Using Laser Doppler Imaging and Monitoring to Analyze Spinal Cord Microcirculation in Rat
Summary
Here we present a combination of laser Doppler perfusion imaging (LDPI) and laser Doppler perfusion monitoring (LDPM) to measure spinal cord local blood flows and oxygen saturation (SO2), as well as a standardized procedure for introducing spinal cord trauma on rat.
Abstract
Laser Doppler flowmetry (LDF) is a noninvasive method for blood flow (BF) measurement, which makes it preferable for measuring microcirculatory alterations of the spinal cord. In this article, our goal was to use both Laser Doppler imaging and monitoring to analyze the change of BF after spinal cord injury. Both the laser Doppler image scanner and the probe/monitor were being employed to obtain each readout. The data of LDPI provided a local distribution of BF, which gave an overview of perfusion around the injury site and made it accessible for comparative analysis of BF among different locations. By intensely measuring the probing area over a period of time, a combined probe was used to simultaneously measure the BF and oxygen saturation of the spinal cord, showing overall spinal cord perfusion and oxygen supply. LDF itself has a few limitations, such as relative flux, sensitivity to movement, and biological zero signal. However, the technology has been applied in clinical and experimental study due to its simple setup and rapid measurement of BF.
Introduction
The tissue of the spinal cord is highly vascularized and extremely sensitive to hypoxia induced by spinal cord injury (SCI). Our previous studies showed that blood flow of the spinal cord was significantly decreased after concussion injury1,2, which might be related to the deficit of motor function. Recent studies have shown that the integrity of blood vessels following SCI is well-correlated with the improvement of sensory motor function3. It has been reported that improved vascularity might rescue white matter, indirectly leading to improved function4. Therefore, the maintenance of post-injury spinal cord perfusion appeared to be of primary importance for preserving viability and functionality.
The effects of various treatments on perfusion after SCI have been examined by numerous investigators using a variety of techniques in experimental models of SCI5,6,7. Laser Doppler, as a well-established technique, was undoubtedly a useful method for quantifying perfusion in several animal and human studies8,9,10,11. The technique is based on measuring the Doppler shift12 induced by moving red blood cells to the illuminating light. Since the commercialization of the technique in the early 1980s, great progress has been made in laser technology, fiber optics and signal processing for measuring perfusion by laser Doppler instruments13, which made LDF into a reliable technology.
In the current study, both methods of laser Doppler measurement were applied to evaluate blood flow (BF) in the spinal cords of concussive rats. Due to the noninvasive nature of the technology and its simple setup, our protocol provides a sensitive, rapid and reliable method for BF measurements of the spinal cord. More importantly, this method allows longitudinal study of BF post concussive SCI without animal sacrifice at each time point.
Due to the ability to assess the BF of the tissue and fast changes of perfusion during stimulation, it is possible to apply this protocol to evaluate cerebral BF14,15 as well as measure other tissues such as liver16,17, skin18,19, and bowel20. In a rat model of transient occlusion of the middle cerebral artery, the laser Doppler readings were used to ensure proper reduction of the BF rate to levels that are expected in the ischemic penumbra14. In rats which have undergone critical limb ischemia (CLI) induction, laser Doppler scanning was applied to observe hind limb BF before and after the CLI procedure and during different periods after treatment21. Additionally, the bioavailability and metabolic clearance of some drugs depended on hepatic BF, which was detected by LDF16. Therefore, LDF could be widely used in experimental model, pharmacodynamic, and pharmacokinetic evaluation.
Protocol
Animal protocols involving experimental animals followed guidelines established by the National Institutes of Health (NIH) and were approved by the Animal Care and Use Committee of Capital Medical University.
The procedures of introducing SCI and measuring BF of spinal cord using laser Doppler equipment described below were used in a published study1.
1. Preparation for the Surgery
- Prepare pentobarbital sodium solution 3% (w/v) in saline and administrate at the dose of 35 mg/kg.
Caution: pentobarbital sodium is a controlled substance. Detailed records should be kept and solutions stored in a safe, locked location. - Sterilize equipment and prepare surgery area.
- Clean the surgery equipment with the following steps: 75% ethanol cleaning, then autoclave at 121 °C for 30 min, then dry in a 60 °C oven overnight. Sterilize the surgery area with 75% alcohol.
2. Preparation of Rat for Surgery
- Anesthetize the rat with an intraperitoneal injection of pentobarbital sodium (35 mg/kg). The entire procedure should take 30 – 40 min including surgery, BF measurements, and sutures.
- Shave the dorsal area of the rat from the lower back to the neck. The hair should be cut as short as possible. Place the rat on a 40 °C heating pad to maintain a constant body temperature.
3. Laminectomy and the Concussion to the Spinal Cord
NOTE: To perform laminectomy only for the sham group, follow steps 3.1 to 3.6.
- Position the animal dorsal side up. Sterilize the shaved part with iodine followed by 75% alcohol using sterile cotton balls. Make a skin incision (4 cm) with the scalpel over the laminectomy site covering thoracic vertebrae T7 to T11.
- Cut the attached muscles on both sides from T8 to T10 to expose the spinous processes, the laminae, and the facet joints.
- Use the scalpel to make incisions that disconnect the junction between T10 and T11. Further expose the junction by carefully by dissecting the muscle layer away to expose the bone.
- Use the scissors to further clear muscle away from the lamina and around the pedicle with small snips. This will open a small space between the vertebrae at T10 and T11 (Figure 1A). Slowly and delicately insert one hemostatic forceps into this gap and break the pedicle (Figure 1B). Make sure the curvature of the forceps is always positioned laterally, away from the cord. Repeat on the other side.
- Expose the spinal cord (Figure 1C) and carefully lift and break off the lamina. Be sure not to leave any free or jagged bone fragments behind.
- Repeat the process to further remove T9 and T8 laminae.
- Move the animal to the impactor equipment table and use the pair of Adson forceps attached to the table to stabilize the animal's spine by clamping on the spinous process of T7 and T11, then adjust the forceps to straighten the spine (Figure 1D).
- Put the animal under the impactor, aim the strike rod to the center of the exposed spinal cord and lower the rod to within 3-5 mm of the surface of the spinal cord.
- Set the impact parameters such as the impact force (160 KD) and dwell time (1 s)
- Induce the SCI by clicking the "Start Experiment" button on the software interface, then click "yes" on the following interface to start the impact automatically. After the impact, the software will display the actual data of the impact next to the set parameters, check the data to make sure it was close to the setting point (Figure 1E).
NOTE: A typical sign for the success of the experiment was a short period of involuntary tail swing and limb movement after the impact. Stimuli to the tail to check for limb reflection could also be done. However, locomotor assessment such as the Basso, Beattie, and Bresnahan (BBB) locomotor scale22,23 is necessary to determine the effectiveness of induced injury.
- Induce the SCI by clicking the "Start Experiment" button on the software interface, then click "yes" on the following interface to start the impact automatically. After the impact, the software will display the actual data of the impact next to the set parameters, check the data to make sure it was close to the setting point (Figure 1E).
4. Laser Doppler Scanning
- See the Table of Materials for the details of the laser Doppler scanner used in this study. To scan the exposed spinal cord, place the rat dorsal side up on a black, non-reflective background.
- Set up scanning parameters: Open the scanning software, click "Measure" to enter the measurement graphical user interface and click on the "Scanner setup" button to open the scanner setup interface. To scan small areas such as in this experiment, select "High Resolution" under the "Scan Size and Display Options" for a fine scanning mode with higher resolution (256 × 256 points covering 4 × 10 cm2) (Figure 2A). Click on the "Image Scan" option to check the scanning perimeters (Figure 2B).
- Click on the "Video and Distance" option to check the live video image. Position the scanner 10-13 cm above the surgical window and move the background with the animal to center the exposed spinal cord on the scanning window (Figure 2C).
- Use the "auto distant" function to fine adjust the scanning height, note the height of scanning should be kept consistent across all measurements in the experiment Figure 2C.
- Use a nonreflective cover with a window to expose only the surgical area to further minimize background and mark the animal's direction.
- Click on the "Repeat Scan", set the number of scans (we use 8 repeat scans in this case) then click "OK" to open the repeat scan interface. Click the start button to start scanning and the whole process will take approximately 3 – 4 min (Figure 2D).
5. Laser Doppler Monitoring
- We used a scanner monitor with VP3 Blunt needle end delivery probe to monitor BF and SO2 over time. Attach the Laser Doppler probe perpendicular to a stereotaxic instrument to set up the monitoring equipment.
- Put the rat on the stereotaxic apparatus dorsal side up, underlay the animal with a small piece of Styrofoam when necessary to level the exposed spinal cord.
- Lower the probe to the spinal cord to monitor BF.
NOTE: Step 5.3 is crucial for the reproducibility of the measurement as the data readings are sensitive to the pressure applied to the probe, hence extra caution is required to not over- or under-position the probe.- Examine the incision and remove any excessive liquid or blood using a sterile cotton pad.
- Use the apparatus's X and Y axis to locate the probe to 2 mm rostral to the center point of the exposed spinal cord or lesion point and avoid the central vein.
- Use the Z axis to slowly lower the probe to the level just touching the surface of the spinal cord. The probe should just touch the surface of the spinal cord but not so loose to allow any bright light to escape from the side of the contact point.
- Data recording
- Open the data acquisition software, click on the "new experiment" button to open the setup interface. Under the "General" option check for the system configuration and click "Next" (Figure 3A), in the Display Setup select the channel for BF and SO2 and click "Next" (Figure 3B).
- Input file information and click "Next" (Figure 3C) to enter the data recording interface, click on the green triangle button to start recording data from the probe (Figure 3D).
- Once the signal is stable, record data for 8 consecutive min. Then lift the probe and remove the animal from stereotaxic apparatus to suture the incision and put the animal into post-operative care.
6. Sutures and Post-Operation Care
- Suture the incision: Insert a suture needle into the muscle on both sides of the incision. Pull the thread through, pulling the tissues together, thereby covering the exposed spinal cord at the site of removed laminae. Using the needle holder, pull the entire thread through, form three square knots and trim the thread as close to the knots as possible.
- Suture the skin with 3 – 4 square knots in the same way as suturing the incision, then trim the threads approximately 1 cm from the knots.
- Place the rat on its side in its cage, avoiding contact between the surgery site and the cage bottom. Cages should be placed on heating pads.
- Monitor the animal until it wakes up from anesthesia to ensure no post-surgery bleeding and that the sutures remain closed.
- Subcutaneously inject Benzyl Penicillin Sodium in rat for 3 days following surgery, 120 mg/kg per day. Intraperitoneally inject Buprenorphine (0.05 mg/kg) immediately after surgery and every 6 hours post-surgery for 1 day.
- To make sure animals have access to sufficient food and water, fit water bottles with extended spouts and put food close to the animal in the cage.
NOTE: We conducted BBB rating scale to evaluate the hindlimb locomotor function of the animal 24 h post-injury to exclude animals with a BBB score above 0, therefore ensuring that the animal was paralyzed by the induced injury. - Post-surgery, provide manual empty of urinary bladder by gently applying pressure on the abdomen twice daily, if necessary.
Representative Results
LDPI was used to measure BF in the spinal cord, which was quantified along the rostral-caudal axis of the spinal cord by extracting linear profiles (Figure 4). Figure 5A and Figure 5B represent the flux imaging of the spinal cord of the sham group and SCI group, respectively. Figure 5C and Figure 5D represent the altering BF along the rostral-caudal axis of the spinal cord of sham group and SCI group, respectively. A comparison of Figure 5A and Figure 5B demonstrated that SCI induced reduction of BF, and BF of the epicenter was lower than rostral cord and caudal cord.
LDPM showed the time-domain LD signal and SO2 and Figure 6 illustrated the acquiring and processing of the LDPM data. After the data were recorded, an 8 min stretch of continuous Region of Interest (ROI) data was selected, which was then filtered by a built-in filter to minimize any non-biological signals. Subsequently, the ROI was statistically analyzed and the results were exported in a raw data format. Figure 7 recorded the periodic variation of BF and SO2 over time in the sham group and SCI group. As shown in Figure 7A, the spinal cord BF of the SCI group significantly decreased compared with the sham group. Simultaneously, the SO2 of the spinal cord was remarkably lower after spinal cord concussion (Figure 7B), which was consistent with the change of BF after injury. To reduce disturbance, measurements were taken repeatedly and the data were normalized.
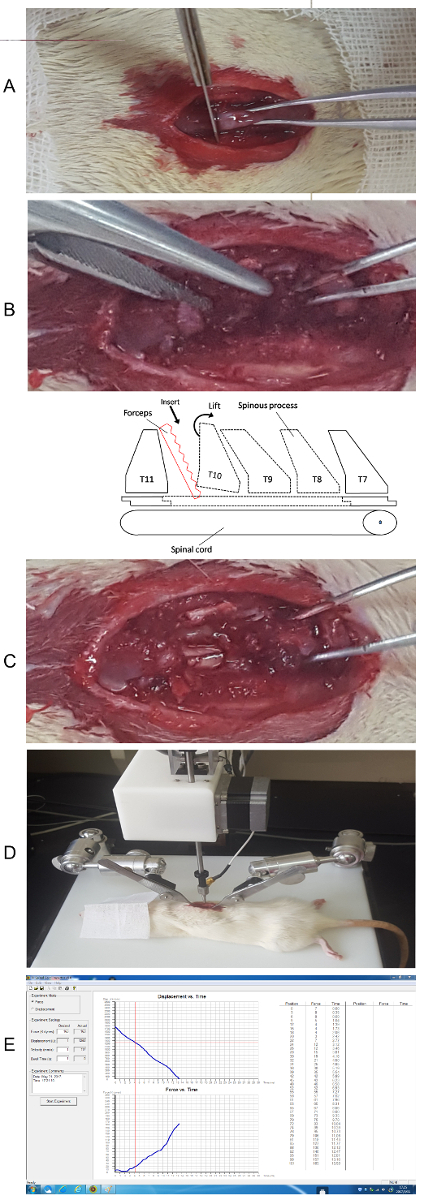
Figure 1. Laminectomy and the concussion to the spine. (A) Disconnect the junction between T10 and T11. (B) Insert the forceps to break the pedicle. (C) Break the lamina and expose the spinal cord. (schematic sketch of the anatomy) (D) Stabilize the spine on the experiment table. (E) Initial impact using the software and check the data. Please click here to view a larger version of this figure.
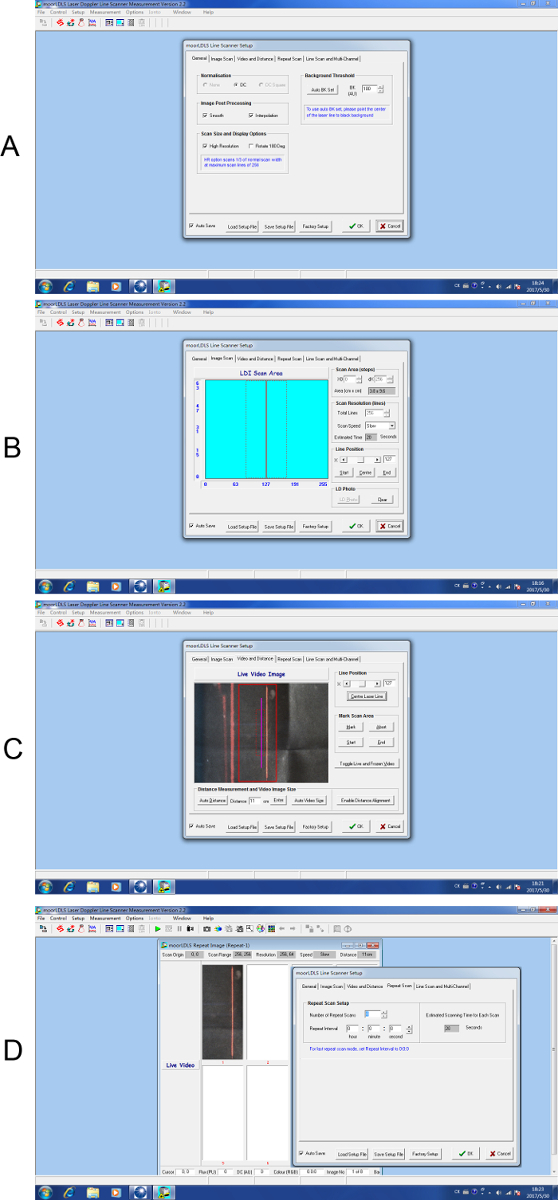
Figure 2. Step by step setup for laser Doppler scanning. (A) General setup for scanning. (B) Setup interface for image scan parameters. (C) Setup interface for video and distance. (D) Setup interface for repeat scan. Please click here to view a larger version of this figure.
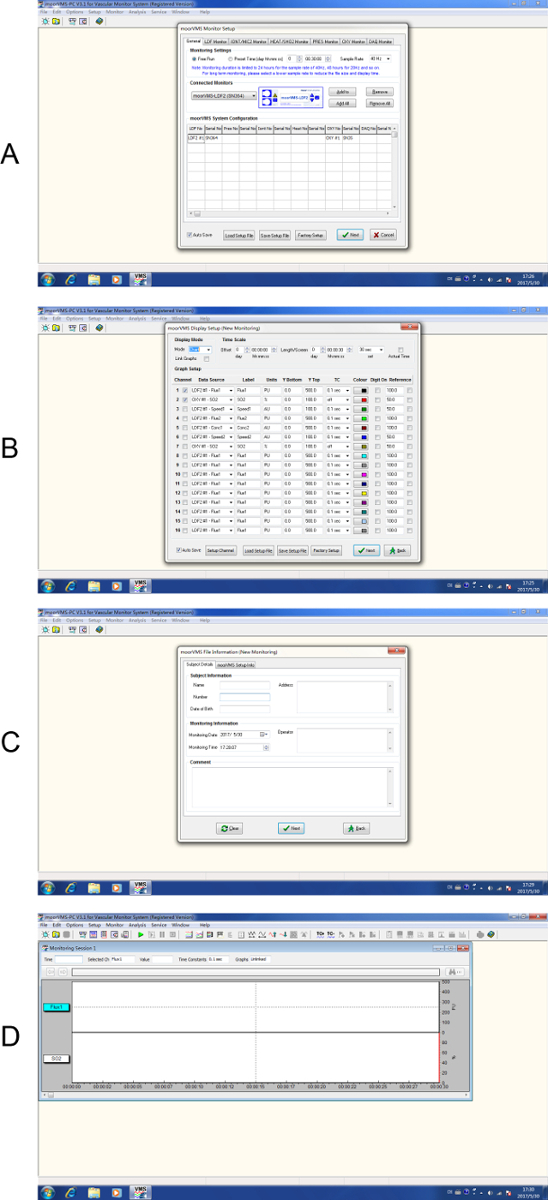
Figure 3. Step by step setup for laser Doppler monitoring. (A) Start a new experiment. (B) Select channel display. (C) Input subject details. (D) To start data recording. Please click here to view a larger version of this figure.
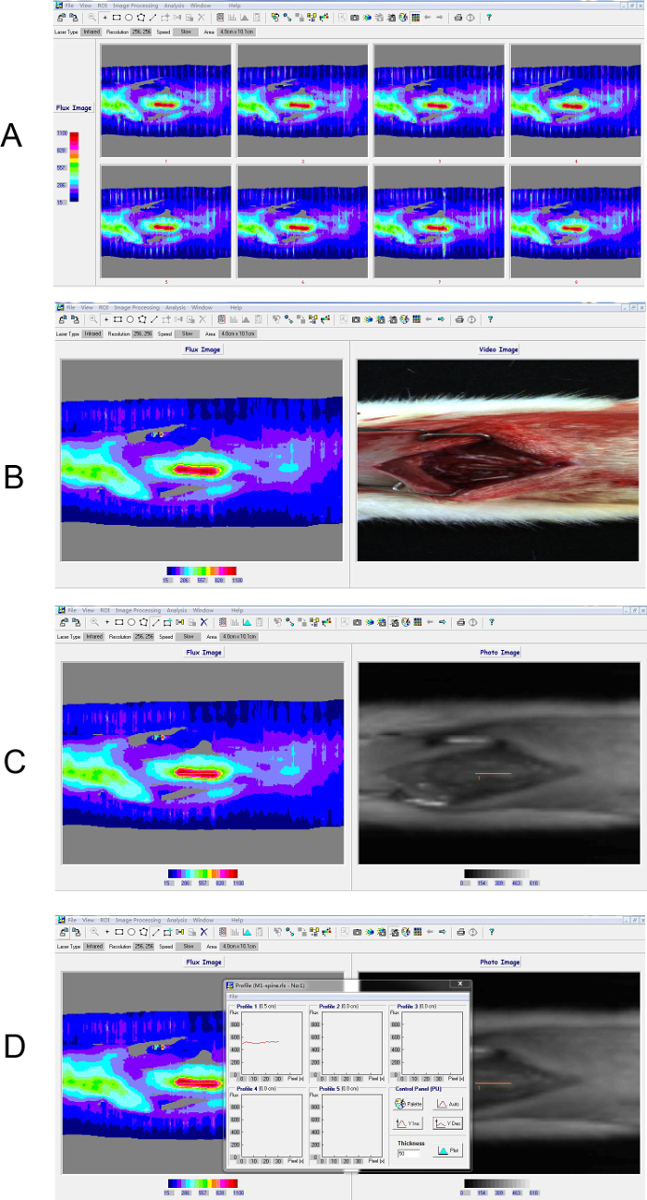
Figure 4. Process of laser Doppler perfusion imaging. (A) 8 continuous scans derived by scanning rats in the sham group. (B) The average image of the continuous scans. (C, D) Region of Interest (ROI) was selected based on the infrared image to extract the intensity profile along the central axis of the spine. The inset box shows the profiling result of the ROI. The color bar indicated perfusion units measured by laser Doppler scanner where blue represents the lowest value and red represents the highest value. The instrument detected the relative value of perfusion, namely "flux". Please click here to view a larger version of this figure.
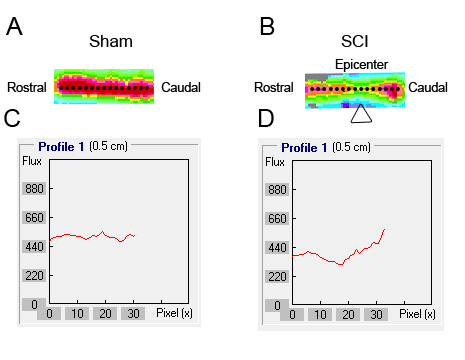
Figure 5. BF of the spinal cord was detected using laser Doppler perfusion imaging. (A, B) A 5 mm ROI was drawn on the flux map along the axis of the spine from the rostral to the caudal cord. (C, D) The intensity profile of each ROI along a line centered on the spinal cord axis was extracted for quantification.
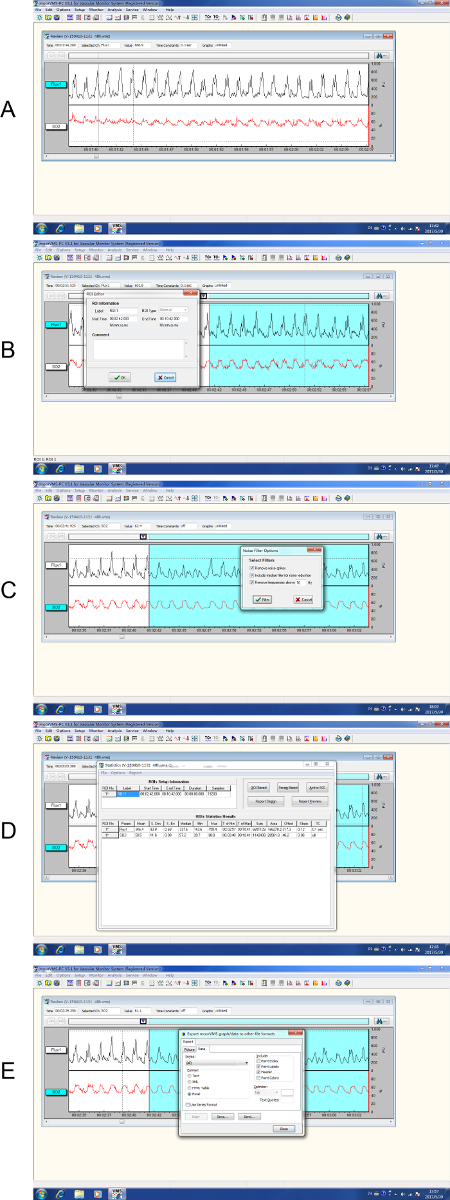
Figure 6. Process of laser Doppler perfusion monitoring. (A) The recording of raw data where the time marker indicated the starting point. (B) Selection of an 8 min ROI. (C) The selected data were then filtered by a built-in filter. (D) Statistical analysis of ROI. (E) Export of the raw data. Please click here to view a larger version of this figure.
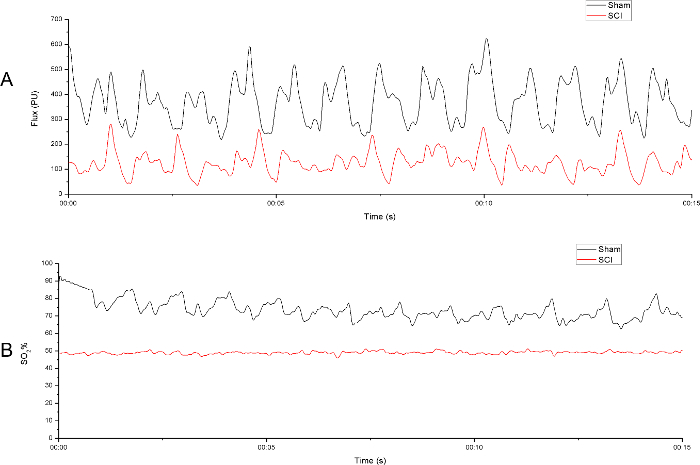
Figure 7. Spinal cord perfusion was evaluated by laser Doppler perfusion monitoring. (A) A 15 s sample of the raw blood flow output from both sham group and SCI group. (B) A 15 s sample of the raw oxygen saturation output from both sham group and SCI group. The laser Doppler probe was positioned 2 mm rostrally to the middle point at the level just above the surface of the spinal cord to the right side of the central vein. Please click here to view a larger version of this figure.
Discussion
A few details should be noticed when performing this protocol. Firstly, the process of anesthesia and surgery should be carried out as quickly and elegantly as possible to minimize the introduced stress to the animal. To reduce disturbance to the results, keep the animal in a relatively peaceful and stable state. Secondly, more attention should be paid to bleeding during the measurement using laser Doppler equipment, since blood could potentially interfere with the reading. Finally, during the data recording, animals should be kept in a temperature controlled environment to avoiding inconsistent results caused by temperature variance.
There are several important factors researchers should consider when using laser Doppler scanning. As mentioned in protocol, the distance of the scan should be kept consistent throughout the experiment for comparable results. For small areas, we suggest high resolution with multiple scans to produce reliable data of the BF. Additionally, we recommend putting a sterile gauze with the marked direction of the animal covering the surgical area with a small window only exposing the spine to further minimize background.
Probe positioning is the critical consideration in adapting and implementing the monitoring protocol. The probe should be perpendicular to the measured surface and excessive pressure should be avoided. To achieve this goal, the rat spine should be straightened and leveled by underlaying the animal with Styrofoam if necessary, and the probe should be positioned using the apparatus and the coordinates to make sure measurements are taken from roughly the same area.
As discussed in our previous article1, there are some limitations to this technology, such as the disability of calibration with absolute flow and sensitivity to movement artifact24. Another well-noticed limitation is the biological zero signal – that is, the presence of signal without BF25,26. To minimize the influence of these limitations to the results, measurements should be taken repeatedly and normalization is recommended to reduce disturbance.
Other techniques such as radioactive microsphere technique and Doppler ultrasound technique have been developed for BF measurement. However, the former is not in real time since a radioactive substance must be injected into the blood and the tissue needs to be excised for the measurement27. As for the technology of contrast enhanced ultrasound imaging, although it is non-invasive like LDF, contrast agent (microbubbles) must be injected intravenously and catheterization of the jugular or femoral is necessary for consistent microbubble infusion28. Compared with these techniques, LDF is capable of non-invasively measuring the microcirculatory flux of the tissue.
LDF signals consist of different features of both time and frequency. To capture these features, methods of wavelet analysis and fourier analysis have been applied to reveal periodic frequency fluctuations29,30. These oscillations manifested the influence of heart beat, respiration, intrinsic myogenic activity of the vascular smooth muscle, neurogenic activity on the vessel wall, and endothelial related metabolic activity31,32. In clinical applications and fundamental research, LDF can not only obtain the signals of BF, but also the evaluation of microvascular BF can provide a platform from which to investigate microvascular impairment and, more generally, the pathogenesis of microvascular disease.
In the current study, both methods of LDF were applied to evaluate BF in the spinal cord. The data of LDPI provided a geographic distribution of BF, which gave an overview of perfusion around the area and made it possible to perform comparative analysis of BF in different locations. By intensely measuring the probing area over time, the data derived from LD monitoring provided a more detailed description of the local blood flow, allowing in-depth analyses, such as spectrum and wavelet analysis, to gain a deeper understanding of the BF in the area, which is a promising future research topic.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Laser Doppler Line Scanner | Moor Instruments | moorLDLS2 | |
| Laser Doppler Monitor | Moor Instruments | moorVMS-LDF | |
| Probe for Monitor | Moor Instruments | VP3 | Blunt needle end delivery probe |
| Impactor | Precision Systems and Instrumentation | IH-0400 | |
| Phenobarbital sodium | Sigma-Aldrich | P3761 | |
| Buprenorphine | Sigma-Aldrich | B-908 | |
| Syringe | Becton Dickinson Medica (s) Pte.Ltd | 300841 | |
| Surgical suture needles with thread | Shanghai Pudong Jinhuan Medical Products Co., Ltd | 18T0329 (batch number) /4-0 | |
| Scalpel | Operation instrument factory of Shanghai Medical Instrument Co., Ltd. | J11030 4# | |
| Scalpel blade | Operation instrument factory of Shanghai Medical Instrument Co., Ltd. | J12130 20# | |
| Ophthalmic forceps | Operation instrument factory of Shanghai Medical Instrument Co., Ltd. | JD1040 | |
| Hemostatic forceps | Operation instrument factory of Shanghai Medical Instrument Co., Ltd. | J31050 | |
| Benzyl penicillin sodium | North China Pharmaceutical Co., Ltd | F6072116 (batch number) | |
| 75% alcohol | Dezhou Anjie Gaoke disinfection products Co., Ltd | 150421R (batch number) | |
| Iodine | Shandong Lierkang Medical Technology Co., Ltd | 20170102 (batch number) | |
| Rat | Laboratory Animal Center, The Academy of Millitery Medical Sciences | Sprague-Dawly (rat strain) |
References
- Jing, Y. L., Bai, F., Chen, H., Dong, H. Meliorating microcirculatory with melatonin in rat model of spinal cord injury using laser Doppler flowmetry. Neuroreport. 27 (17), 1248-1255 (2016).
- Jing, Y. L., Bai, F., Chen, H., Dong, H. Melatonin prevents blood vessel loss and neurological impairment induced by spinal cord injury in rats. J Spinal Cord Med. , 1-8 (2016).
- Han, S., et al. Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain. 133 (Pt 4), 1026-1042 (2010).
- Gerzanich, V., et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 15 (2), 185-191 (2009).
- Phillips, J. P., Cibert-Goton, V., Langford, R. M., Shortland, P. J. Perfusion assessment in rat spinal cord tissue using photoplethysmography and laser Doppler flux measurements. Journal of Biomedical Optics. 18 (3), 037005 (2013).
- Garcia-Lopez, P., Martinez-Cruz, A., Guizar-Sahagun, G., Castaneda-Hernandez, G. Acute spinal cord injury changes the disposition of some, but not all drugs given intravenously. Spinal Cord. 45 (9), 603-608 (2007).
- Brown, A., Nabel, A., Oh, W., Etlinger, J. D., Zeman, R. J. Perfusion imaging of spinal cord contusion: injury-induced blockade and partial reversal by β2-agonist treatment in rats. Journal of Neurosurgery-Spine. 20 (2), 164-171 (2014).
- Olive, J. L., McCully, K. K., Dudley, G. A. Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord. 40 (12), 639-645 (2002).
- Yamada, T., et al. Spinal cord blood flow and pathophysiological changes after transient spinal cord ischemia in cats. Neurosurgery. 42 (3), 626-634 (1998).
- Gordeeva, A. E., et al. Vascular Pathology of Ischemia/Reperfusion Injury of Rat Small Intestine. Cells Tissues Organs. , (2017).
- Liu, M., et al. Insulin treatment restores islet microvascular vasomotion function in diabetic mice. J Diabetes. , (2016).
- Drain, L. . The laser Doppler technique. , (1980).
- Rajan, V., Varghese, B., van Leeuwen, T. G., Steenbergen, W. Review of methodological developments in laser Doppler flowmetry. Lasers Med Sci. 24 (2), 269-283 (2009).
- Dohare, P., et al. The neuroprotective properties of the superoxide dismutase mimetic tempol correlate with its ability to reduce pathological glutamate release in a rodent model of stroke. Free Radic Biol Med. 77, 168-182 (2014).
- Bai, H. Y., et al. Pre-treatment with LCZ696, an orally active angiotensin receptor neprilysin inhibitor, prevents ischemic brain damage. Eur J Pharmacol. 762, 293-298 (2015).
- Vertiz-Hernandez, A., et al. L-arginine reverses alterations in drug disposition induced by spinal cord injury by increasing hepatic blood flow. J Neurotrauma. 24 (12), 1855-1862 (2007).
- Garcia-Lopez, P., Martinez-Cruz, A., Guizar-Sahagun, G., Castaneda-Hernandez, G. Acute spinal cord injury changes the disposition of some, but not all drugs given intravenously. Spinal Cord. 45 (9), 603-608 (2007).
- Li, Z., et al. Post pressure response of skin blood flowmotions in anesthetized rats with spinal cord injury. Microvasc Res. 78 (1), 20-24 (2009).
- Boyle, N. H., et al. Scanning laser Doppler is a useful technique to assess foot cutaneous perfusion during femoral artery cannulation. Critical Care. 3 (4), 95-100 (1999).
- Emmanuel, A. V., Chung, E. A. L., Kamm, M. A., Middleton, F. Relationship between gut-specific autonomic testing and bowel dysfunction in spinal cord injury patients. Spinal Cord. 47 (8), 623-627 (2009).
- Sheu, J. J., et al. Combination of cilostazol and clopidogrel attenuates rat critical limb ischemia. J Transl Med. 10, 164 (2012).
- Basso, D. M., Beattie, M. S., Bresnahan, J. C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Experimental Neurology. 139 (2), 244-256 (1996).
- Basso, D. M., Beattie, M. S., Bresnahan, J. C. A Sensitive and Reliable Locomotor Rating-Scale for Open-Field Testing in Rats. Journal of Neurotrauma. 12 (1), 1-21 (1995).
- Oberg, P. A. Tissue motion–a disturbance in the laser-Doppler blood flow signal?. Technol Health Care. 7 (2-3), 185-192 (1999).
- Tenland, T., Salerud, E. G., Nilsson, G. E., Oberg, P. A. Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp. 2 (2), 81-90 (1983).
- Kernick, D. P., Tooke, J. E., Shore, A. C. The biological zero signal in laser Doppler fluximetry – origins and practical implications. Pflugers Arch. 437 (4), 624-631 (1999).
- Rudolph, A. M., Heymann, M. A. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 21 (2), 163-184 (1967).
- Dubory, A., et al. Contrast Enhanced Ultrasound Imaging for Assessment of Spinal Cord Blood Flow in Experimental Spinal Cord Injury. Jove-Journal of Visualized Experiments. (99), e52536 (2015).
- Kuliga, K. Z., et al. Dynamics of Microvascular Blood Flow and Oxygenation Measured Simultaneously in Human Skin. Microcirculation. 21 (6), 562-573 (2014).
- Li, Z. Y., et al. Post pressure response of skin blood flowmotions in anesthetized rats with spinal cord injury. Microvascular Research. 78 (1), 20-24 (2009).
- Muck-Weymann, M. E., et al. Respiratory-dependent laser-Doppler flux motion in different skin areas and its meaning to autonomic nervous control of the vessels of the skin. Microvasc Res. 52 (1), 69-78 (1996).
- Stefanovska, A., Bracic, M., Kvernmo, H. D. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. Ieee Transactions on Biomedical Engineering. 46 (10), 1230-1239 (1999).

