Two Techniques to Create Hypoparathyroid Mice: Parathyroidectomy Using GFP Glands and Diphtheria-Toxin-Mediated Parathyroid Ablation
Summary
Mice with acquired hypoparathyroidism would be useful for studying novel drug therapies for hypoparathyroidism. Two procedures to create such mice are demonstrated. The GFP-PTX mouse is generated by surgical parathyroidectomy guided by green fluorescing parathyroid glands. A second, non-surgical approach is based on parathyroid-specific expression of the diphtheria toxin receptor.
Abstract
Hypoparathyroidism (HP) is a disorder characterized by low levels of PTH which lead to hypocalcemia, hyperphosphatemia, and low bone turnover. The most common cause of the disease is accidental removal of the parathyroid glands during thyroid surgery. Novel therapies for HP are needed, but testing them requires reliable animal models of acquired HP.
Here, we demonstrate the generation of two mouse models of acquired HP. In the GFP-PTX model, mice with green fluorescent protein (GFP) expressed specifically in the parathyroids (PTHcre-mTmG) were created by crossing PTHcre+ mice with Rosa-mTmGfl/fl mice. Green fluorescing parathyroid glands are easily identified under a fluorescence dissecting microscope and parathyroidectomy is performed in less than 20 min. After fluorescence-guided surgery, mice are profoundly hypocalcemic. Contrary to the traditional thyro-parathyroidectomy, this precise surgical approach leaves thyroid glands and thyroid function intact. The second model, which does not require surgery, is based on a diphtheria-toxin approach. PTHcre-iDTR mice, which express the diphtheria toxin (DT) receptor specifically in the parathyroids, were generated by crossing the inducible DTR mouse with the PTHcre mouse. Parathyroid cells are thus rendered sensitive to diphtheria toxin (DT) and can be selectively destroyed by systemically injecting mice with DT. The resulting hypocalcemic phenotype is stable.
Introduction
Since the first systematic description of the parathyroid glands in human and in several other species by Sandström in 1880 1, understanding the significance of this small endocrine organ for human physiology and disease has continuously improved. Parathyroid glands secrete parathyroid hormone (PTH), the principal regulator of calcium metabolism. PTH is also an important hormone for phosphate homeostasis and bone turnover 1,2. Accidental removal or damage of the parathyroids during neck surgery is the most common cause of hypoparathyroidism, a disease characterized by low blood calcium, low PTH, and elevated phosphorus 1.
To better study acquired hypoparathyroidism and test novel therapies, reliable and easily accessible mouse models are required. Mice are widely used in research because a wide variety of genetic tools are available in this species permitting sophisticated mechanistic studies in vivo. However, while surgical parathyroidectomy (PTX) can be used in rats 2,3,4,5, and larger mammals 6,7,8, it is technically very challenging in mice because of the small size of the glands and their variable anatomic distribution 9. Therefore, thyro-parathyroidectomy (TPTX) is typically performed in mice, in which the thyroid and parathyroid glands are removed together 10. However, low thyroid hormone levels are a potential confounder in experiments, complicating this model. In addition, C-cells within the thyroid gland, which produce calcitonin, a hormone important in calcium homeostasis in rodents, are also lost upon removal of the thyroids 11.
Several genetic mouse models of hypoparathyroidism exist, which include the PTH-null mouse 12, the GCM2-null mouse 13, and the Nuf mouse with an activating mutation in the calcium-sensing receptor (CaSR) 14,15. However, these genetic defects are already present during embryonic development, and the function of the parathyroids is therefore already impaired during embryogenesis. This can affect the development of organs such as the skeleton. This contrasts with patients with postsurgical hypoparathyroidism who acquire the disease later in life. Moreover, some of these mouse models exhibit early lethality and reduced fertility, which further complicates their use 12,13,14.
We developed two new mouse models for acquired hypoparathyroidism. Using genetically engineered mice that express GFP specifically in the parathyroids allows the parathyroid glands to be easily identified for surgical removal without removing the thyroid gland. This mouse was generated by crossing the PTH-Cre mouse, which expresses Cre recombinase under the control of the 5.5 kb PTH promoter 16 with the ROSAmTmG mouse. The resulting PTHcre;mTmG mice express green fluorescent protein specifically in parathyroid cells. The second mouse model uses the same PTH-Cre, this time to remove a STOP cassette from the inducible DTR mouse, resulting in expression of the diphtheria toxin receptor specifically in the parathyroids. Systemic administration of DT destroys parathyroid cells, rendering the animals hypoparathyroid without surgery.
The two mouse models presented in this study demonstrate a stable hypocalcemic phenotype over the three-month observation period. The procedures are easy to perform, the phenotype is reproducible, and the hypoparathyroid mice exhibit a high survival rate 17.
Protocol
This study has been approved by the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts General Hospital. Obtain appropriate institutional approval for this animal study before beginning. Some techniques might have to be adjusted according to local IACUC requirements.
1. GFP-PTX Mice
- Obtain PTHcre+ mice and Rosa-mTmG mice at 8 – 10 weeks old for further mating.
- Backcross the PTHcre+ mice (mixed 129;FVB background) with C57BL/6 mice for 6 generations to obtain mice with C57BL/6 background.
- Inbreed PTHcre+ mice to obtain PTHcre+/+ mice17.
NOTE: To perform genotyping, extract the DNA from the tip of the tail and use it for PCR. The PCR primers to check for heterozygosity and homozygosity of the PTHcre transgene are as follows: forward primer A, CCTGTCAAGGATGTGGAAGA, reverse primer A', TCAGATCACACCACACAGCA, forward primer B, CAGTTGTCTTTAGTTTACTCAGCATCAG, reverse primer B', GATAATCGCGAACATCTTCAGGTT17. - Cross PTHcre+/+ mice16 with Rosa-mTmG mice18. Wean PTHcre;mTmG pups when 4 weeks old.
- Anesthetize 8 – 10 weeks old PTHcre;mTmG mice by i.p. injection of tribromoethanol (Avertin) at 0.6 mg/g body weight, or other agents such as ketamine/xylazine. Use buprenorphine 0.1 mg/kg s.c. every 12 h as an analgesic for 48 h after surgery according to an approved protocol. Ensure appropriate depth of anesthesia by the toe pinch withdrawal reflex or other means. Place the animal in the supine position.
- Extend and prepare the ventral neck region by shaving the skin using single edge blades. Disinfect the shaved skin with a povidone iodine antiseptic pad.
- Cover the animal with sterile surgical drapes to reduce contamination of the surgical site and use autoclaved microsurgical instruments for the surgical procedure.
- Cut a 2 cm longitudinal incision in the skin with a surgical scalpel. Dissect fascia and push the salivary glands to the side by blunt dissection with curved serrated forceps.
- Under the dissection microscope using halogen light and a 4 – 5X magnification, cut and separate the paratracheal muscles using sharp forceps tips and expose the trachea.
- Identify the thyroid gland with the right and left lobe located next to the trachea.
- Switch the light source to fluorescent light and visualize the two green-fluorescing parathyroid glands.
NOTE: Typically, the two parathyroid glands are located at or near the surface of the thyroid glands, but occasionally, they are located further away. - Carefully remove the green parathyroid glands using surgical forceps and scissor. Use sterile gauze for hemostasis and carefully check along the trachea to ensure that all green tissue has been removed.
- Close the paratracheal muscles by interrupted suture using 6-0 polyglactin 910 sutures. Close the skin incision by Halsted suture using 6-0 polyglactin 910 sutures.
- Optional: Inject 10 µL/g body weight sterile normal 0.9% sodium chloride solution to the mice for fluid replacement.
- Place postsurgical animals to a separate cage on a warm incubator (37 °C) for body temperature recovery. Once the mice are awake and in the recumbent position, put pellet chow and water with some gelatin food on the cage floor.
- After a postsurgical observation period of 2 h, return mouse to the animal facility and follow local requirements for postsurgical care.
- 3 days after parathyroidectomy, obtain 10 µL tail blood and measure blood ionized Ca++ using an analyzer such as the blood gas system Ca++/pH analyzer. Successful parathyroidectomy results in blood ionized Ca++ equal or lower than -2SD of sham-operated control mice (1.20 mmol/L in our experiments of n = 30).
2. PTH-cre-iDTR Mice
- Obtain PTHcre+ and DTRfl/fl mice at 8 – 10 weeks old for mating.
- Backcross the PTHcre mice (mixed 129;FVB background) with C57BL/6 mice for 6 generations to obtain mice with C57BL/6 background.
- Inbreed the PTHcre+ (C57BL/6 background) mice to obtain PTHcre+/+ mice17.
NOTE: To perform genotyping, DNA from mice tail tips was extracted and used for PCR. Genotyping Primers: forward primer A, CCTGTCAAGGATGTGGAAGA, reverse primer A', TCAGATCACACCACACAGCA, forward primer B, CAGTTGTCTTTAGTTTACTCAGCATCAG, reverse primer B', GATAATCGCGAACATCTTCAGGTT17. - Mate PTHcre+/+ mice16 with DTRfl/fl mice19 to obtain PTHcre-iDTR mice.
- Prepare DT solution by diluting diphtheria toxin powder with sterile saline to a concentration of 0.5 µg/mL. Sterile filter, and store aliquots at -80 °C.
- Choose 8 – 10 weeks old PTHcre-iDTR mice for injection experiment. Thaw DT aliquot to room temperature, administer DT intraperitoneally at 5 µg/kg (10 µL/g) bodyweight of the animal. For maximal efficiency and least toxicity, DT administration is repeated for a total of 2 injections in a 3-day interval17.
- 3 days after the second injection, take 10 µL tail blood from each PTHcre-iDTR/PTHcre-iDTR-mTmG mouse and measure blood Ca++ using a Blood Gas System Ca++/pH analyzer.
NOTE: We define mice with blood ionized Ca++ lower than 1.18 mmol/L as hypoparathyroid mice, which equals 2SD below the mean of vehicle-injected control mice (n = 22).
Representative Results
Location of the Parathyroid Glands
First, we recorded the distribution of the parathyroid glands of 54 PTHcre-mTmG mice as observed under the fluorescent dissection microscope. 74% (40/54) mice had two green parathyroid glands (Figure 1A, C, E), 26% (14/54) mice had an additional third parathyroid gland (Figure 1B, D, F). No mouse with a single gland or more than three glands were observed. Usually, parathyroid glands were located near the superior border of the thyroid gland (58%, 71/122; Figure 1A(1,2), B(1,2), C(2), D(1,2), E(2), F(3)). 27% (33/122) glands were located near the inferior border of the thyroid gland (Figure 1C(1), D(3), F(1)), and 13% (16/122) were located away from the thyroid gland (Figure 1B(3), E(1), F(2)). Our findings are consistent with and extend previous findings of varying locations of the parathyroid glands20.
GFP-PTX Mice
The entire surgery from anesthesia to closing the skin incision took approximately 20 min per mouse. The survival rate of postsurgical mice over a 3-month observation period was 96.3% (53/55). 92.4% (49/53) GFP-PTX mice exhibited ionized calcium levels that were 2 SD below the mean of sham-operated control mice or lower. The hypoparathyroid phenotype in the GFP-PTX mice (hypocalcemia, low PTH and elevated serum phosphate) was stable for the entire observation time of 3 months. Importantly, thyroid function was not different from sham-operated animals 3 months after surgery (TSH = 42 ± 24 vs. 30 ± 15 mU/L, p = 0.171; T4 = 3.0 ± 0.6 vs. 3.1 ± 0.9 µg/dL, p = 0.707 (Figure 2).
PTH-cre-iDTR Mice
DT injected PTH-Cre-iDTR mice developed hypoparathyroidism (low blood ionized calcium, elevated blood phosphorus, and inappropriately low-normal PTH levels). We have previously reported that a few PTH positive cells escape the ablation by diphtheria toxin, explaining the measurable circulating PTH and the therefore the somewhat milder phenotype of these mice17.
Hypoparathyroidism in GFP-PTX Mice and PTH-cre-iDTR Mice
Significant reductions of blood Ca++ and serum PTH levels were observed in GFP-PTX mice 3 days after surgery, compared to levels found in sham-operated mice (Ca++ = 1.05 ± 0.40 vs. 1.30 ± 0.03 mmol/L, p < 0.05; PTH=32 ± 22 vs. 580 ± 137 pg/mL, p < 0.05). The hypoparathyroidism phenotype was stable over the 3-month observation period (Figure 3A, reprinted with permission from (reference#17)). After 2 injections of 5 µg/kg DT at 3 days interval, a dose and regimen optimized to use the least amount of DT to give the maximal hypoparathyroid phenotype, the DT injected PTH-cre-iDTR mice showed significant hypocalcemia and reduced PTH compared to vehicle control mice, which persisted over the 90 days observation period (Ca++ = 1.10 ± 0.07 mmol/L vs. 1.26 ± 0.05 mmol/L, p < 0.05; PTH = 218 ± 156 pg/mL vs. 572 ± 164 pg/mL, p < 0.05) (Figure 3B, reprinted with permission from (reference#17)).
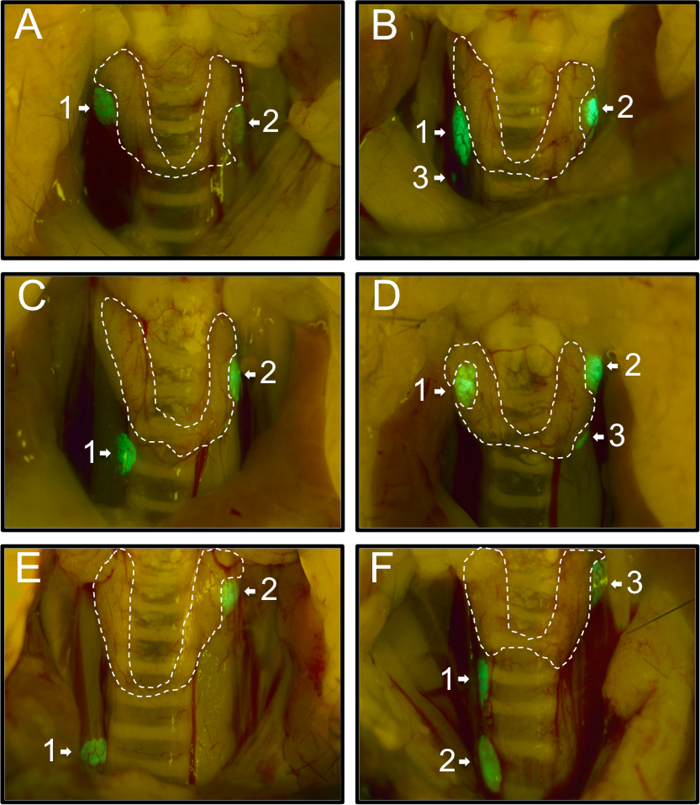
Figure 1: Representative Images of the Various Locations of the Parathyroid Glands in 54 PTHcre-mTmG Mice under Fluorescent Dissection Microscope. Mice had either two (A, C, E) or three (B, D, F) green parathyroid glands. Most parathyroid glands were located near the superior border (A(1,2), B(1,2), C(2), D(1,2), E(2), F(3) or near the inferior border of the thyroid gland (C(1), D(3), F(1)). In rare cases, some were located ectopically (B(3), E(1), F(2)). Please click here to view a larger version of this figure.
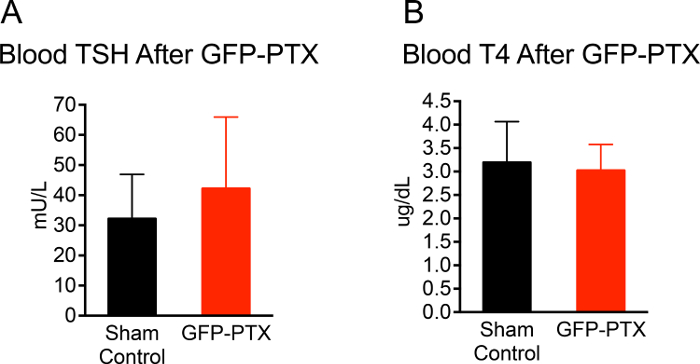
Figure 2: TSH and T4 Level of GFP-PTX Mice. 3 months after GFP-PTX, serum was obtained for TSH and T4 measurements. GFP-PTX mice showed TSH concentrations (42 ± 24 mU/L) (A) and T4 concentrations (3.0 ± 0.6 μg/dL) (B) that were not different from control mice (TSH = 30 ± 15 mU/L, p = 0.171, n =11, T4 = 3.1 ± 0.9, p = 0.707, n = 11). Please click here to view a larger version of this figure.
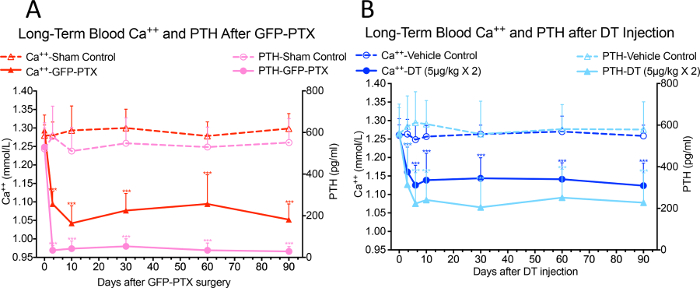
Figure 3: Blood Ca++ and PTH Levels in GFP-PTX Mice and DT Injected PTH-cre-iDTR Mice. Both GFP-PTX mice (A) and DT injected PTH-cre-iDTR mice (B) exhibited stable hypocalcemia and reduced PTH levels over 3 month observation period (N = 6 – 8, ***p < 0.001) (re-printed with permission from reference#17). Please click here to view a larger version of this figure.
Discussion
We demonstrate the technique of GFP-guided parathyroidectomy using transgenic mice with GFP expression selectively in the parathyroid glands. In the PTHcre;mTmG mice, the double fluorescence (the green GFP in Cre-expressed cells and red Tomato in non-Cre expressed cells) enabled us to clearly identify, and to precisely remove all parathyroid glands without removing the thyroids. While we prefer the use of the double-fluorescence mTmG mice for the ability to identify fluorescent red non-parathyroid tissue, our procedure should also work well using single fluorescent animals such as the tomato red (B6.Cg-Ct(ROSA)26Sortm14(CAG-tdTomato)Hze/J) mouse. The procedure is relatively easy to perform, requires about 20 min per mouse, and results in a severe and sustained hypoparathyroid phenotype.
In addition, another advantage of the GFP-positive parathyroid glands is the ease of detection of aberrant parathyroid glands. Our studies demonstrate mislocalization of the parathyroids in about a quarter of B6 mice (Figure 1), which are readily detected in our mouse model by the green fluorescence. Contrary to thyro-parathyroidectomy, our technique avoids the removal of the thyroid with subsequent need for thyroid hormone replacement and the unknown effect of removal of calcitonin-producing thyroid C-cells. Fluorescently labeled parathyroid glands could be also used for other investigations that require the isolation of the parathyroid glands. The limitation of this model includes the requirement for surgery. Basic microsurgery skills and a dissection microscope with a fluorescent light source are required.
A second technique for generating hypoparathyroid mice that eliminates the need for surgery is demonstrated. Diphtheria toxin-treated PTHcre-iDTR mice exhibit a milder hypoparathyroidism phenotype, but simply require injecting DT intraperitoneally into the mouse. The critical part for generating this model was the optimization of the dose and dosing regimen. High doses of DT led to toxicity but low doses decreased the efficacy of the approach. We determined that the dose regiment of 2 injections at 5 µg/kg, administered 3 days apart, resulted in reliable and stable hypocalcemia with no/minimal mortality.
Ultimately, we hope that these two approaches provide useful mouse models for acquired hypoparathyroidism. Using a novel long-acting PTH, we reported the first use of both of these models in determining the efficacy of new drugs for hypoparathyroidism17.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the NIH grants R01-DK100584 and China State Key Laboratory of Oral Diseases Open Funding SKLOD2015OF01 (RB). We thank Wenping Zhao, Tadatoshi Sato, and Kelly Lauter for help.
Materials
| ROSAmT/mG mice | The Jackson Laboratory | 7676 | |
| PTH-Cre mice | The Jackson Laboratory | 5989 | |
| iDTRmice | The Jackson Laboratory | 7900 | |
| 6-0 polyglactin 910 suture with needle | Ethicon, Inc | J510G | |
| Safety Single Edge Razor Blades | American Safety Razor Company | 66-0089 | |
| Disposable Scalpel | Feather Safety Razor Co., LTD | 72042-11 | |
| Povidone-Iodine Prep Pads | Dynarex Corporation | 1108 | |
| Ply gauze | Busse. Inc | BHD707 | |
| 0.9% Sodium Chloride Solution | HOSPIRA Worldwide, Inc | 07983-09 | |
| 1mL 29G Insulin Syringe | BECTON DICKINSON | 329622 | |
| Surgical Incise Drapes | 3M | 6640EZ | |
| Dumstar Biology forceps | Roboz Surgical Instrument Co., Inc | RS-4984 | |
| Micro Dissecting Spring Scissors | Roboz Surgical Instrument Co., Inc | RS-5605 | |
| Needle Holder | MILTEX.,Inc | V98-42 | |
| 2,2,2-Tribromethanol | Sigma-Aldrich | T48402 | |
| 2-Methyl-2-Butanol | Sigma-Aldrich | 152463 | For dissolve the 2,2,2-Tribromethanol |
| Diphtheria Toxin Powder | Sigma-Aldrich | D0564 | Dissolve in 0.9% sodium chloride solution at 1mg/mL as the stock solution in -80C |
| Multicap Blood Collection Capillary tubes | Siemens Healthcare Diagnostics Ltd | 855578 | For collecting blood in iCa2+ analysis using RapidLab 348 Ca2+/pH analyzer |
| RapidLab 348 Ca2+/pH analyzer | Siemens Healthcare | For iCa2+ analysis |
References
- Brandi, M. L., Brown, E. M. . Hypoparathyroidism. , (2015).
- van Abel, M. Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int. 68, 1708-1721 (2005).
- Sebastian, E. M., Suva, L. J., Friedman, P. A. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 43, 1022-1030 (2008).
- Rodriguez-Ortiz, M. E. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 23, 1190-1197 (2012).
- Liao, H. W. Relationship between Fibroblast Growth Factor 23 and Biochemical and Bone Histomorphometric Alterations in a Chronic Kidney Disease Rat Model Undergoing Parathyroidectomy. PloS one. 10, e0133278 (2015).
- Fox, J., Care, A. D. Effect of low calcium and low phosphorus diets on the intestinal absorption of water in intact and parathyroidectomized pigs. Calcif Tissue Int. 31, 253-255 (1980).
- Finco, D. R., Brown, S. A., Ferguson, D. C., Crowell, W. A. Selective parathyroidectomy of the dog. Can J Vet Res. 57, 288-292 (1993).
- Can, I. Parathyroid allotransplantation in rabbits without cultivation. Int J Clin Exp Med. 7, 280-284 (2014).
- Dunn, T. B. Melanoblasts in the stroma of the parathyroid glands of strain C58 mice. j Natl Cancer Inst. 10, 725-733 (1949).
- Sakai, A. Osteoclast development in immobilized bone is suppressed by parathyroidectomy in mice. J Bone Miner Metab. 23, 8-14 (2005).
- Miao, D. Skeletal abnormalities in Pth-null mice are influenced by dietary calcium. Endocrinology. 145, 2046-2053 (2004).
- Gunther, T. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 406, 199-203 (2000).
- Hough, T. A. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci U S A. 101, 13566-13571 (2004).
- Hannan, F. M. The Calcilytic Agent NPS 2143 Rectifies Hypocalcemia in a Mouse Model With an Activating Calcium-Sensing Receptor (CaSR) Mutation: Relevance to Autosomal Dominant Hypocalcemia Type 1 (ADH1). Endocrinology. 156, 3114-3121 (2015).
- Libutti, S. K. Parathyroid gland-specific deletion of the mouse Men1 gene results in parathyroid neoplasia and hypercalcemic hyperparathyroidism. Cancer Res. 63, 8022-8028 (2003).
- Bi, R. Diphtheria Toxin- and GFP-Based Mouse Models of Acquired Hypoparathyroidism and Treatment with a Long-Acting Parathyroid Hormone Analog. J Bone Miner Res. , (2015).
- Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L., Luo, L. A global double-fluorescent Cre reporter mouse. Genesis. 45, 593-605 (2007).
- Buch, T. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2, 419-426 (2005).
- Dunn, T. B. Melanoblasts in the stroma of the parathyroid glands of strain C58 mice. J Natl Cancer Inst. 10, 725-733 (1949).

