec at maximum speed before use). Incubate for 3 min at room temperature.
NOTE: Read the chloroform MSDS before use. Handle chloroform reagent under a hood and wear recommended Personal Protective Equipment. Avoid contact with skin, eyes and clothing. Keep away from incompatibles such as metals, alkalis. - Centrifuge the sample at 15,700 x g for 15 min at 4 °C and transfer the aqueous phase into a new 1.5 ml tube.
- Add 5-10 µg glycogen and 250 µl precooled (at -20 °C) isopropanol; mix well and incubate over night at -20 °C.
- Centrifuge the sample at 15,700 x g for 30 min at 4 °C and discard the supernatant.
- Wash the pellet with 1 ml 75% ethanol, centrifuge at 15,700 x g for 15 min at 4 °C and discard the supernatant.
- Let the ethanol evaporate by drying the pellet at room temperature until it becomes white (e.g. for 10-15 min).
- Add 20 µl RNase-free ddH2O to the pellet and incubate for 10-15 min at 55 °C to dissolve the RNA; then keep on ice.
- Assess the RNA concentration and purity by absorbance measurement. Assess the RNA integrity by running the sample on a 1% agarose gel.
Representative Results
Here, we describe a representative heart dissection experiment using the zebrafish Tg(myl7:GFP)twu34 transgenic line9, which expresses green fluorescent protein (GFP) exclusively within the myocardium (Fig. 1). We collected both the dissected hearts and the embryos from which they were derived to assess the purity of the heart sample. Briefly, homozygous Tg(myl7:GFP)twu34 zebrafish9 were outcrossed with wild-type so that all embryonic hearts were GFP labeled. Some 500 embryos (56 hpf) were transferred into 1.5 ml tubes (approx. 100 embryos in each) (Fig. 1A1, 1B). Each tube was processed as described in the Protocol section (step 2) and in Figure 1A. Hearts were released by pipetting up and down several times (Fig. 1A2) and then applied onto a 100 μm filter (Fig. 1A3). Large pieces of embryonic tissue were retained in this filter; this fraction was retrieved and put in Trizol for RNA extraction to obtain an “embryo without heart” RNA sample (Fig. 1A3). The flow-through containing hearts was then applied onto a 30 μm filter (Fig. 1A4), which retained the hearts and tissue debris of similar size. GFP-positive hearts were flushed out of the filter into an agarose-coated petri dish (Fig. 1A5, 1C), quickly gathered in the middle of the dish under a fluorescent stereomicroscope and transferred into 0.75 ml RNAlater (Fig. 1A6). Within this tube with RNAlater, we pooled hearts derived from 5 dissection rounds (approx. 300 hearts from 500 embryos representing an extraction efficiency of about 60%). A comparison of the heart samples prior to sorting from the debris from embryos at 56 hpf (Fig. 1C) versus 36 hpf (Fig. 1C’) is shown in Figure 1. At 56 hpf, the disruption of embryos yielded more embryonic debris (such as lenses, arrowhead, Fig. 1C) than at 36 hpf following the 30 μm filtration step (Fig. 1C’).
To determine the purity of the 56 hpf “heart” sample, we compared the expression levels of cardiac versus extra-cardiac transcripts in “heart” and “embryo without heart” samples by RT-qPCR. To this end, we extracted mRNA from those two samples and assessed RNA quality on an agarose gel (Figure 2A) and quantity by absorbance measurement (Table 1) as described in the protocol section (step 3). The approx. 300 hearts yielded 660 ng RNA. Next, cDNA was synthesized using M-MLV Reverse transcriptase RNase H(-) and random hexamers according to Manufacturer´s instructions, and RT-qPCR experiments were performed as described12. Relative gene expression levels were calculated for different tissue-specific markers such as myosin light polypeptide 7 [myl7, a myocardial marker]9, kinase insert domain receptor like [kdrl, an endothelial/endocardial marker]13, hemoglobin beta embryonic-1.1 [hbbe1.1, an erythrocyte marker]14, crystallin-alpha A [cryaa, an eye lens marker]15, and glial fibrillary acidic protein [gfap, a central nervous system marker]16 (Figure 2B). The zebrafish eukaryotic translation initiation factor 1B [eif1b]12 was used as an internal reference gene (see Table 2 for GenBank ID numbers, primer sequences, PCR product sizes and tissue specificity for each gene). As expected, myl7 was highly enriched in the “heart” sample as compared to the “embryo without heart” sample (Fig. 2B). The endothelial cell marker kdrl was found to be moderately enriched in the heart sample, as expected (about 40% of the cardiac cells at 56 hpf are kdrl-expressing endocardial cells) (Fig. 2B). The erythrocyte marker hbbe1.1 was overrepresented in the “heart” sample, even though the hearts were still beating after dissection, which should expel red blood cells from the heart (Fig. 2B). In contrast, the expression levels of the CNS and lens markers (gfap and cryaa, respectively) were extremely low in the “heart” sample (Fig. 2B). Altogether, we conclude that the heart dissection protocol yields high quality and cardiac-specific RNA from entire zebrafish embryos.
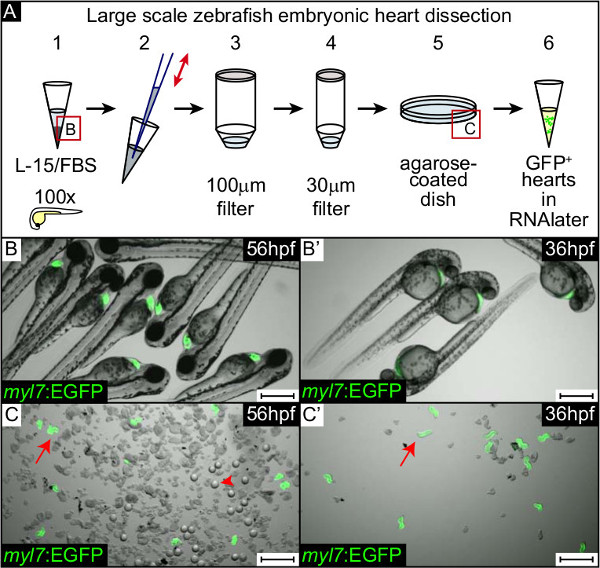
Figure 1. Heart extraction from zebrafish embryos. (A) Scheme depicting the heart extraction procedure. About 100 live embryos are collected in a 1.5 ml tube (A1, B,B’), anesthetized and then pipetted up and down several times through a narrow pipette tip to release the hearts (A2). The resulting solution containing the embryonic tissues (A2) is then applied onto a 100 μm filter (A3). Embryonic tissues without yolk and hearts, and large embryonic debris remain in the filter (A3). The flow-through is collected in a 50 ml tube (A3) and then applied onto a 30 μm filter (A4). The hearts and small debris are retained in the 30 μm filter and transferred into a small agarose-coated petri dish (A5, C,C’). EGFP labelled hearts (C,C’; arrow) are manually sorted from the remaining debris, such as lenses (C, arrowhead), and are collected in RNAlater (A6). This procedure is repeated at least 5 times and all hearts are pooled together for further processing. (B-C’) Overlay of fluorescence (EGFP in green) and DIC images of live transgenic Tg(myl7:EGFP)twu34 embryos at 56 hpf (B,C) and 36 hpf (B’,C’) at two steps of the heart dissection procedure: prior to embryo dissection (B,B’) and just after flushing from the 30 μm filter, prior to sorting the hearts from the debris in the petri dish (C,C’). Scale bar: 500 μm.
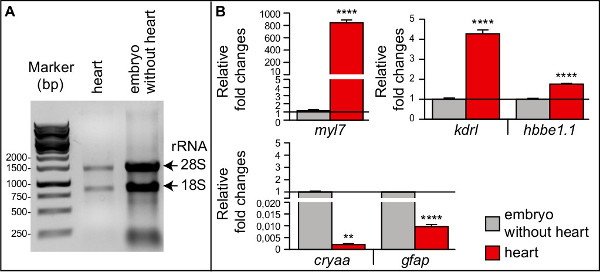
Figure 2. Analysis of RNA quality and purity by electrophoresis and RT-qPCR, respectively. (A) Total RNA from the “heart” sample (2 μl) and from the “embryo without heart” sample (1 μl), derived from 56 hpf embryos, resolved on a 1% agarose gel. Prominent S18 and S28 rRNA bands indicate that RNAs are not degraded. (B) Relative expression levels of the tissue specific markers myl7 (myocardium), kdrl (endothelium), hbbe1.1 (erythrocytes), cryaa (lens), and gfap (CNS) as determined by RT-qPCR for the “heart” sample normalized to the “embryo without heart” sample. The RT-qPCR experiments were performed as technical triplicates and data are given as means ± SEM. An unpaired t-test analysis was performed. **** p<0,0001; ** p<0,005. bp, base pair.
| sample ID | ng/μl | A260 | A280 | 260 / 280 | 260 / 230 | cursor abs. | 340 raw |
| hearts | 32.97 | 0.82 | 0.41 | 2.01 | 0.35 | 2.355 | 0.031 |
| embryos without hearts | 568.82 | 14.22 | 7.00 | 2.03 | 2.04 | 6.965 | 0.018 |
Table 1. RNA quantity and quality by spectrophotometric measurement. The quantity and quality of the RNA of the “heart” and “embryo without heart” samples obtained from 56 hpf embryos as measured by spectrophotometry.
| gene | GenBank # | primer sequence | PCR product size (bp) | Tissue-specific marker |
| myl7 | BX248505 | F: 5'-ACAGCAAAGCAGACAGTGAA-3' | 163 | myocardium |
| R: 5'-TAACTCCATCCCGGTTCTGA-3' | ||||
| kdrl | CR759732 | F: 5'-ACAACGACACTGGCATCTAC-3' | 170 | endothelium/ endocardium |
| R: 5'-TGTTCTACAGGGGACCACAA-3' | ||||
| gfap | BX324157 | F: 5'-AGACAACTTGGCCTCAGAC-3' | 247 | central nervous system |
| R: 5'-ATCCACATGAACCTGTTGGG-3' | ||||
| cryaa | BX248514 | F: 5'-ATCCAACACCCTTGGTTCAG-3' | 190 | eye lens |
| R: 5'-TCAGACCTCACCTCAGAGAC-3' | ||||
| hbbe1.1 | BC095024 | F: 5'-TGGTTGTGTGGACAGACTTCGA-3' 8 | 102 | erythrocyte |
| R: 5'-CGATAAGACACCTTGCCAGAGC-3' 8 | ||||
| eif1b | BX323079 | F: 5'-CAGAACCTCCAGTCCTTTGATC-3' 12 | 195 | reference gene |
| R: 5'-GCAGGCAAATTTCTTTTTGAAGGC-3' 12 |
Table 2. Primers used for the RT-qPCR experiments. Name, GenBank accession number, sequence, PCR product sizes and tissue-specificity are shown for all genes analyzed.
Discussion
This protocol allows the rapid enrichment of zebrafish embryonic heart tissue for gene expression analyses. The quantity and quality of the cardiac-specific RNA sample greatly depends on a few crucial steps: first, the quantity of the sample is greatly improved if loss of hearts is prevented at every step of the protocol, since RNA purification will only work with sufficient starting material. Second, the purity of the sample, which depends entirely on the experimenter, is determined by sorting and collecting hearts within the petri dish while strictly excluding other tissues. Third, sample quality critically depends on limiting RNA degradation which can occur upon disruption of the embryos; this is achieved by using cell culture medium to avoid cell death and by keeping the samples on ice. RNA degradation is stopped once the hearts have been transferred into RNAlater or Trizol. That hearts are beating in the petri dish powerfully demonstrates that the cardiac tissue is physiologically normal after dissection.
We recommend starting with at least 300 hearts (i.e. around 500 embryos), from which enough RNA can safely be precipitated (together with 5-10 µg glycogen). However, we cannot exclude than fewer hearts would suffice for small-scale experiments. It is simply important to have sufficient RNA to determine the concentration, purity and integrity of the sample. Please, note that RNA contents can vary depending on the developmental stage and on the genetic background of the embryo (e.g. in the case of mutants with abnormal hearts). Hence, the minimal amount of embryos required for an experiment would have to be determined in each individual case.
There are few technical differences between this protocol and an earlier protocol by Burns and MacRae, and we don’t think that there is a significant difference in efficiency or speed between the two protocols. However, we have introduced improvements: Most importantly, we suggest the use of RNAlater stabilization solution. This is important for two reasons: first, to collect high quality RNA for further experiments such as RT-qPCR by reducing RNA degradation to a minimum, as RNAlater immediately inactivates RNases and stabilizes RNA within tissues or cells. Second, RNAlater is crucial for allowing the collection of hearts on different days, which is important when the number of embryos is a limiting factor. It also allows pooling of the hearts which may be necessary to obtain a sufficient amount of starting material for efficient isolation of high-quality RNA with Trizol. Transferring the hearts directly into Trizol would bypass the use of RNAlater, however, due to health hazards, this step would have to be performed under a chemical hood, which cannot be assumed to be located in the direct vicinity of the fluorescence microscope for all experimenters.
We found that the heart dissection works well with embryos between 20-72 hpf [12; unpublished data for 72 hpf]. However, the extraction procedure becomes more difficult with increasing embryonic age and length: additional and more forceful pipetting is required to successfully introduce the embryos into the long tip and to separate the hearts from other embryonic tissues. As a result, more embryonic debris is present within the sample preparation (see Figure 1C, C’) and the experimenter has to be more careful in sorting the hearts in the petri dish.
The heart dissection protocol presented here allows an analysis of the entire cardiac expression profile of different cell types, including myocardium and endocardium. Further separation of cardiac cell types could be achieved by using endocardial- and myocardial-specific-reporter lines [e.g. Tg(myl7:GFP)twu34 and Tg(kdrl:mCherry)is5]9,17 for heart extraction combined with FACS sorting. In contrast to the Translating Ribosome Affinity Purification (TRAP) method18,19, which allows tissue-specific transcriptional analysis without tissue dissection, our approach is not limited to actively-translated mRNAs but also includes not-actively-translated mRNAs or noncoding RNAs. An alternative application of the heart dissection protocol is to analyze protein expression within hearts: protein levels can be assessed by Western blot and protein localization can be analyzed by immunohistochemistry, as the anatomy of the heart is preserved during the dissection procedure.
In summary, we present here a detailed protocol for dissecting functional/beating hearts from hundreds of embryos in a short time, which can be used for obtaining heart-specific RNA (or protein) samples of high purity for further analyses.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank C. Burns, C. McRae for outlining the basic principle of this purification protocol, and F. Priller for initial implementation of this method in our lab. S.A.-S. is supported by a Heisenberg professorship of the Deutsche Forschungsgemeinschaft (DFG). This work was supported by DFG grant SE2016/7-1.
Materials
| Equipment for raising fish and collecting eggs | see the Zebrafish Book11 for details | ||
| Fluorescence stereomicroscope | |||
| Refrigerated Microcentrifuge | |||
| UV-Spectrophotometer | eg. Thermo Scientific Nanodrop 2000 | ||
| Nucleic acid electrophoresis chamber | |||
| Petri dishes 4cm Ø, coated with 1% agarose in E3 medium | |||
| Micropipettes and tips (P20, P100, P1000) | |||
| 1,5ml centrifugation tubes | |||
| 15ml and 50ml centrifugation tubes | |||
| Pair of Dumont #5 forceps | |||
| ExactaCruz™ Round Gel Loading Tips in Sterile Rack, 1-200μl | Santa Cruz | sc-201732 | |
| 100 μm filter (BD Falcon 100 mm Cell Strainer) | BD Biosciences | 352360 | |
| 30 μm filter (Pre-Separation Filters-30 µm) | Miltenyi Biotec | 130-041-407 | |
| Phase lock gel, heavy, 1,5ml tubes | Prime | 2302810 | |
| Egg water medium | 60μg/ml Instant Ocean Sea Salts in ddH2O, 0.00001% (w/v) Methylene Blue | ||
| E3 medium | 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4 | ||
| Tricaine (3-amino benzoic acidethylester) | Sigma-Aldrich | A-5040 | 4mg/ml Tricaine stock solution, pH 7 |
| 1% agarose (in E3 medium) | |||
| 1% agarose gel (in TBE buffer) | |||
| Leibovitz´s L-15 medium | Gibco | 21083-027 | |
| FBS (Fetal Bovine Serum) | Sigma | F4135 | |
| RNAlater | Ambion | AM7020 | |
| Trizol | Ambion | 1559606 | |
| Glycogen | Invitrogen | 10814-010 | 20 µg/µL in RNase-free water |
| chloroform | |||
| isopropanol | |||
| 75% ethanol (in DEPC-ddH2O) | |||
| Nuclease-free water or sterilized DEPC treated ddH2O | |||
| Nucleic acid loading buffer | |||
| TBE (Tris/Borate/EDTA) buffer for electrophoresis |
References
- Stainier, D. Y., et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 123, 285-292 (1996).
- Chen, J. N., et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 123, 293-302 (1996).
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res. 91, 279-288 (2011).
- Jowett, T., Lettice, L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 10, 73-74 (1994).
- Gibson, U. E., Heid, C. A., Williams, P. M. A novel method for real time quantitative RT-PCR. Genome Res. 6, 995-1001 (1996).
- Epstein, C. B., Butow, R. A. Microarray technology – enhanced versatility, persistent challenge. Curr Opin Biotechnol. 11, 36-41 (2000).
- Qian, X., Ba, Y., Zhuang, Q., Zhong, G. RNA-Seq Technology and Its Application in Fish Transcriptomics. OMICS. 18, 98-110 (2014).
- Burns, C. G., MacRae, C. A. Purification of hearts from zebrafish embryos. Biotechniques. 40, 274-278 (2006).
- Huang, C. J., Tu, C. T., Hsiao, C. D., Hsieh, F. J., Tsai, H. J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 228, 30-40 (2003).
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., Schilling, T. F. Stages of embryonic development of the zebrafish. Dev Dyn. 203, 253-310 (1995).
- Westerfield, M. . The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). , (2000).
- Veerkamp, J., et al. Unilateral dampening of Bmp activity by nodal generates cardiac left-right asymmetry). Dev Cell. 24, 660-667 (2013).
- Jin, S. W., Beis, D., Mitchell, T., Chen, J. N., Stainier, D. Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 132, 5199-5209 (2005).
- Brownlie, A., et al. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 255, 48-61 (2003).
- Marvin, M., et al. Developmental expression patterns of the zebrafish small heat shock proteins. Dev Dyn. 237, 454-463 (2008).
- Lam, C. S., Marz, M., Strahle, U. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev Dyn. 238, 475-486 (2009).
- Wang, Y., et al. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 137, 3119-3128 (2010).
- Heiman, M., et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 135, 738-748 (2008).
- Tryon, R. C., Pisat, N., Johnson, S. L., Dougherty, J. D. Development of translating ribosome affinity purification for zebrafish. Genesis. 51, 187-192 (2013).

