The Complete and Updated “Rotifer Polyculture Method” for Rearing First Feeding Zebrafish
Summary
Larval zebrafish are adapted to feed on zooplankton. It is possible to capitalize on this natural feature in the laboratory by growing first feeding fish together in the same system with live saltwater rotifers. This “polyculture” strategy promotes high growth and survival with minimal labor and disturbance to the larvae.
Abstract
The zebrafish (Danio rerio) is a model organism of increasing importance in many fields of science. One of the most demanding technical aspects of culture of this species in the laboratory is rearing first-feeding larvae to the juvenile stage with high rates of growth and survival. The central management challenge of this developmental period revolves around delivering highly nutritious feed items to the fish on a nearly continuous basis without compromising water quality. Because larval zebrafish are well-adapted to feed on small zooplankton in the water column, live prey items such as brachionid rotifers, Artemia, and Paramecium are widely recognized as the feeds of choice, at least until the fish reach the juvenile stage and are able to efficiently feed on processed diets. This protocol describes a method whereby newly hatched zebrafish larvae are cultured together with live saltwater rotifers (Brachionus plicatilis) in the same system. This polyculture approach provides fish with an “on-demand”, nutrient-rich live food source without producing chemical waste at levels that would otherwise limit performance. Importantly, because the system harnesses both the natural high productivity of the rotifers and the behavioral preferences of the fish, the labor involved with maintenance is low. The following protocol details an updated, step-by-step procedure that incorporates rotifer production (scalable to any desired level) for use in a polyculture of zebrafish larvae and rotifers that promotes maximal performance during the first 5 days of exogenous feeding.
Introduction
The zebrafish (Danio rerio) is a pre-eminent laboratory animal utilized in a growing number of scientific disciplines, including but not limited to developmental genetics, toxicology, behavior, aquaculture, regenerative biology, and the modeling of many human disorders1–5. Although the species is relatively easy to maintain in the laboratory, there are a number of management challenges associated with their culture6. The most prominent of these is larval rearing, particularly when the fish first begin to feed subsequent to gas bladder inflation7. Under normal, controlled conditions, this developmental event occurs at ~5 days post-fertilization (dpf), with the following 3 – 5 days of growth being particularly critical7. The central technical difficulty during this stage is to adequately meet the nutritional demands of the first feeding larvae – feed items must be appropriately sized, digestible, attractive, and available on a nearly continuous basis, without creating excessive waste in culturing tanks. Historically this has been achieved typically by delivering numerous small amounts of feed to the fish in tanks, along with routine water exchange8,9. While these methods are to some degree successful, they are inefficient, require high labor inputs, and return only variable and limited rates of growth and survival10.
In nature, zebrafish larvae presumably feed on abundant small zooplankton present in the water column11. For this reason, larviculture protocols that incorporate live feeds such as Paramecium, rotifers, and Artemia are typically most efficient7. In 2010, Best and co-authors demonstrated that it was possible to grow larval zebrafish in static, brackish water along with saltwater rotifers for the first 5 days of exogenous feeding12. This approach, which harnesses the natural high productivity of rotifer cultures to provide ample, highly nutritious prey without polluting the water, yields very high rates of larval growth and survival with low labor input12,13. In recent years, an increasing number of laboratories around the world have adopted variations of this protocol, and many are now culturing rotifers in a continuous fashion to support nursery systems14.
Over the past several years, methods for both rotifer/zebrafish polyculture and rotifer production have been refined and improved to become more standardized and readily scalable. This article provides step-by-step instructions for 1) continuous and robust rotifer production and 2) the establishment of the rotifer/zebrafish polyculture system used to support robust growth of fish for the first 5 days of exogenous feeding.
Protocol
1. Rotifer Culture
- Basic Components of a Culture System using a 100 L Culture Vessel
- Gather all the components for the rotifer culture setup . The rotifer culture setup consists of a culture vessel (CV) to grow the rotifers; a similar vessel to maintain feedout rotifers (feedout culture vessel, FCV); a round-bottomed hatching jar (Feed Reservoir, FR) for storage of the algae feed mixture (AFM); an air supply (AS) to aerate the CV, FCV and the FR; a peristaltic pump with a metering timer (PMT) to control delivery of algae feed into the CV and FCV; and a floss particle filter (FPF) that sits inside the CV.
NOTE: A complete list of supplies and components is provided in the Materials List.
- Gather all the components for the rotifer culture setup . The rotifer culture setup consists of a culture vessel (CV) to grow the rotifers; a similar vessel to maintain feedout rotifers (feedout culture vessel, FCV); a round-bottomed hatching jar (Feed Reservoir, FR) for storage of the algae feed mixture (AFM); an air supply (AS) to aerate the CV, FCV and the FR; a peristaltic pump with a metering timer (PMT) to control delivery of algae feed into the CV and FCV; and a floss particle filter (FPF) that sits inside the CV.
- Configuration
- Elevate the CV and FCV on a stand or table so that the cultures may be easily harvested via a drain fitting into a collection container (Figure 1). Use flexible air supply tubing to connect the AS to a length of rigid tubing in each culture vessel. Make sure that the tubing is long enough to deliver air to the bottom of the CV or FCV.
- Use a small-capacity air line to connect the AS to a length of rigid tubing that extends to the bottom of the FR that contains the AFM. Install a valve into each air line to regulate air flow. Connect the FR to the PMT with feed delivery tubing, and run the tubing from the PMT into a hole drilled into the side of the CV/FCV, near the top. Figure 1.
- Startup
- Fill the culture vessel to 90% of the available volume with Reverse Osmosis water (RO). If RO is not available, use clean, dechlorinated municipal water; however, a biosecurity risk assessment should be performed to ensure that no potentially pathogenic organisms are present in the source water. NOTE: Such an analysis can be performed by any qualified water testing laboratory.
- Dose the culture vessel water with aquarium salt to reach a salinity of 15 g/L. Set the air flow into the vessel so that it maintains a "rolling boil", and then slowly add the measured amount of salt to the culture vessel until it is completely dissolved by the aeration. Continue aerating the water for >1 hr to ensure that it is fully oxygenated.
- Make the algae feed mixture. To 3 L of clean, dechlorinated fresh (0 ppm) water add 100 g of NaHCO3 and 100 g of ammonia neutralizer (sodium hydroxymethylsulfonate). This last reagent provides the additional benefit of neutralizing any residual chlorine from tap water or bleach residues from sanitizing of culture equipment. It is critical to ensure that these compounds are fully dissolved. Then add 1 L of algae concentrate (biomass dry weight ~15%). Add the feed mixture to the FR and store at 4 °C.
- Add a starter culture of 5 – 10 million Brachionus plicatilis rotifers to the CV containing the aerated 15 g/L salinity water. If the rotifers have been chilled during shipping or storage, they should be gradually (during 30 min or longer) acclimated to the temperature of the water in the culture vessel (25 – 27 °C).
- Turn on the PMT and begin pumping the algae feed into the rotifer culture vessel. Using the timer feature of the PMT, set the delivery rate of algae feed mixture so that ~1.6 ml of algae feed mixture is delivered per million rotifers in the culture, per day. Distribute the feedings in small portions at regular intervals over the course of a 24 hr period; the more frequent the feedings, the better.
- Calibrate the delivery rate of the pump by manually turning on the PMT for a set period (e.g., 1 min) and collect the algae that it pumps during this interval into a graduated cylinder or beaker. For example, if the PMT doses 5 ml of algae in 1 min, then the dose rate would be 5 ml algae/min.
- Calculate the required daily feeding rate by multiplying the number of rotifers present, in millions, by 1.6 ml. For example, a rotifer culture with a population size of 100 million rotifers would require ~160 ml of feed per day (100 x 1.6 ml).
- Set the PMT to dose the total daily feed requirement at regular intervals throughout a 24 hr period. For example, delivery of a total daily feed amount of 160 ml could be delivered in portions once every 3 hr over a 24 hr period using a PMT set with a dosing pump rate of 5 ml/min for 4 min, 8 times daily (5 ml/min x 4 min = 20 ml x 8 feedings = 160 ml).
- Allow the culture to grow until it generates the required population, typically for 48 – 72 hr, before harvesting. At 24 h post-start up, add the floss particle filters to the culture vessel and begin normal maintenance.
- Maintenance
NOTE: The culture operates on a continuous basis and requires routine maintenance that ideally should be performed at the same time each day, in the following sequence.- Fill the FCV to 90% of available volume with clean, dechlorinated fresh water, dosed with 10 g/L aquarium salts. Ensure that the water is well mixed, and that all of the salt is fully dissolved. Set the air flow into the vessel so that it maintains a "rolling boil". Measure the salinity with a refractometer and ensure that the salinity is 10 g/L. It is critical to meet this target and not to exceed it.
- Sample the rotifers in the CV: Ensure that the culture is well-mixed, then collect 3 samples of a 2 – 3 ml each using a transfer pipette or autopipettor, from different parts of the culture. Combine these samples in a tube or vial of convenient size (e.g., 10 ml).
- Transfer 1 – 2 ml of the combined sample onto a petri dish so that it can be visualized under a dissecting microscope. Check the quality of the culture (swimming behavior of the rotifers, presence of detached eggs, contaminating protozoa).
- Immobilize the rotifers in the remaining combined sample by adding 100 µl of 50% Lugols iodine solution to the sample. Within seconds after addition of the Lugols, observe the rotifers to stop swimming. Now, easily count the rotifers .
NOTE: Ethanol, diluted bleach, or vinegar can be used in place of Lugols. Vinegar (2 drops/10 ml) has the advantages of being non-hazardous, not losing strength in storage as bleach and iodine solutions can, and not making the rotifers contract, so the corona of cilia and the "foot" remain extended and the animals look more natural. - Ensure that the sample is well mixed (immobilized rotifers will settle rapidly), then quickly take a ~2 ml subsample in a plastic pipet and dispense 1 ml into a Sedgewick-Rafter counting slide (20 x 50 1-mm squares) (Figure 2). Using a dissecting or compound microscope, count the intact rotifers and the total number of eggs attached to these rotifers (Figure 2). Count as much of the slide area as necessary to count ~100 rotifers. Calculate the number of rotifers per ml, and record this in a spreadsheet or logbook.
- Harvest ~30% of the volume of the rotifers in the CV: Remove the air supply and floss filter, slowly open the valve at the bottom of the CV and allow the water to flow into a plankton collector with a 53 µm mesh screen. Collect the water flowing out of the bottom of the collector after filtering into a bucket or drain. Use a gentle to moderate flow to avoid damaging the rotifers. Do not allow the rotifers to dry on the screen.
- For consistency reasons, it is advisable to establish the FCV with a standard number of rotifers each day. Therefore, based on the known CV rotifer density and harvest volume, adjust the FCV total volume to achieve a consistent final density (e.g., 1,500 rotifers/ml). Add the harvested rotifers to the FCV: Gently transfer the rotifers from the collection screen using a wash bottle filled with clean salt water (10 – 15 g/L). Invert the screen over the FCV and wash the rotifers into the FCV with a gentle stream of salt water. Start the PMT to deliver feed (~1.57 ml per million rotifers per day) to the FCV.
- Scrub the entire inside of the CV with a clean, soft nylon brush or scrub pad.
- Make a new mix of 15 g/L water by adding the appropriate amount of salt to a measured amount of clean RO water in a 5 gallon bucket to replace the volume of water lost to harvest. Add the salt to the water in the bucket and stir vigorously until it is completely dissolved, and then add to the CV.
- Using a high-pressure spray in a sink, rinse the floss filter until it is free of debris, and then return it to the CV.
- Adjust feed rates of algae delivered to the CV by changing the duration of each dosing event, according to the daily count of rotifers/ml. Use the calculations provided in step 1.3.8, above to determine the appropriate amount of feed to be delivered.
- Approximately 24 hr later, repeat the process. Start by harvesting the rotifers remaining in the FCV (that were not needed for the previous day) in the same manner as described above (steps 1.4.2 – 1.4.10). Concentrate them in 2 L of fresh, clean dechlorinated water (5 g/L salt). These can be stored at 4 °C as a backup supply, or used to feed later stages of fish, beyond what is described in this protocol.
NOTE: This protocol permits up to 2 – 3 days of reduced culture maintenance (with normal automatic feeding), because the rotifers in the CV can tolerate omission of harvests without serious consequences.
2. Polyculture
- Setup
- Collect zebrafish embryos from a spawning event by pouring spawned embryos through a tea-strainer and then gently rinsing with sterile fish water (or any other non-contaminated source of appropriately conditioned solution; e.g., embryo medium, E3, etc.) from a wash bottle into petri dishes.
- Incubate the embryos at 25 – 28 °C in petri dishes at a density of 40 – 50 embryos per dish for 5 days.
- Begin the polyculture phase on day 5 post-fertilization, or when greater than 90% of the hatched larvae are actively swimming up in the water column.
- Inoculation
- Add 500 ml of rotifer culture directly from the FCV to a 3.5 L nursery tank; inclusion of rotifer culture water provides algal feed that maintains the nutritional content of the rotifers during polyculture.
- Gently pour the larvae from one petri dish into the nursery tank. Ensure that no larvae remain in the dish.
- Add 500 ml of clean, conditioned fish water from a recirculating system or dedicated water source to the tank to reach a final volume of 1 L and a final salinity of 5 g/L.
NOTE: This salinity is critical because zebrafish larvae survival will be negatively impacted if salinity is >7 g/L and rotifer survival will be negatively impacted if salinity <2 g/L.
- Polyculture phase
NOTE: The polyculture phase should last for up to 4 days post-inoculation (a total of 5 days, corresponding to days 5-9 post-fertilization).- Observe the polyculture tank at least once per day during this period to ensure that rotifers and fish are present and growing. Ensure that the rotifers are visible throughout the water column. Ensure that the the fishs are also visible within the water column, swimming among the rotifers.
- Start normal water flow through the tank. Place a screen or baffle over the drain port to ensure that larvae are not flushed out of the tank.
NOTE: At the end of this phase, the fish will be large enough to consume larger prey items such as Artemia nauplii or processed feed items in the size range of 75 – 125 µm.
NOTE: The rotifer population dynamics within a representative polyculture tank were measured by sampling/counting rotifers from the tank in the same manner as described in steps 1.4.2 – 1.4.5. This was done once per day from the beginning of the polyculture phase until it was completed.
Representative Results
The continuous rotifer culture system described here is dynamic, and it is normal for rotifer numbers to fluctuate to a small extent over time if there are variations in daily feeding and harvest rates. The population of the rotifers in one of the active cultures in the aquaculture facilities at Boston Children's Hospital, maintained in the manner described above, was monitored for 30 days (Figure 3). The mean culture density during this period was 932 rotifers/mL, with a maximum of 1,330 rotifers/ml and a minimum of 510 rotifers/ml. This represents the typical performance of a rotifer culture maintained in accordance with this protocol.
The dynamics of the rotifer population in a polyculture tank with zebrafish is shown in Figure 4. A 3.5 L fish tank was inoculated with 500 ml of rotifer culture from the FCV, 500 ml of sterilized fish water, and 40 5-day old wild-type (Tubingen strain) larvae, to demonstrate how rotifers and zebrafish perform in a typical polyculture tank. The rotifer population in the polyculture tank peaked on the second day with a density of 747 rotifers/ml (Figure 4). The density of the rotifers in the polyculture tank decreased over the 5 day polyculture period to a minimum of 481 rotifers/ml on day 5. However, the mean rotifer density throughout the entire period was 589.4 rotifers/ml, and the minimum value of 481 rotifers/ml is ~10 times that of the rotifer density at the end of the polyculture period in previously published work12.
Zebrafish growth during the five day period is shown in Figure 5. The fish showed steady growth each day, from a mean total length of 3.904 mm ± 0.063 SEM when they were added to the polyculture tank to a mean total length of 5.423 ± 0.063 SEM on the final day of the polyculture phase. Survival was 95% (38/40).
Water quality parameters were measured daily in the representative polyculture tank, and are shown in Table 1. The values of these parameters are similar to previously published work12, and represent conditions that are conducive to the growth and survival of both the rotifers and the fish during the 5 day period of the polyculture.
| Day | Day | Salinity | TAN | NO2 | NO3 | pH | Alkalinity | Hardness | Temperature |
| (Actual) | (post-fertilization) | g/L | mg/L | mg/L | mg/L | mg/L (CaCO3) | mg/L (CaCO3) | °Celsius | |
| Monday | 5 | 5 | 2 | 0 | 0 | 8.4 | 120 | 425 | 23.2 |
| Tuesday | 6 | 5 | 3 | 0 | 0 | 8.4 | 180 | 425 | 23.3 |
| Wednesday | 7 | 5 | 3 | 0 | 0 | 8.3 | 240 | 425 | 22.3 |
| Thursday | 8 | 6 | 3 | 0 | 0 | 8.4 | 240 | 425 | 22.8 |
| Friday | 9 | 7 | 3 | 0 | 0 | 7.9 | 240 | 425 | 22.2 |
Table 1. Water Quality Parameters in a Representative Zebrafish Polyculture Tank.
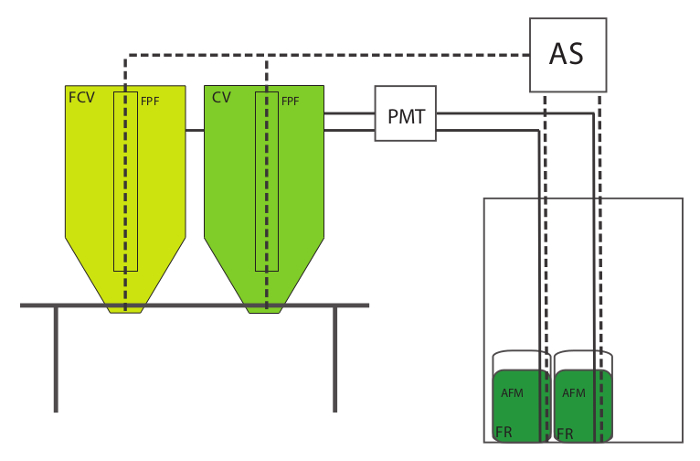
Figure 1. Rotifer Culture Setup. Legend: Culture Vessel (CV), Feedout Culture Vessel (FCV), Air Supply (AS), Floss Particle Filter (FPF), Algae Feed Mixture (AFM), Feed Reservoir (FR), Programmable Metering Timer (PMT). Solid lines represent algae feed delivery. Dashed lines represent air delivery. Please click here to view a larger version of this figure.
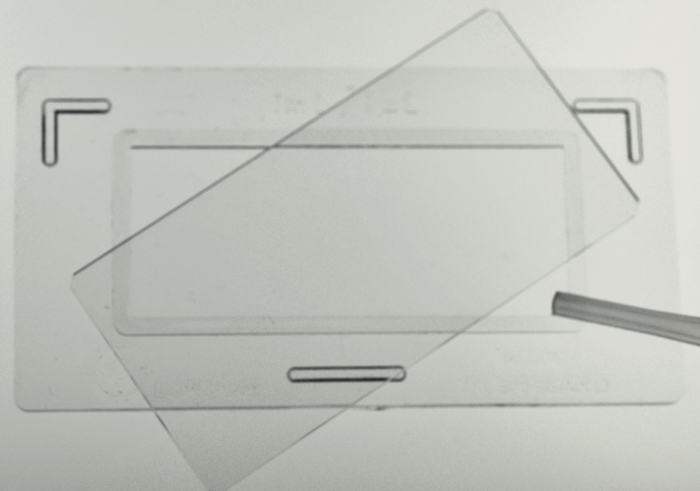
Figure 2. Filling of a Sedgewick-Rafter Counting Slide. Please click here to view a larger version of this figure.
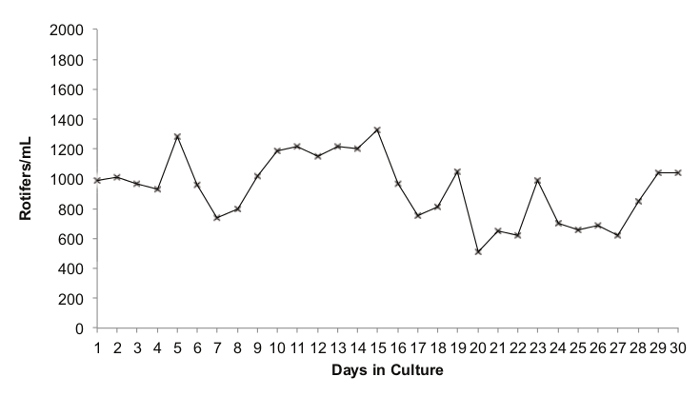
Figure 3. Rotifer Population Dynamics in Culture over a 30 day Period. Legend: The number of counted rotifers (females + eggs) per ml is on the x-axis. The number of days in culture is on the y-axis. Please click here to view a larger version of this figure.
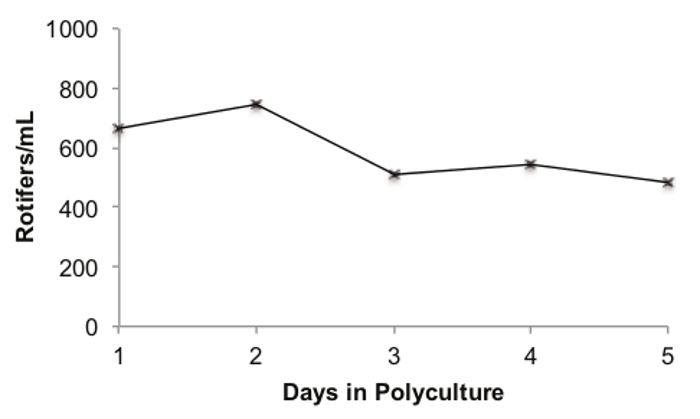
Figure 4. Rotifer Population Dynamics during a 5 day Polyculture. Legend: the number of counted rotifers (females + eggs) per ml is on the x-axis. The number of days in polyculture is on the y-axis. Please click here to view a larger version of this figure.
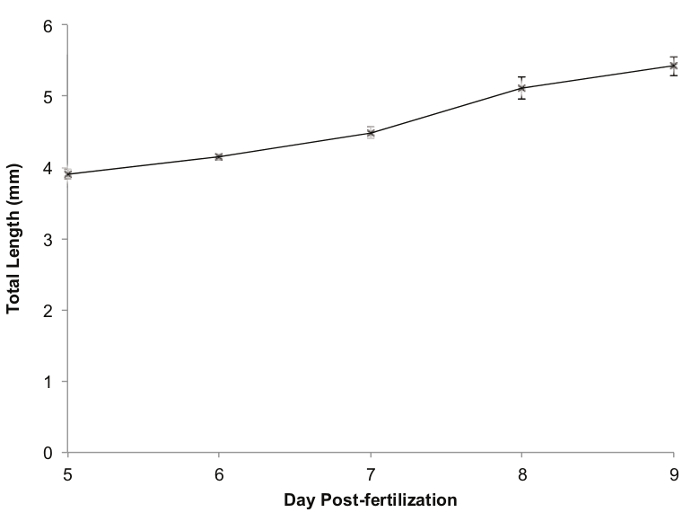
Figure 5. Zebrafish Growth during a 5 day Polyculture. Legend: Values are mean total length in mm; error bars represent standard error of the mean (SEM). Please click here to view a larger version of this figure.
Discussion
Successful implementation of the rotifer polyculture method for feeding early larval zebrafish requires effective protocols for two tasks: the establishment and maintenance of a continuous rotifer culture system to feed the fish, and culturing first-feeding zebrafish larvae along with rotifers in the same tank.
The setup for a continuous saltwater rotifer production system for zebrafish laboratories first described by Lawrence and co-authors14 has been modified and enhanced in a number of ways to make it both more robust and universal in application. The new protocol incorporates improvements both in the equipment and methodology.
The storage system for the algae feed mixture now employs hatching jars used in catfish production systems15. Storage of the feed in these round-bottomed jars facilitates thorough mixing of the algae feed so that it does not stratify or clump and lead to clogging of delivery lines, a problem which frequently plagued the original protocol. Similarly, the use of an all-in-one programmable timer/metering pump reduces the total number of components, and simplifies the metered delivery of feed to the rotifers. It also permits the dosing of feed to the rotifers on an hourly basis over the 24-hr period, which is preferable to fewer, larger feedings because the feed is assimilated more efficiently.
A method for counting rotifers that permits fine-tuning of feed delivery to the rotifer culture has been included in this protocol. Feeding the rotifers based on an accurate, real time assessment of their population size ensures steady, high-level production. A secondary Feedout Culture Vessel has also been added to the protocol. A continuous culture system may produce a daily excess of tens of millions of rotifers beyond what is needed for even a large nursery operation, due to variations in nursery activity. This Feedout Culture Vessel is used to hold rotifers for daily inoculation of polycultures , and the remaining rotifers can be fed as a high-quality nutritional supplement to later larval and early juvenile stages of fish. The reduced salinity (10 g/L) in the FCV also pre-acclimates the rotifers to the conditions in the polyculture tanks, so that they maintain vigorous swimming activity when transferred.
Importantly, these changes to the rotifer culturing protocol lead to direct improvements in the polyculture approach, particularly with regard to labor input. The previous method published by Best and co-authors involved not only an initial inoculation of rotifers at day 5, but also daily additions of rotifers until the static polyculture phase ended on day 912. Mean rotifer densities during this period peaked at 333 rotifers/ml on the day of inoculation and ended below 50 rotifers/ml at the termination of the polyculture phase on day 912. In contrast, the current protocol maintains much higher densities throughout, typically starting at ~665 rotifers/ml, and ending with ~481 rotifers/ml on day 9.
There are two factors enabling these much higher rotifer densities. 1) The overall robustness of the rotifer culture used for inoculation is far greater. Furthermore, the revised step of inoculating the polyculture with equal volumes of algae/rotifers at 10 g/L salinity and ~0 g/L salinity fish water, results in an intermediate salinity of 5 g/L that is maintained throughout polyculture. Starting with a greater total volume of water (1L) also is more conducive to rotifer growth and survival in the polyculture tanks. In the previously published method, lower starting rotifer densities in a smaller volume necessitated daily additions of rotifers to replenish losses by attrition (presumably due to poor water quality) and consumption by developing fish larvae. The results of these adjustments to the protocol dramatically reduce labor inputs (one feeding over 4 days compared to 4 feedings in 4 days with the previous method). Larval growth and survival performance over the initial 5 day polyculture period is comparable to that published previously12.
The productivity of rotifer cultures is necessarily limited by the feed input. Rotifer densities of 2,000 – 3,000/ml can be achieved with these methods simply by increasing the feed delivery. When rotifer yield per unit of feed is known, feed rate can be adjusted to match rotifer production to projected needs. During weekends and holidays, feeding can be reduced and harvests omitted for a day or two without harming the rotifers, although their productive potential will be suboptimal until the normal daily feeding and harvest routine resumes.
When there is an unanticipated need for more rotifers than usual, a larger portion of the population can be harvested without harming the culture, but depending on the size of the harvest the culture may require a day or more to recover its usual density. If rotifer demand will be reduced for more than a day or two, production is best reduced by reducing the feed delivery, rather than decreasing the harvest. A harvest rate of at least 25%/day maintains a younger rotifer age profile so that the rotifers are more vigorous and fecund, improving their performance in the polyculture tanks.
This protocol is easily adapted to smaller scales for smaller zebrafish facilities. For example, a 15 L culture, which can be conveniently set up in a standard 5 U.S. gallon (20 L) bucket with hand-feeding twice per day, can readily achieve a density of 1,000 rotifers/ml. Use of a feed pump enables densities of 2,000-3,000/ml. A single bucket harvested at 30%/day can therefore provide 5-15 million rotifers/day, enough to start 10 – 30 polycultures. Two buckets can be used in the same CV + FCV protocol as described above. An algae + ammonia controller + pH buffer pre-mix feed is available (see Table of Specific Reagents/Equipment) that is convenient for small-scale applications. It has sufficient viscosity that the algae cells do not settle out quickly and so it does not require continuous mixing in the feed reservoir.
If rotifer culture productivity declines, or an unusual accumulation of dead rotifers is found in the culture, all water quality parameters (temperature, salinity, pH, NH3 concentration) should be checked. Brachionus plicatilis normally carries its eggs until they hatch, and the presence of many unattached eggs (which usually do not hatch) in the culture is a sign of stress, most often due to accumulated ammonia.
If the rotifer culture water is a more intense green than usual, then either the feed rate is too high or the rotifers are not consuming food at their usual rate. Usually this is caused by either a stress factor, or the culture has been overharvested without adjusting feed rate according to the reduced population. In dense cultures it is normal for a brown coloration to accumulate in the water as algal pigments are metabolized by the rotifers.
The use of saltwater rotifers during the first feeding phase has been widely adopted across the zebrafish research community since the method was first published in 201012. The modifications made to the polyculture rearing method that are outlined in this protocol will permit an even greater number of laboratories to adopt this approach, regardless of scale. These advances are timely; developments in gene knockout technology (e.g., CRISPR, TALENs, etc.) for zebrafish will require streamlined and efficient rearing protocols to grow thousands of new strains of genetically modified fish for usage in various fields of science.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The care and usage of fish generated for representative results described in this protocol was performed in full accordance with the guidelines set forth by the Institutional Animal Care and Use Committee at Boston Children's Hospital, protocol # 14-05-2673R.
Materials
| Rotifer Culture Infrastructure | |||
| 100 Liter Culture Vessel | Aquaneering | Custom | Polycarbonate culture vessel, conical bottomed, with drain valve |
| 5 Gallon Culture Bucket Kit | Reed Mariculture | CCS Starter Kit | Small volume culture vessel for small facilities |
| Rigid Clear Tubing 1/2" O.D., 36” | Pentair Aquatic Ecosystems | 16025 | Rigid clear tubing for air delivery |
| Mesh tube | Pentair Aquatic Ecosystems | RT444X | Mesh tube support for floss filter |
| Rotifer Floss | Reed Mariculture | Rotifer floss 12” x 42” | Particulate waste trap |
| Peristaltic Metering Timer Pump, 5 GPD | Grainger | 38M003 | Metering pump with timer for dosing feed to rotifers |
| Peristaltic Metering Timer Pump, 1-100 mL/h (for smaller-scale culture) | Coral Vue | SKU: IC-LQD-DSR | Metering pump with timer for dosing feed to rotifers |
| Silicone Tubing | Cole Parmer | Tubing for algae delivery to rotifer vessel | |
| Rigid Clear Tubing " O.D.,36” | Pentair Aquatic Ecosystems | 16025 | Rigid clear tubing for air delivery to algae paste |
| Rigid Clear Tubing O.D., 36” | Pentair Aquatic Ecosystems | 16025 | Rigid clear tubing for algae delivery |
| Rotifers | |||
| Live Rotifers Brachionus plicatilis Type L | Reed Mariculture | Type L 5 million | Rotifer stock culture for system startup |
| Rotifer Feed | |||
| Sodium hydroxymethylsulfonate | Reed Mariculture | ClorAm-X® 1lb tub | Ammonia reducer for algae feed mix |
| Sodium Bicarbonate | Fisher Scientific | S25533B | pH buffer for algae feed mix |
| Microalgae concentrate | Reed Mariculture | Rotigrow Plus® 1 liter bag | Nutritionally optimized rotifer feed |
| Water Preparation | |||
| Reef Crystals Reef Salt | That Fish Place | 198210 | Salt for making culture water (NOTE: this item is an example only; any contaminant free salt formulations may be used). |
| Refractometer | Pentair Aquatic Ecosystems | SR6 | measuring salinity |
| Rotifer Culture Equipment | |||
| Plankton Collectors 12" Dia, 53 microns | Pentair Aquatic Ecosystems | BBPC20 | Mesh screen for collecting rotifers |
| Scrub Pads | Pentair Aquatic Ecosystems | SCR-58 | Scrub pad for cleaning inside of culturing vessels |
| Scrub Brush | |||
| Bucket | Grainger Supply | 43Y530 | Graduated bucket for mixing culture water |
| Hatching Jar | Pentair Aquatic Ecosystems | J30 | Storage of algae feed mix |
| Lugol’s Solution, Dilute | Fisher Scientific | S99481 | Agent used to immobilize live rotifers for counting |
| Sedgewick-Rafter plankton counting slide with grid | Pentair Aquatic Eco-Systems | M415 | Counting rotifers |
| Miscelleneous | |||
| Tea Strainer | Kitchenworks | 971972 | Used for collecting zebrafish embryos after spawning |
References
- Ribas, L., Piferrer, F. The zebrafish (Danio rerio) as a model organism, with emphasis on applications for finfish aquaculture research. Reviews in Aquaculture. 6, 209-240 (2014).
- Poss, K. D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nature reviews. Genetics. 11, 710-722 (2010).
- Gemberling, M., Bailey, T. J., Hyde, D. R., Poss, K. D. The zebrafish as a model for complex tissue regeneration. Trends in genetics TIG. 29, 611-620 (2013).
- Santoriello, C., Zon, L. I. Hooked! modeling human disease in zebrafish. Journal of Clinical Investigation. 122, 2337-2343 (2012).
- Selderslaghs, I. W. T., Blust, R., Witters, H. E. Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reproductive Toxicology. 33 (2), 142-154 (2012).
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 269, 1-20 (2007).
- Harper, C., Lawrence, C. . The Laboratory Zebrafish (Laboratory Animal Pocket Reference). , (2010).
- Nusslein-Volhard, C., Dahm, R. . Zebrafish, A Practical Approach. , (2002).
- Westerfield, M. . The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish. , (2007).
- Carvalho, P., Arau, L. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aquaculture Research. 37, 1107-1111 (2006).
- Spence, R., Gerlach, G., Lawrence, C., Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 83 (1), 13-34 (2008).
- Best, J., Adatto, I., Cockington, J., James, A., Lawrence, C. A novel method for rearing first-feeding larval zebrafish: polyculture with Type L saltwater rotifers (Brachionus plicatilis). Zebrafish. 7 (3), 289-295 (2010).
- Lawrence, C. Advances in zebrafish husbandry and management. Methods in Cell Biology. 104, 429-451 (2011).
- Lawrence, C., Sanders, E., Henry, E. Methods for culturing saltwater rotifers (Brachionus plicatilis) for rearing larval zebrafish. Zebrafish. 9, 140-146 (2012).
- Tucker, C. S., Hargreaves, J. A. . Biology and Culture of Channel Catfish. 34, 634-657 (2004).

