Rodent Working Heart Model for the Study of Myocardial Performance and Oxygen Consumption
Summary
Isolated working heart models can be used to measure the effect of loading conditions, heart rate, and medications on myocardial performance and oxygen consumption. We describe methods for preparation of a rodent left heart working model that permits study of systolic and diastolic performance and oxygen consumption under various conditions.
Abstract
Isolated working heart models have been used to understand the effects of loading conditions, heart rate and medications on myocardial performance in ways that cannot be accomplished in vivo. For example, inotropic medications commonly also affect preload and afterload, precluding load-independent assessments of their myocardial effects in vivo. Additionally, this model allows for sampling of coronary sinus effluent without contamination from systemic venous return, permitting assessment of myocardial oxygen consumption. Further, the advent of miniaturized pressure-volume catheters has allowed for the precise quantification of markers of both systolic and diastolic performance. We describe a model in which the left ventricle can be studied while performing both volume and pressure work under controlled conditions.
In this technique, the heart and lungs of a Sprague-Dawley rat (weight 300-500 g) are removed en bloc under general anesthesia. The aorta is dissected free and cannulated for retrograde perfusion with oxygenated Krebs buffer. The pulmonary arteries and veins are ligated and the lungs removed from the preparation. The left atrium is then incised and cannulated using a separate venous cannula, attached to a preload block. Once this is determined to be leak-free, the left heart is loaded and retrograde perfusion stopped, creating the working heart model. The pulmonary artery is incised and cannulated for collection of coronary effluent and determination of myocardial oxygen consumption. A pressure-volume catheter is placed into the left ventricle either retrograde or through apical puncture. If desired, atrial pacing wires can be placed for more precise control of heart rate. This model allows for precise control of preload (using a left atrial pressure block), afterload (using an afterload block), heart rate (using pacing wires) and oxygen tension (using oxygen mixtures within the perfusate).
Introduction
The study of isolated organs permits control of physiologic conditions beyond what is possible in vivo. Ex vivo heart preparations were first described by Otto Langendorff,1 who described an isolated model with retrograde perfusion. Subsequently, others described the "working heart" model, in which the myocardium performs both pressure and volume work.2 Such preparations have been instrumental in elucidating mechanisms of myocardial action,3 myocardial metabolism,4-6 and effects of cardiotonic medications.7-9
The use of medications that enhance myocardial contractility is common in critically ill patients. However, few data are available comparing the relative effects of these medications on contractility and myocardial oxygen consumption, data which may be useful in the care of patients with clinical signs of heart failure of in the postoperative setting.10 However, because most cardiotonic medications affect not only the myocardium, but also arteriolar resistance, venous capacitance11, and a patient's metabolic rate,12 ex vivo isolated heart models remain the optimal means by which to study the effects of such medications on the myocardium proper.
We describe the use of an ex vivo model for the load-independent study of inotropic medications on myocardial function and oxygen consumption. Hearts from Sprague Dawley rats were cannulated using a left ventricular working heart model and perfused using a modified Krebs Henseleit perfusate. Aortic and left atrial pressures were controlled. Pressure-volume impedance catheters were placed into the left ventricle via apical puncture for the continuous monitoring of systolic and diastolic function. Oxygen consumption was continuously measured as the indexed difference in oxygen content between left atrial perfusate and the pulmonary artery effluent. Medications to be tested were infused into the left atrial block, and changes in cardiac performance and oxygen metabolism were measured and compared with an immediately preceding baseline.
Protocol
This protocol is performed under a current protocol under the institution's animal care and use committee.
1. Preparation for the Study
- Turn on the water bath to heat the Krebs-Henseleit buffer (KHB) reservoir (set to 42 °C).
- Prepare 16 L of KHB containing (in mM) 128 NaCl, 5.7 KCl, 1.3 MgSO4, 25 NaHCO3, 2.7 CaCl2, 0.53 EDTA, 0.54 NaC3H3O3, and 10.8 dextrose.13 The substrate masses are as follows: 27.584 g NaCl, 1.58 g KCl, 0.578 g MgSO4, 8.401 g NaHCO3, 1.47 g CaCl2, 0.744 g EDTA, 0.22 g NaC3H3O3, and 7.208 g dextrose.
Note: these components may be stored in conical tubes in powdered form for constitution on the day of experimentation.- Filter 4 L of deionized water through a 0.22 micron filter.
- Add 3.7 L of this water to a 4 L beaker. Add all components except for CaCl2 to the water.
- Dissolve the CaCl2 in the remaining 300 ml of water using a separate beaker.
- Oxygenate the solution with 95% O2 / 5% CO2 at 1 L/min (LPM) for 5 min. This corrects the pH to 7.40 and enhances dissolution of CaCl2.
- Add the CaCl2 to the remainder of the KHB.
- Add the completed KHB to a reservoir and circulate through all tubing for 30 min. Ensure that the system is free of macroscopic bubbles. Oxygenate with 95% O2 / 5% CO2 at 0.5 LPM.
NOTE: KHB may be stored overnight in the refrigerator for no more than 1 – 2 days, brought back to room temperature and re-filtered for use. Do not reuse KHB between experiments. - Prepare 2 x 50 ml clean beakers with ice cold KHB and place them in a bucket of ice near the dissection station. Ensure that the KHB is ice cold (rather than chilled) before explanting the heart.
- Place the micro pressure-volume (PV) catheter in a 10 ml syringe filled with filtered KHB for 30 min prior to calibration, per manufacturer's instructions.
NOTE: Temperature of KHB used to soak the PV catheter should be as close to 37 °C as possible.
- Prepare the anesthesia and dissecting station for the animal.
- Ensure adequate isoflurane in the reservoir. Draw up 500 U of heparin in a 1 ml syringe; place a 26 gauge (1/2") needle on this syringe. Prepare a mask for anesthetizing the animal.
- Set the aortic block perfusion pressure to 80 mmHg and the left atrial (LA) block perfusion pressure to 10 mmHg. Open both the aortic block and LA block to allow warm KHB to drip out. When ready to dissect the animal, open the aortic block to allow a steady slow drip of KHB out.
- Calibrate the PV catheter according to manufacturers' instructions.
2. Animal Preparation and Dissection
NOTE: For best results, ensure animal is between 300 and 500 g; we have found that an animal weight between 425 to 450 g is ideal for our system.
- Anesthetize the animal in a chamber using isoflurane (1 – 2%) until the animal is unconscious. Transfer the animal to the dissection station and place the anesthesia mask with isoflurane and oxygen on the animal. Perform toe pinch to assess level of sedation. Apply vet ointment on eyes to prevent dryness while under anesthesia.
- Inject heparin, 500 units intraperitoneal in the abdominal cavity. Allow 10 min for the Heparin to be absorbed. Secure the limbs of the animal with tape to enhance visualization of the thorax.
- Dissection of the heart.
- Once ensuring that there is no response to a toe pinch, lift the skin away from the abdominal cavity with forceps and then use scissors to incise the peritoneal cavity, following the curve of the diaphragm back to the posterior angle of the ribs.
- Once the diaphragm is visible, using small scissors, cut along the anterior surface of the diaphragm following the direction of the prior cuts to allow for entry into the thorax. Extend each cut along the axillary line bilaterally to the axilla.
NOTE: The next steps should be performed efficiently since ventilation will be compromised once the diaphragm is incised. - Retract the ribcage anteriorly from the xiphoid process using forceps. Incise the pericardium and pleura.
- Identify the inferior vena cava (IVC) and aorta just above the diaphragm and retract them en bloc anteriorly using blunt forceps.
- Using large, curved scissors, rapidly make an incision across the IVC and the aorta, pulling the heart and lungs out of the chest en bloc. Cut the esophagus, trachea, brachiocephalic arteries and veins cephalad to remove the heart and lungs from the thorax. Excise the thymic tissue with this block of tissue. Take care not to cut the proximal portion of the ascending aorta.
- Immediately immerse the heart and lungs in ice-cold KHB and move to the Langendorff apparatus, previously set up as described in step 1.
3. Aortic Cannulation
- Place the heart-lung complex in a flat dish and orient the heart with the thymus and great vessels facing the experimenter and the posterior aspect of the lungs facing the table. Pull apart the two lobes of the thymus and identify the takeoff of the brachiocephalic arteries from the aorta.
- Drape the aorta over the edge of the dish and transect the aorta using small scissors approximately 5 mm above the aortic valve, just proximal to the takeoff of the right subclavian artery.
NOTE: The incision should yield a clean round circle — the aorta in cross-section. If it is off-angle (i.e., a wide oval) or incomplete, repeat the cut to achieve the desired result. This will facilitate efficient aortic cannulation.
- Drape the aorta over the edge of the dish and transect the aorta using small scissors approximately 5 mm above the aortic valve, just proximal to the takeoff of the right subclavian artery.
- Using 2 pairs of curved forceps on either side of the aorta, guide the aorta over the aortic cannula (which should be slowly dripping with KHB). The aortic valve should sit 1 – 2 mm below the tip of the cannula.
- After aorta cannulation, reposition the forceps perpendicularly across the aorta to hold the aorta in place. Alternatively, place a small clamp across the aorta to hold the heart lung complex in place, enabling a single experimenter to complete this model.
- Have an assistant pass a silk 4-0 suture just below the forceps and tie into place, looping around the cannula and tying multiple times both in front of and behind the heart. Open the cannula fully to begin full retrograde aortic flow. Observe the heart beat vigorously.
NOTE: If the heart does not begin to beat rapidly (~ 200 BPM) and vigorously, the tie or cannula may be occluding one or both of the coronary arteries. If this is suspected, remove the tie and reposition it away from the coronary arteries. If the heart distends and does not beat, the cannula may be across the aortic valve. If the coronary artery leaks (KHB sprays from the aortic root), advance the cannula closer to the aortic valve (this phenomenon can occur if a brachiocephalic artery is cannulate in place of the ascending aorta).
4. Pulmonary Vein Occlusion and Preparation of the Pulmonary Artery for Cannulation
NOTE: The purpose of this step is to create a closed left atrial system to ensure that all volume and pressure from the left atrial block is transmitted to the left heart structures. Failure to completely occlude the pulmonary veins could result in preload deficiency and may falsify results or create an unstable working heart preparation.
- Remove the thymus to improve exposure of the pulmonary artery (PA).
- Manually rotate the aortic cannula so that the posterior aspect of the heart is facing the operator. Dissect out the vessels leading to the right lung. Suspend the right lung tissue using forceps to further delineate these vessels. Using medium-large surgical clips (or suture), occlude the right pulmonary artery and vein and bronchus with a single clip. Resect the right lung distal to the clip.
NOTE: Due to difficulty in dissecting the pulmonary artery free, our practice is to occlude the pulmonary veins to distend the pulmonary artery, making it easier to incise without injuring the nearby structures in a beating heart model. - Repeat step 4.2 on the left lung.
NOTE: Potential pitfalls and problem solving: Once both pulmonary arteries are occluded, the right atrium will visibly distend and the heart may become bradycardic. This is because the right ventricle becomes pressurized. If this does not occur, it is likely that the pulmonary veins are not completely occluded, and that preload will be insufficient for working heart mode. If the heart is not able to maintain cardiac output after left atrial (LA) cannulation and attempted transition to working heart (see below), consider placing additional clips or a tie around the pulmonary vein stumps to occlude any residual leak. Once the PAs are occluded, however, Step 5 should be performed immediately to minimize myocardial ischemia. Note that some investigators incise the pulmonary artery prior to ligation of the pulmonary veins to avoid pressurization of the right ventricle. - Pulmonary arterial incision
- Rotate the aortic cannula so that the anterior aspect of the heart is facing the operator. Identify the pulmonary artery. Again, this artery may be distended. Using small scissors make a transverse incision approximately 3 mm above the pulmonary valve.
NOTE: This will immediately relieve the pressure and the heart rate may increase. Because this cannula is easy to dislodge, cannulate the pulmonary artery after left atrial cannulation is complete.
- Rotate the aortic cannula so that the anterior aspect of the heart is facing the operator. Identify the pulmonary artery. Again, this artery may be distended. Using small scissors make a transverse incision approximately 3 mm above the pulmonary valve.
5. Left Atrial Cannulation
- Rotate the aortic cannula so that the left atrium is facing the operator. Using the small scissors, make a 2 – 3 mm incision in the upper body of the left atrium, approximately 3 mm above the atrioventricular groove.
- Position the left atrial cannula perpendicular to the plane of the mitral valve and pointed towards the atrial septum.
- Open the LA cannula until KHB flows. Ensure that the KHB is warm to the touch (it gets cold quickly when sitting in any non-jacketed tubing) in order to avoid myocardial dysfunction due to hypothermia following transition to working mode. Transition to a drip rate during cannulation.
- Using forceps to hold counter-traction, insert the atrial cannula into the body of the left atrium, taking care not to use excessive force, which can tear the atrium.
NOTE: The LA cannula should be positioned so that it sits in the middle of the atrium without any tension on the atrial wall. - Pass a 4-0 silk suture around body of the left atrium and tie a knot to create a seal of the atrium around the cannula. Take care to ensure that the posterior aspect of the left atrium is included in the suture. Add additional sutures as necessary. Once sealed, pull the cannula back 1 – 2 mm so that it sits in the middle of the atrium rather than against the atrial septum.
NOTE: The most common reason that the heart becomes malperfused upon transition to working heart mode is that the LA cannula abuts the atrial septum, which occludes left atrial inflow. The LA tracing often changes to demonstrate a proper a wave and v wave when the cannula is in proper position (see Figure 2E). - Open the LA cannula valve entirely to administer the full preload to the left atrium. Monitor the drip rate from the heart (which comes from coronary effluent). Ensure that the drip rate does not change when the LA cannula is open. If it does, retie the atrium around the cannula as described in step 6.4, as this represents a leak in the system.
6. Pulmonary Artery Cannulation and Transition to Working Heart Mode
- If measuring myocardial oxygen consumption (or other substances in coronary effluent, such as drug levels or cytokines), insert 1/32" flexible tubing into the prior incision in the pulmonary artery.
NOTE: Oxygen consumption is measured as the difference in oxygen content between left atrial perfusate and pulmonary artery effluent.2- For continuous measurement of myocardial oxygen consumption, use an in-line oxygen electrode to compare left atrial and coronary sinus effluent.
- Collect coronary sinus effluent (from both the pulmonary artery and dripping from the heart) in a graduated cylinder for timed quantification of coronary flow.
- Calculate myocardial oxygen consumption as previously described. 2
- Transition to working heart mode by turning off the retrograde aortic pump.
NOTE: When this is done, the LA pressure becomes the pre-load pressure and the resistor which was previously providing resistance to the retrograde pump in Langendorff mode now provides resistance to cardiac output, creating a mean arterial pressure. If the mean arterial pressure declines below ~ 80 mmHg, the cause is likely related to either preload or myocardial function. The most likely problem is the left atrial cannula, which should be adjusted after restarting the retrograde pump.
7. Insertion of the Left Ventricular Pressure Volume Catheter
NOTE: The PV catheter may be placed either retrograde (through the aortic valve) or via apical puncture. The benefit of retrograde is that position is more consistent and it obviates the need for apical puncture and the concomitant risks of coronary injury or loss of preload. However, retrograde placement can sometimes be very challenging, so we describe both techniques herein.
- Attach a 1.4 French pressure-volume catheter to the pressure volume loop system. Calibrate the system in warm KHB according to manufacturer's instructions. Ensure the waveform is visible in real time. Bring the catheter and cables close to the surface of the LV so as not to dislodge it following placement.
- For retrograde placement, open the adjustable valve and feed the PV catheter gently across the aortic valve until a stable pressure and volume waveform are identified. Avoid excessive use of force which can damage the aortic valve or puncture the ventricular apex.
NOTE: We have found that it is important to minimize the length of tubing and number of turns that the PV catheter must navigate to approach the AV. It may be helpful to cut down the tubing which comes with the system. - For apical placement, use a 24 G angio-catheter to create an apical puncture in the LV. Make sure to avoid the left anterior descending coronary artery. Aim the needle towards the aortic valve from the ventricular apex. Advance the pressure-volume catheter into the body of the LV. Stop advancing the catheter as soon as the LV pressure and volume waveform is identified.
- Once the pressure-volume catheter is in place, move the water jacket into position around the heart. Secure the catheter to the wall of the water jacket with a small piece of tape.
- Ensure at least a 30 min period of stability prior to beginning any measurements or interventions.
8. Infusion of Medication
- (Optional) Infuse medications (e.g., dopamine) into the left atrial block using a standard medication pump.
NOTE: We have dosed medications according to the whole animal body weight since flow equivalent to a whole cardiac output passes through the atrial block; only a small portion of this passes through the coronary circulation, as it does in vivo. Alternatively, a second set of perfusate can be created with a preset concentration of medication and used to perfuse the heart.
NOTE: In our protocol, we infuse medications over a 12 min period, collecting physiologic data during the final 10 min of each infusion and comparing it to an immediately preceding 10 min baseline.
9. Physiologic Manipulations
- Heart rate
- (Optional) suture two pacing wires onto the right atrial wall and attach to a temporary pacing device.
NOTE: This permits precise control of the heart rate (above the native sinus rate) and an understanding of the relationship between heart rate and contractility independent of a cardiotonic medication.
- (Optional) suture two pacing wires onto the right atrial wall and attach to a temporary pacing device.
- Preload
- Vary the preload (defined as left ventricular end diastolic volume) by varying the height of the column feeding the left atrial block.
- Blood pressure
- Manipulate the blood pressure (the primary determinant of afterload in this model) using the pressure valves on the IH-51.
- Coronary oxygen content
- Accomplish varying degrees of myocardial hypoxia by perfusing the heart with KHB saturated in various gas mixtures. Do this by using separate jacketed reservoirs (each with its own gas mixture) to ensure equilibrium between the gas and KHB.
- Accomplish coronary ischemia by suture ligating a distal coronary artery.
NOTE: The ligation of proximal coronary arteries in the working heart mode can result in lethal myocardial dysfunction. - Induce global coronary ischemia by interrupting or delaying retrograde perfusion for a defined period of time.
Representative Results
A schematic of a fully instrumented heart in retrograde perfusion (Figure 1A) and in left ventricular working heart (Figure 1B). Typical aortic, left atrial and left ventricular pressure and volume tracings are shown in Figure 2A – D. The typical end diastolic pressure is approximately 3 – 5 mmHg in this model, and the peak systolic pressure is approximately 100 mmHg. Figure 2E demonstrates the change in left atrial tracing when the LA cannula is moved away from the atrial septum during placement and positioning of the cannula. In these experiments, aortic pressure was set at 90 mmHg, and LA pressure was set to 10 mmHg.
To test the effects of catecholamines, each physiologic parameter (derived primarily from the pressure-volume catheter and associated software) was compared to the immediately preceding baseline period. In the example shown, dopamine was infused at 15 μg/kg/min into the left atrial block. Although the end diastolic pressure is identical in the two conditions (given the fixed atrial pressure in this model), the left ventricular end diastolic volume decreases by 2.5%, and the left ventricular end systolic volume decreases by 4.9%, yielding an increased stroke volume (Figure 3A). Compared with placebo infusions, the left ventricular stroke work, identified as the area within the pressure-volume curve, increased by 32% during treatment with dopamine (Figure 3B, P < 0.001, t test, n = 10 per group). This was associated with a greater increase in myocardial oxygen consumption relative to placebo infusions (Figure 3C). In this way, the relative potency and energy costs of different cardiotonic medications and doses can be compared to one another independent of their effects on loading conditions.
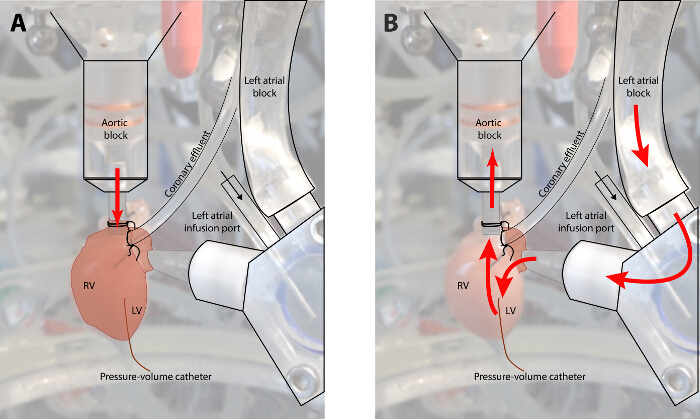
Figure 1: Diagram of Flow in a Fully Instrumented Heart in Retrograde Perfusion and Working Heart Mode. (Panel A: Langendorff mode; Panel B: working heart mode. In retrograde mode, KHB is infused at a set perfusion pressure into the aortic root. This mode is utilized to recover the myocardium following ischemic time and during instrumentation. In working heart mode, perfusate flows through the left heart before perfusing the coronary circulation. In this mode, the myocardium must generate its own perfusion pressure. Please click here to view a larger version of this figure.
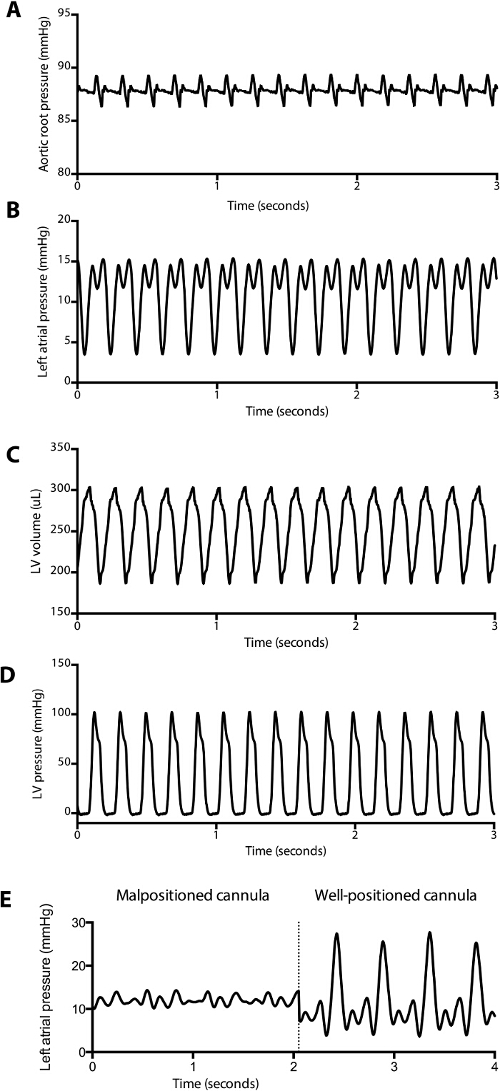
Figure 2: Representative Pressure and Volume Tracings Obtained during Baseline Measurements. (A) Aortic root pressure, (B) left atrial pressure, (C) left ventricular pressure and (D) left ventricular volume tracings during a baseline measurement are displayed. Stroke volume, stroke work, cardiac output, tau, and other parameters can be automatically calculated and displayed in real-time by the software. A blunted left atrial tracing (E) associated with a poor cardiac output in working heart mode can be a clue that the cannula is malpositioned in the left atrium. Note that the prominent v wave in the well-placed left atrial pressure tracing is common, likely due to a decreased left atrial compliance in the fully instrumented animal. Please click here to view a larger version of this figure.
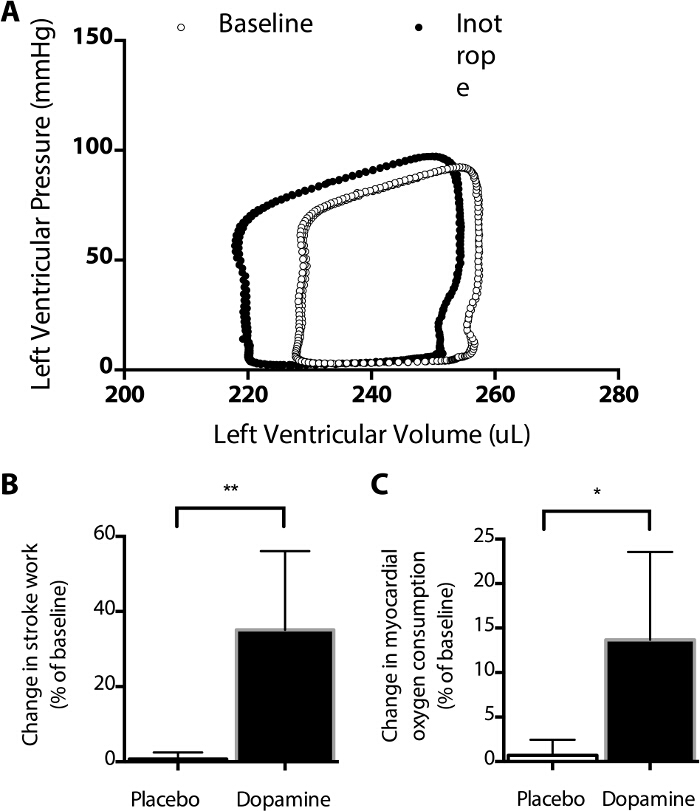
Figure 3: Effect of Dopamine on the Pressure-Volume Curve. Dopamine infusion results in a leftward shift in the PV curve (A), including an increased stroke volume, decreased end systolic volume, compared with baseline measurements. Note that the shape of some components of these PV curves differ from those typically measured in vivo (see Figure 4) due to the absence of arterial and venous elastance. (B) Relative to an immediately preceding baseline, stroke work increased significantly more during infusions of dopamine than placebo (**, P = 0.0017, t-test), as did myocardial oxygen consumption (*, P = 0.013, t-test, C). Using this model, the average myocardial oxygen consumption at baseline was 0.22 ± 0.02 mmol O2/gram tissue/minute, using an estimated dissolved oxygen content of 165 µmol/L in saline at 40 °C. Such measurements can be used to compare the myocardial oxygen consumption of various medications. Please click here to view a larger version of this figure.
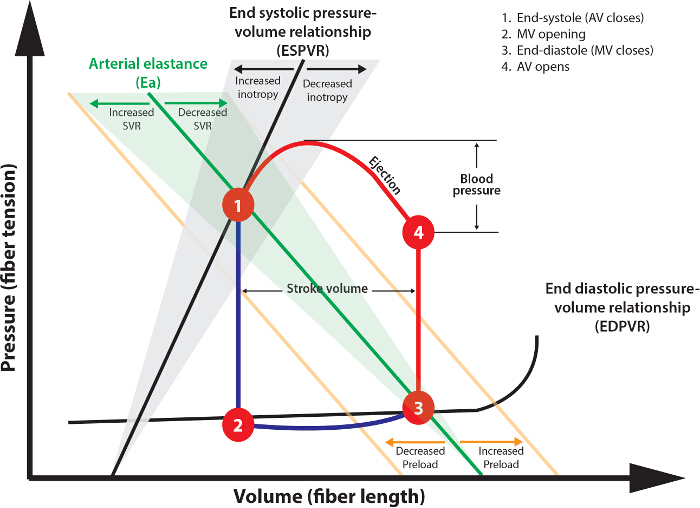
Figure 4: Analysis of Pressure Volume Loops. The Theoretical Pressure-Volume Loop Shown Describes the Normal Cardiac Cycle. Following aortic valve (AV) closure (1), isovolemic contraction occurs (1 – 2) as ventricular pressure decreases below atrial pressure. The duration of this phase is represented by Tau. The mitral valve (MV) then opens contemporaneously with atrial systole, filling the ventricle (2 – 3). Systole then commences with isovolemic contraction (3 – 4) until ventricular pressure exceeds diastolic arterial pressure, at which time the AV opens. Stroke volume is the difference between lines 1 – 2 and 3 – 4. Stroke work is the area within the 1 – 2 – 3 – 4 curve. Please click here to view a larger version of this figure.
Discussion
This working heart model enables assessment of ventricular performance with full control of ventricular preload and afterload, oxygen tension of the perfusate, as well as heart rate. Among other factors, it permits assessment of the intrinsic myocardial effects of inotropic medications independent of afterload and preload, which ways that are not possible using an in vivo model. Because this model utilizes a crystalloid perfusate, it permits assessment the myocardium without interference from hemoglobin, simplifying spectroscopic analysis of myocardial energy states, for example.14 In this model, the right atrium is not cannulated as part of our instrumentation, though it is possible to do so. We intentionally chose not to do so in order to facilitate sampling of coronary sinus flow for the assessment of myocardial oxygen consumption. Importantly, though, the right heart still performs pressure and volume work in this model as it pumps the coronary sinus flow into the pulmonary artery cannula. Providing some right ventricular preload improves positioning of the ventricular septum and enhances left ventricular performance, and is an important component of this model.15
There are several experimental pitfalls to mention. The first is the initial retrograde cannulation, which should be performed expediently (i.e., in less than 2 min) to minimize the period of ischemia. The most important skill to master is the efficient isolation, preparation and handling of the ascending aorta. It is important that the aortic stump not be cut excessively short, leaving insufficient room for cannulation above the aortic valve. However, it is also important that the aortic stump not be too long, which can cause torqueing of the aorta around the cannula. It is also important that the aorta cannula and aortic root be appropriately size-matched. An excessively large aorta on a small cannula can also lead to torqueing of the aortic root on the cannula. The right subclavian artery typically takes off from the ascending aorta approximately 7 mm above the aortic valve. Identifying the brachiocephalic vessels (approximately 1 mm in diameter) during dissection and trimming of the aorta serve as important landmarks for the transverse aortic incision. Trimming the aorta just below the takeoff of the first brachiocephalic artery is advisable. Inclusion of this vessel in the trimmed aortic root typically leads to a leakage of KHB, and loss of aortic root pressure upon transitioning into working heart mode.
Another technically challenging aspect of cannulation is the left atrial cannulation. Although it is feasible to cannulate the left atrial appendage, we found that the cannula frequently gets stuck within the appendage, and does not pass easily into the body of the left atrium. Thus, we prefer to make the incision in the body of the left atrium, approximately 2 mm superior to the atrioventricular groove. It is important to position the left atrial cannula in the proper plane before insertion in order to avoid tearing the thin-walled atrium when securing the cannula.
We found that the ideal size of the left atrium incision was approximately 3 mm. Creating too small of an incision may also make the placement of the left atrial cannula more difficult, and may lead to tearing of the left atrium. We use a straight, 8 mm, beveled piece of oxygen-impermeable tubing (inner diameter 2.9 mm) on the left atrial block. We have found that using this, rather than a cannula with a beveled edge, leads to most consistent atrial cannulation and facilitates the process of securing the left atrial block. Regardless of the tubing used, it is important to ensure that the end of the tubing is not occluded by the atrial septum or the mitral valve (as depicted above, we found that the left atrial pressure tracing was helpful in this regard), as even subtle movement of the atrial cannula can significantly alter left ventricular preload and resulting hemodynamic measurements. For the same reason, it is important to ensure that the left atrium does not leak following after opening the left atrial block. It is important regardless of the type of tubing used to ensure that the tubing within this system is oxygen impermeable to ensure adequate oxygen delivery to the heart.
Another technically challenging aspect of the procedure was the placement of the pressure-volume (PV) catheter. We initially favored a retrograde placement of the catheter through the aortic block. Though technically feasible, we found it to be much simpler and expedient to place the PV catheter via transapical puncture. Care must be taken to monitor the position of the catheter throughout the duration of the experiment, as at times the catheter may move in or out of the left ventricle. This can be done by monitoring the pressure and volume tracings over time.
Finally, care should be taken to ensure that KHB solution is created fresh for each experiment. It is possible to weigh out the constituents of KHB and store them in conical tubes in powdered form ahead of time. On the day of experimentation, these may be mixed with sterile, filtered water, carbon dioxide/oxygen, and then calcium added to the mixture. It is also important to wash the system with enzyme active powdered detergent such as Tergazyme (or similar) and replace the perfusate filter regularly.
Several limitations of this experimental preparation should be noted. First, similar to all crystalloid-perfused Langendorff preparations, KHB and other asanguinous perfusates have a significantly diminished oxygen carrying capacity relative to blood. Although this is partially compensated for through coronary vasodilation and supraphysiologic coronary flow, the preparation is not entirely physiologic for this reason. Second, because of the nearly infinite compliance of the Windkessel chamber used in this instrument, the systolic and diastolic pressures are only minimally separated (see Figure 2A) and thus the coronary perfusion pressure is non-physiologic. This may be overcome in future models by incorporating an elastance component to the afterload block. Third, as with all isolated heart preparations, the heart undergoes a defined period (2 – 3 min) of warm ischemia which is likely to create myocardial injury or dysfunction. Minimizing this injury through practice of the technique is of utmost importance to representative results. Further, although necessary for animal welfare, inhaled anesthetics may serve as a myocardial suppressant early in the reperfusion process, though it is expected that this effect is quickly abolished as the heart is reperfused with KHB.
The working heart system described allows for a wide variety of physiologic investigations relevant to patient care, research and teaching. With a few additional modifications, the system can also be used to simulate important physiology relevant to congenital heart disease, including pulmonary hypertension and single ventricle physiology. Limitations include that it is an ex vivo preparation, that the heart is being perfused by a buffer instead of a higher-oxygen content blood.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The equipment and experiments described here were funded by the Department of Cardiology, Boston Children's Hospital and by philanthropic donations from the Haseotes family. We are grateful to Drs. Frank McGowan and Huamei He for providing us with early experiences with this model, and to Lindsay Thomson for assistance with artwork.
Materials
| Sodium bicarbonate | Sigma-Aldrich | S5761 | 8.401 g/4 L |
| Ethylenediaminetetraacetic acid | Sigma-Aldrich | E6758 | 0.744 g/4 L |
| Potassium chloride | Sigma-Aldrich | P9333 | 1.580 g/4 L |
| Magnesium sulfate | Sigma-Aldrich | M7506 | 0.578 g/4 L |
| Sodium pyruvate | Sigma-Aldrich | P2256 | 0.220 g/ 4 L |
| Sodium chloride | Sigma-Aldrich | S3014 | 27.584 g/4 L |
| Dextrose | Sigma-Aldrich | D9434 | 7.208 g/4 L |
| Calcium chloride dihydrate | Sigma-Aldrich | C7902 | 1.470 g/4 L |
| Biventricular working heart model | Harvard Apparatus | IH-51 | |
| Pressure volume catheter | Millar, Inc | SPR-944-1 | 6 mm spacing catheter used |
| LabChart Pro 8 | AD Instruments | Version 8.1 |
References
- Langendorff, O. Untersuchungen am uberlebenden saugethierherzen [investigations on the surviving mammalian heart. Arch Ges Physiol. 61, 291-332 (1895).
- Neely, J. R., Liebermeister, H., Battersby, E. J., Morgan, H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 212 (4), 804-814 (1967).
- Friehs, I., Cao-Danh, H., et al. Adenosine prevents protein kinase C activation during hypothermic ischemia. Circ. 96 (9 Suppl), 221-226 (1997).
- Aoyagi, T., Higa, J. K., Aoyagi, H., Yorichika, N., Shimada, B. K., Matsui, T. Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion. Am J Physio. Heart Circ Physiol. 308 (12), H1530-H1539 (2015).
- Kitahori, K., He, H., et al. Development of left ventricular diastolic dysfunction with preservation of ejection fraction during progression of infant right ventricular hypertrophy. Circ Heart Fail. 2 (6), 599-607 (2009).
- Cowan, D. B., Noria, S., et al. Lipopolysaccharide internalization activates endotoxin-dependent signal transduction in cardiomyocytes. Circ Res. 88 (5), 491-498 (2001).
- Broadley, K. J. An analysis of the coronary vascular responses to catecholamines, using a modified Langendorff heart preparation. Br J Pharmacol. 40 (4), 617-629 (1970).
- Schmidt, H. D., Hoppe, H., Heidenreich, L. Direct effects of dopamine, orciprenaline and norepinephrine on the right and left ventricle of isolated canine hearts. Cardiol. 64 (3), 133-148 (1979).
- Fawaz, G., Tutunjini, B. The effect of adrenaline and noradrenaline on the metabolism and performance of the isolated dog heart. Br J Pharm Chemother. 15, 389-395 (1960).
- Allen, L. A., Fonarow, G. C., et al. Hospital variation in intravenous inotrope use for patients hospitalized with heart failure: insights from Get With The Guidelines. Circ Heart Fail. 7 (2), 251-260 (2014).
- Furnival, C. M., Linden, R. J., Snow, H. M. The inotropic and chronotropic effects of catecholamines on the dog heart. J Physiol. 214 (1), 15-28 (1971).
- Li, J., Li, J., et al. Adverse effects of dopamine on systemic hemodynamic status and oxygen transport in neonates after the Norwood procedure. J Am Coll Cardiol. 48 (9), 1859-1864 (2006).
- Gillis, A. M., Kulisz, E., Mathison, H. J. Cardiac electrophysiological variables in blood-perfused and buffer-perfused, isolated, working rabbit heart. Am J Physiol. 271 (2 Pt 2), H784-H789 (1996).
- Asfour, H., Wengrowski, A. M., Jaimes, R., Swift, L. M., Kay, M. W. NADH fluorescence imaging of isolated biventricular working rabbit hearts. J Vis Exp. (65), (2012).
- Demmy, T. L., Magovern, G. J., Kao, R. L. Isolated biventricular working rat heart preparation. Ann Thor Surg. 54 (5), 915-920 (1992).

