Three-dimensional Quantification of Dendritic Spines from Pyramidal Neurons Derived from Human Induced Pluripotent Stem Cells
Summary
Dendritic spines of pyramidal neurons are the sites of most excitatory synapses in mammalian brain cortex. This method describes a 3D quantitative analysis of spine morphologies in human cortical pyramidal glutamatergic neurons derived from induced pluripotent stem cells.
Abstract
Dendritic spines are small protrusions that correspond to the post-synaptic compartments of excitatory synapses in the central nervous system. They are distributed along the dendrites. Their morphology is largely dependent on neuronal activity, and they are dynamic. Dendritic spines express glutamatergic receptors (AMPA and NMDA receptors) on their surface and at the levels of postsynaptic densities. Each spine allows the neuron to control its state and local activity independently. Spine morphologies have been extensively studied in glutamatergic pyramidal cells of the brain cortex, using both in vivo approaches and neuronal cultures obtained from rodent tissues. Neuropathological conditions can be associated to altered spine induction and maturation, as shown in rodent cultured neurons and one-dimensional quantitative analysis 1. The present study describes a protocol for the 3D quantitative analysis of spine morphologies using human cortical neurons derived from neural stem cells (late cortical progenitors). These cells were initially obtained from induced pluripotent stem cells. This protocol allows the analysis of spine morphologies at different culture periods, and with possible comparison between induced pluripotent stem cells obtained from control individuals with those obtained from patients with psychiatric diseases.
Introduction
Dendritic spines of cortical pyramidal neurons are small and thin protrusions which are distributed along the basal and apical dendrites of these neuronal subtypes in rodent, primate, and human brain. They are the sites of most excitatory synapses and display key functions in learning and cognitive processes. The detailed structures of human dendritic spines have been technically studied by electron microscopy 2. However, such approach is time-consuming and represents heavy workload. More recently, a three-dimensional (3D) reconstruction of the morphology of dendritic spines has been reported in human brain cortex using specific software combined to large manual spine analysis3.
Green fluorescence protein (GFP) technology coupled to immunofluorescence represents an accurate tool for spine identification and shape measurement by fluorescence microscopy. This approach can be easily applied to cultured neurons. However, no data have been reported on the analysis of spine maturation and morphology on human neurons derived from induced pluripotent stem cells (iPSC).
The objective of this study was to describe a protocol, which allows dendritic spine imaging from cultured human neurons in vitro. GFP labeling, confocal microscopy and 3D analysis with the Filament Tracer module of Imaris software were used in the present protocol. Culture steps that are necessary to obtain cortical glutamatergic neurons of layers II to IV from neural stem cells (NSC) are also briefly described here. The entire protocol for human NSC production has already been published elsewhere 4.
Protocol
1. Neuronal Culture
Note: Fibroblast reprogramming in pluripotent stem cells, commitment to the dorsal telencephalon lineage, derivation, amplification, and banking of late cortical progenitors (LCP) were described in Boissart et al4. Neuronal differentiation of LCP-like cells was also performed according to Boissart et al4 with slight modifications. Other procedures have been developed for direct reprogramming of fibroblasts into induced pluripotent stem cells followed by their differentiation into neurons. This protocol was retained since it allows the selective production of pyramidal glutamatergic neurons.
- Treat 6-well culture plates with glass coverslips with poly-ornithine (diluted to 1/6 in DPBS, stock concentration 0.01%) O/N, followed by three washes in DPBS. Then add laminin (stock concentration 1 mg/ml, diluted 500 times in DPBS) for at least 10 hr under the flow hood.
- Plate and dispatch NSC at low density (50,000 cells/cm2) in 6-well culture plates with glass coverslips in 3 ml of culture medium consisting of DMEM/F12 (500 ml), 2 vials (5 ml each) of N2 supplement, 2 vials (10 ml each) of B27 supplement, 10 ml of Pen-Streptomycin (Penicillin = 10,000 units/ml and Streptomycin = 10,000 units/ml), 1 ml of 2-mercaptoethanol (Stock solution: 50 mM) and laminin (1/500), without growth factors. CRITICAL STEP: Carry out this step carefully by adding cells with slow rotating movements in order to reduce cell clustering.
- Remove the culture medium. Add fresh N2B27 medium containing 2 µg/ml of fresh laminin solution to keep the neuron attached on the glass coverslips and avoid clumping. Change the medium every 3 days. Keep some of the remaining medium (200 µl) before adding fresh medium in order to prevent the cell from drying. Alternatively, proceed rapidly and change the total volume (3 ml).
2. Lentiviral Transduction
Note: GFP lentiviral vectors were kindly provided by Dr. Uwe Maskos Laboratory at Institut Pasteur (Paris) and prepared according to the published protocol 5. GFP expression is driven by the mouse phosphoglycerate kinase (PGK) promoter. For this study, viral titer was of 400 ng/µl (stock solution in PBS 1x).
- Transduce human iPSC-derived neurons at any step of maturation by viral particles by adding 1 µl of the stock solution containing 40 ng of GFP lentiviral vector per culture well (6-well plates) and incubate for 48 hr in fresh culture medium. Note: For this protocol, the duration of incubation allows a good labeling of spine structures.
3. Immunofluorescence
Note: In order to improve the labeling of the whole spine morphology, immunofluorescence labeling was performed using an anti-GFP antibody in permeabilized conditions.
- Remove culture medium and fix transduced cells on coverslips in 4% paraformaldehyde for 10 min at RT, then wash 3 times in 1x PBS (10 min each).
- Immerge coverslips in PBS supplemented with 0.05% Triton (100x) and 10% horse serum for 1 hr at RT, then wash 3 times in 1x PBS.
- Add 100 µl of 1x PBS supplemented with 4% horse serum and primary antibody (1/1,000) raised against GFP and diluted by a factor of 1,000 on each coverslip. Incubate in a dark box O/N at 4°C, then wash 3 times with 1x PBS.
- Dilute Alexa Fluor 488-conjugated antibody (1/200) in PBS supplemented with 0.5% of Tween 20 and incubate for 1 hr at RT. Then wash 3 times in 1x PBS and mount coverslips on glass slides with mounting medium for fluorescence microscopy.
4. Dendritic Spine Imaging
- Perform confocal Imaging through a confocal laser-scanning microscope.
- Select healthy neurons with pyramidal morphology and a full dendritic arborization, and quantify at least 10 neurons per condition from separate experiments. Quantify 60 – 100 µm per dendrite.
- Acquire images using a 40X oil NA = 1.3 objective and a 488 nm laser line for GFP excitation, with a typical peak power at sample level around 20 µW. Set pixel size around 80 nm to properly sample dendritic spines.
Note: The subsequent analysis call for an image in which the noise does not compromise the proper segmentation of dendrites and spines. With the spatial sampling and power settings mentioned above, we observed that a pixel dwell-time of 3.15 µs is sufficient to collect enough photons to build such an image. For dim samples, image quality can be improved by averaging 2 to 4 scans. The acquisition of several XY tiles may be needed to cover the region of interest, which then must be stitched together before processing. - To sample the whole neuron volume, acquire a Z-stack, with a Z spacing ranging from 150 nm to 300 nm, yielding 20 to 30 Z slices.
Note: The lateral spatial resolution achieved with these settings is 234 nm and the axial resolution is 591 nm. The sampling chosen here is adequate, but as the axial resolution is larger than the smallest size of the spines to be imaged, the analysis favors the spines that extend laterally from the dendrites.
5. 3D Quantification of Dendritic Spines
Note: The following sections specifically describe the use of the Imaris software for analysis. Alternative implementations exist, including NeuronStudio15 or Metamorph8, which can provide similar results.
Use the following key settings:
- As a pre-processing stage, use Gaussian filtering through the image processing facilities offered by the software. Perform Gaussian filtering via the Image Processing > Smoothing > Gaussian Filter. Set the filter width to be equal to the pixel size in XY.
- Perform a Semi-automatic tracing of dendrites, using the Filament Tracer module of the software.
- First, estimate the dendrite diameter, by using the distance tool in the Slice tab of the software.
- In the Surpass tab, click on the Filaments tool. For a better robustness, this protocol relies on semi-automated tracking; click on Skip automatic creation. The module interface is now showing the Draw tab. Here, select AutoPath as a method, Dendrite as a type, and input the estimated dendrite diameter.
- Use the Select mode of the pointer, turning the cursor into a box. Shift-right-click on the dendrite starting point. Note: the software performs initial calculations.
- Move the pointer along the dendrite. From the starting point (represented as a blue sphere), a yellow line representing the most likely dendrite path is shown. Shift-left-click on the dendrite endpoint.
- Perform the automated spines segmentation. Note: The spines on the traced dendrite are to be found automatically by the module interface.
- In the module interface, click on the Creation tab. In the Rebuild drop-list, pick Rebuild Dendrite Diameter and check the Keep data box. Then click Rebuild.
- Set the Threshold so that the segmented volume corresponds to the actual dendrite volume. As an Algorithm, select Shortest Distance from Distance Map. Click the Next button.
- Determine the smallest spine head diameter and the maximal length, again by using the distance tool in the Slice tab of the software, then come back to the Surpass tab and enter the parameters. For this protocol, values around 200 – 300 nm for minimal diameter and 4 µm for maximal length are good starting points. Do not check the Allow Branch Spines box. Click the Next button.
- Adjust the Seed Points Threshold so that the blue points representing spines localize to actual spine heads. Click the Next button. Note: The core calculation is done now and can be lengthy.
- Classify spines by going to the Tools tab of the module interface. Click on Classify Spines. In the prompted user interface, make sure that there are four classes, defined by their morphology as follows: Stubby: length <1 µm; Mushroom: Length (spine) >3 and Max width (head) >mean width (neck) x 2; Long thin: Mean width (head) ≥ Mean width (neck); Filopodia-like: Length ≤ 4 µm (no head).
Note: The module interface generates four new Filament objects containing the results of the classification. - Export Statistical data: on any of these four object interfaces, go to the 통계학 tab.
Click on the Export All Statistics to File button.
Note: Other statistical values can be exported for dendrites (e.g., length, area, mean diameter, branch depth, branching angle, volume, etc.), and for spines (e.g., straightness, area of attachment, length and volume of distinct spine parts, spine diameter, densities, etc.)
Representative Results
The present study describes a standardized protocol for spine quantification of cultured dendrites of pyramidal neurons derived from iPSC. This protocol allows the analysis of spine maturation on human neurons and its possible comparison with the maturation of spines in standard rodent neuronal cultures as well as in in vivo animal models.
Figure 1A represents a scheme of the different steps of culture which allow the production of cortical pyramidal neurons. Such schematic representation is provided in order to better understand the global time scaling of the production of pyramidal neurons endowed with different categories of spines. Reprogrammation steps, however, are out of the scope of this study and have been described elsewhere 4. Healthy neurons can be kept in culture up to 65 – 70 days. Figure 1B show the workflow of imaging and 3D quantification of spines.
Figure 2A illustrates the culture of human neurons labeled with an anti-beta III tubulin antibody. This figure also shows the tendency of cell clustering. Figure 2B shows a GFP-labeled pyramidal neuron of the superficial cortical layers at 40 days post differentiation of late cortical progenitors (LCP).
Figure 2C and Figure 3A illustrate 3D reconstruction of segments of dendritic spines at different stages of maturation. The quantitative analysis of two selected parameters (spine densities and spine head volume) is represented in Figure 3B. Our results indicate that these two parameters increase over the culture period as expected.
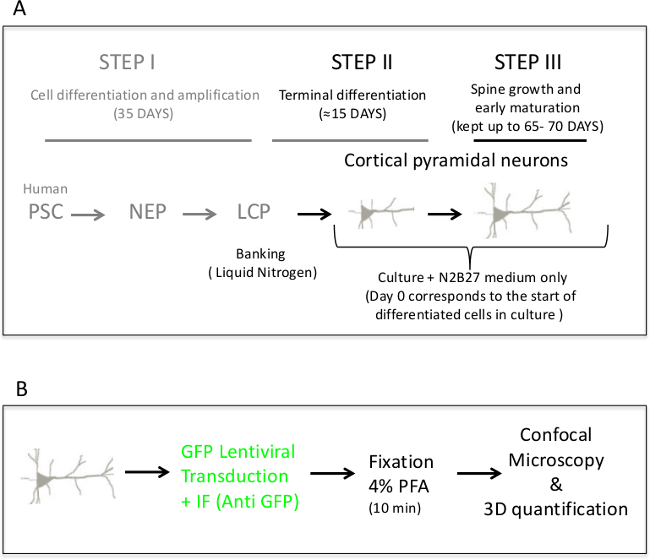
Figure 1. (A) Overview of the timescale of neuronal differentiation. This schematic representation describes the three steps of neuronal differentiation. The protocol is adapted from Boissart et al. 4. In step 1, human iPSC are derived in early cortical progenitors (i.e., early neuro-epithelial cells from the dorsal telencephalon) followed by transformation to late cortical progenitors (LCP). LCP are then amplified for cell banking in liquid nitrogen. In step 2, neural differentiation is achieved within two weeks. Step 3 corresponds to maintaining the neurons in culture for longer periods, in order to follow spine growing and progressive maturation. Under the culture conditions, healthy neurons can be kept up to 65 – 70 days. (B) Workflow of the different steps for imaging and 3D quantification of spines. (IF: immunofluorescence). Please click here to view a larger version of this figure.
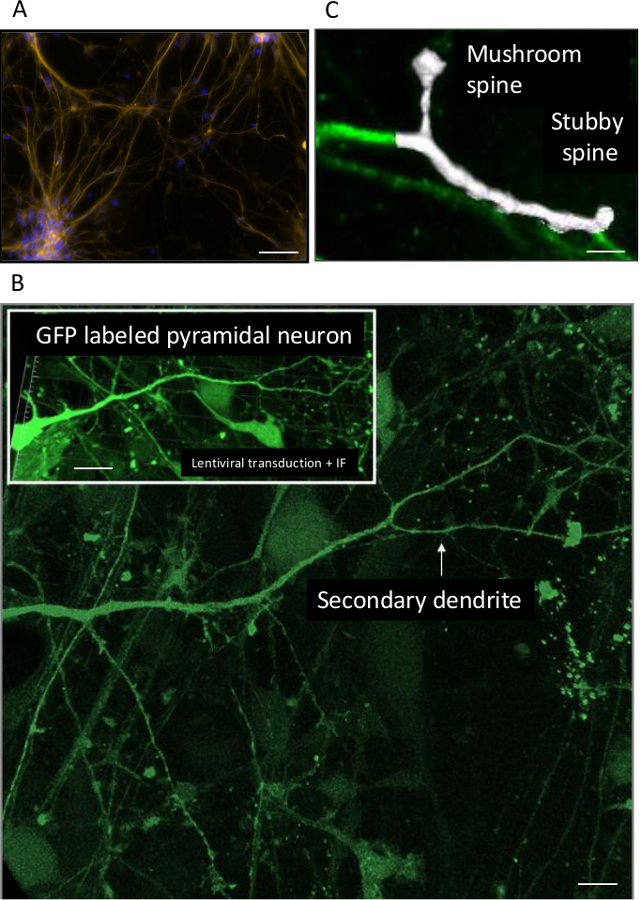
Figure 2. (A) Beta III tubulin positive cells revealed by Immunofluorescence using the same protocol as described, and a polyclonal anti-beta III tubulin antibody used at a dilution of 1/1,000. (B) Primary and secondary dendritic ramification of a GFP-labeled pyramidal neuron cultured 40 days post LCP stage. In the inset, the same neuron is represented at a lower magnification with apparent cell body. (C) 3D reconstruction of two categories of spines on a segment of a secondary dendrite. Scale bars: 250 µm (A), 100 µm (B), 40 µm (B inset), 1.5 µm (C). Please click here to view a larger version of this figure.
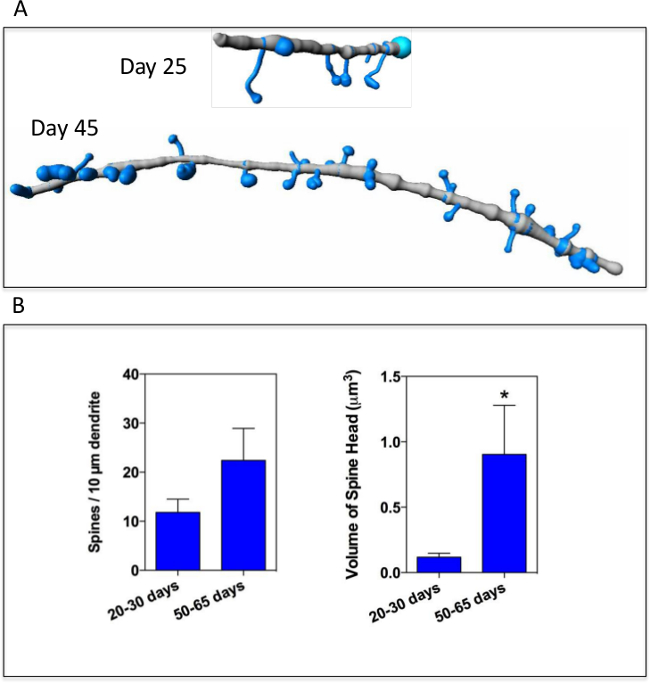
Figure 3. (A) 3D reconstruction of dendritic spines (blue color) with illustrated segments of secondary dendrites (grey color), at two different culture stages (25 and 45 days post-differentiation from LCP). (B) Quantitative analysis of spine densities and morphologies with two selected parameters such as spine density along dendrite and spine head volume and at two culture periods. Results are presented as mean ± SEM of at least 10 distinct neuron segments obtained from secondary dendrites imaged by confocal microscopy. Statistical analysis was performed using a Mann-Whitney test (* p<0.05). Please click here to view a larger version of this figure.
Discussion
The quantification of the morphological features of pyramidal neurons relied on the software. The Filament Tracer interface was used for segmentation of neurons and spines, and the XT module was used for their analysis.
To analyze the accuracy of our technique, we first compared the measured morphologic parameters (length, area, and total spine volume when applicable), with those published using rat mature pyramidal neurons in culture 6, 7 and human brain tissues 3. Densities were comparable in all cases. No volume data were described in rat neurons (a 2D analysis was reported with the software used by the authors) whereas total spine volume was slightly reduced as compared to our data, in the study of Benavides-Piccione 3 using their own protocol for volume reconstruction on selected spines. Such differences could also be explained by the regional origin of cells and the fluorescent dye used for their labeling.
Some critical points should be underlined in our protocol, which mainly refer to threshold definitions for confocal quality of images and subsequent quantifications. The coupling of the transduction of a GFP-lentiviral vector with anti-GFP immunofluorescence ensures a good labeling and visualization of the entire spine structures. We were not able to label the full dendritic arborization by transfecting reprogrammed cells with GFP vectors and by conventional protocols. Typically, 70 – 80% are transduced under our experimental conditions. Such percentage is much greater than what we observed using transfection protocols with GFP plasmids (15% of transfected neurons in our tests).
Depending on the efficiency and specificity of labeling, Gaussian filtering or deconvolution may be useful as a pre-processing stage to diminish noise. We also tested the 3D image reconstruction and quantification after deconvolution. This process was expected to be useful because, even with confocal imaging, deconvolution considerably improves image quality and limits blurring caused by diffraction. Deconvolution however, puts a major strain on the imaging step, imposing harsh spatial sampling in all three directions. With optical setup used in this protocol, the desired pixel size for deconvolution is around 40 nm, while we set for about 80 nm for the images presented here. Moving to 40 nm would quadruple the acquisition time, and lower the photon yield pixel. In addition, under the experimental conditions, no significant improvement in the segmentation results was observed as compared to the results, which may have been obtained with Gaussian filtering. The protocol therefore relies on this simpler post-processing step and relaxed the Z sampling constraints during imaging. This in turn diminished the acquisition time and sample bleaching.
The Filament Tracer module offers a fully automatic segmentation. However in this type of culture, the neurons are often dense, cross over each other, and are imaged from a strong background. This adversely affects the accuracy of automatic segmentation. The semi-automatic segmentation led to more robust results and efficient processing. This consists of a guided tracing of dendrites from the basal body of the neuron, followed by an automatic 3D segmentation of spines along selected dendrites.
Our method could be used to perform time-lapse imaging and directly record spine maturation in living neurons from primary rat cortical neurons, as described previously 8.
Human iPSC-derived neurons have drawn the attention of the scientists and there have been recent attempts to model neurodevelopmental disorders including autism spectrum disorders 9-14. In cortical circuits, dendritic spines play a major role in the establishment of excitatory synapses, but their quantitative analysis has been poorly documented in humans with neurodevelopmental disorders. During development and throughout lifespan, the densities of spines and their morphologies are critical for connectivity of neuronal circuits. In pathologies with genetic causes, the use of iPSC-derived neurons from patient biopsies (e.g., fibroblasts) allows the analysis of circuits development between neurons expressing the mutated gene(s) that could be responsible for disease states. This protocol represents a powerful approach for extensive in vitro analysis of spinogenesis within a population of mutated neurons and comparison of phenotypes with reprogrammed neurons from control individuals.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by the Institut Pasteur, the Bettencourt-Schueller foundation, Centre National de la Recherche Scientifique, University Paris Diderot, Agence Nationale de la Recherche (ANR-13-SAMA-0006; SynDivAutism), the Conny-Maeva Charitable Foundation, the Cognacq Jay Foundation, the Orange Foundation, and the Fondamental Foundation. L.G. is supported by an undergraduate fellowship from the Health Ministry. We acknowledge the help of BitPlane in particular Georgia Golfis, in the early stage of this work.
Materials
| PD-PBS (1X), sans Calcium, Magnesium et Phenol Red | Gibco/ Life Technologies | 14190169 | |
| Poly-L-Ornithine Solution Bioreagent | Sigma Aldrich | P4957 | |
| Mouse laminin | Dutscher Dominique | 354232 | |
| N2 Supplement | Gibco/ Life Technologies | 17502048 | |
| B-27 Supplement w/o vit A (50X) | Gibco/ Life Technologies | 12587010 | |
| DMEM/NUT.MIX F-12 W/GLUT-I | Gibco/ Life Technologies | 31331028 | |
| Neurobasal Med SFM | Gibco/ Life Technologies | 21103049 | |
| 2-mercaptoethanol | Gibco/ Life Technologies | 31350-010 | |
| Pen-Steptomycin | Gibco/ Life Technologies | 15140-122 | |
| GFP Rabbit Serum Polyclonal Antibody | Gibco/ Life Technologies | A-6455 | |
| Horse serum | Gibco/ Life Technologies | 16050130 | |
| Alexa Fluor 488 Goat Anti-Rabbit | Gibco/ Life Technologies | A11034 | |
| Polyclonal Anti-betaIII tubulin antibody | Millipore | AB9354 | |
| Coverglass 13 mm | VWR | 631-0150 | |
| Prolong Gold Antifade Reagent avec DAPI | Gibco/ Life Technologies | P36931 | |
| Tween(R) 20 Bioextra, Viscous Liquid | Sigma Aldrich Chimie | P7949 | |
| Triton X-100 | Sigma Aldrich Chimie | X100-100ML | |
| Human Fibroblasts | Coriell Cell Line Biorepository | GM 4603 and GM 1869 | Coriell Institute for Medical Research, Camden, NJ, USA |
| Confocal laser scanning microscope | Zeiss (Germany) | LSM 700 | |
| Imaris Software | Bitplane AG, Zurich | 6.4.0 version | Filament Tracer and Imaris XT modules are necessary |
| Huygens Software | Huygens software, SVI, Netherlands | Pro version | Optional (for deconvolution testing) |
References
- Durand, C., et al. SHANK3 mutations identified in autism led to modification of dendritic spine morphology via an actin-dependent mechanism. Molecular Psychiatry. 17 (1), 71-84 (2013).
- Arenallo, J. I., Espinosa, A., Fairen, A., Yuste, R., Defelipe, J. Non-synaptic dendritic spines in neocortex. 신경과학. 145, 464-469 (2007).
- Benavides-Piccione, R., Fernaud-Espinosa, I., Robles, V., Yuste, R., DeFelipe, J. Age-based comparison of human dendritic spine structure using complete three-dimensional reconstructions. Cerebral Cortex. 23 (8), 1798-1810 (2013).
- Boissart, C., et al. Differentiation from human pluripotent stem cells of cortical neurons of the superficial layers amenable to psychiatric disease modeling and high-throughput drug screening. Translational Psychiatry. 3, 1-11 (2013).
- Avale, M. E., et al. Interplay of beta 2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proceedings of National Academy of Science USA. 105 (41), 15991-15996 (2008).
- Xie, Z., et al. Coordination of synaptic adhesion with dendritic spine remodeling by AF6 and kalirin-7. Journal of Neuroscience. 28 (24), 6079-6091 (2008).
- Srivastava, D. P., et al. Afadin is required for maintenance of dendritic structure and excitatory tone. Journal of Biological Chemistry. 287 (43), 35964-35974 (2012).
- Srivastava, D. P., Woolfrey, K. M., Penzes, P. Analysis of dendritic spine morphology in cultured CNS neurons. Journal of Visualized Experiments. (53), e2794 (2011).
- Brennand, K. J., Gage, F. H. Modeling psychiatric disorders through reprogramming. Disease Models and Mechanisms. 5 (1), 26-32 (2012).
- Kim, S. S., Ross, P. J., Zaslavsky, K., Ellis, J. Optimizing neuronal differentiation from induced pluripotent stem cells to model ASD. Frontiers in Cellular Neuroscience. 8, 1-16 (2014).
- Inoue, H., Nagata, N., Kurokawa, H., Yamanaka, S. iPS cells: a game changer for future medicine. EMBO Journal. 33 (5), 409-417 (2014).
- Stein, J. L., et al. Aquantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron. 83, 69-86 (2014).
- Sivapatham, R., Zheng, X. Generation and characterization of patient-specific induced pluripotent stem cell for disease modeling. Methods in Molecular Biology. , (2014).
- Xu, X., Miller, E. C., Pozzo-Miller, L. Dendritic spine dysgenesis in Rett Syndrome. Frontiers in Neuroanatomy. 8, 1-8 (2014).
- Rodriguez, A., Ehlenberger, D. B., Dickstein, D. L., Hof, P. R., Wearne, S. L. Automated Three Dimensional Detection and Shape Classification of Dendritic spines from Fluorescence Microscopy Images. PLoS ONE. 3 (4), e1997 (2008).

