Spinal Cord Lateral Hemisection and Asymmetric Behavioral Assessments in Adult Rats
Summary
Here we describe surgical procedures to produce a reliable spinal cord lateral hemisection (HX) at the 9th thoracic level in adult rats and neurobehavioral assessments designed for detecting asymmetric deficits after such a unilateral injury.
Abstract
Incomplete spinal cord injury (SCI) often leads to impairments of sensorimotor functions and is clinically the most frequent type of SCI. Human Brown-Séquard syndrome is a common type of incomplete SCI caused by a lesion to one half of the spinal cord which results in paralysis and loss of proprioception on the same (or ipsilesional) side as the injury, and loss of pain and temperature sensation on the opposite (or contralesional) side. Adequate methodologies for producing a spinal cord lateral hemisection (HX) and assessing neurological impairments are essential to establish a reliable animal model of Brown-Séquard syndrome. Although lateral hemisection model plays a pivotal role in basic and translational research, standardized protocols for creating such a hemisection and assessing unilateralized function are lacking. The goal of this study is to describe step-by-step procedures to produce a rat spinal lateral HX at the 9th thoracic (T9) vertebral level. We, then, describe a combined behavior scale for HX (CBS-HX) that provides a simple and sensitive assessment of asymmetric neurological performance for unilateral SCI. The CBS-HX, ranging from 0 to 18, is composed of 4 individual assessments which include unilateral hindlimb stepping (UHS), coupling, contact placing, and grid walking. For CBS-HX, the ipsilateral and contralateral hindlimbs are assessed separately. We found that, after a T9 HX, the ipsilateral hindlimb showed impaired behavior function whereas the contralateral hindlimb showed substantial recovery. The CBS-HX effectively discriminated behavioral functions between ipsilateral and contralateral hindlimbs and detected temporal progression of recovery of the ipsilateral hindlimb. The CBS-HX components can be analyzed separately or in combination with other measures when needed. Although we only provided visual descriptions of the surgical procedures and behavioral assessments of a thoracic HX, the principle may be applied to other incomplete SCIs and at other levels of the injury.
Introduction
The incomplete spinal cord injuries (SCI) often lead to severe and persistent impairments of sensorimotor functions and are clinically the most frequent type of SCI1. The Brown-Séquard syndrome in humans is caused by a lesion to half of the spinal cord which results in paralysis and loss of proprioception on the same (or ipsilesional) side as the injury, and loss of pain and temperature sensation on the opposite (or contralesional) side2,3,4. Spinal lateral hemisection animal models are used broadly to mimic human Brown-Séquard syndrome and they have been reported in rats5,6,7,8,9, opossums10, and monkeys7,11,12,13 by various laboratories at various spinal levels. However, detailed visualized procedures for producing a standard lateral hemisection have not been described. Providing step-by-step procedures for a lateral hemisection should optimize the model and facilitate the comparison or replication of experimental results in basic and translational research.
A unilateral SCI produces asymmetrical and disproportional behavior deficits that are difficult to measure using conventional assessments for symmetric injuries. An adequate methodology for assessing neurologic impairments for a unilateral SCI is an essential component of developing a unilateral SCI model. Despite the pivotal role of a unilateral spinal injury, standardized protocols for evaluating sensorimotor deficits in animals with such an injury are lacking. The Basso-Beattie-Bresnahan (BBB) locomotor rating scale has been the most frequently used measurement of function after SCI for adult rats 14 which yields a semiquantitative description of locomotion as a whole. However, it does not measure each hindlimb independently.
In this study, we report step-by-step procedures to produce a rodent spinal HX at the 9th thoracic (T9) vertebral level. We also introduce a combined behavior scale for hemisection (CBS-HX) that includes unilateral hindlimb stepping (UHS), coupling, placing contact, and grid walking assessments for evaluating neurological impairments and recovery after a unilateral SCI. We hope this model will be a useful model for examining injury mechanisms and therapeutic efficacies for unilateral SCIs.
Protocol
All surgical and animal handling procedures were performed as approved under the Guide for the Care and Use of Laboratory Animals (National Research Council) and the Guidelines of the Indiana University School of Medicine Institutional Animal Care and Use Committee.
1. General Consideration
- Use adult female Sprague-Dawley (SD) rats (weighing 200 g, n=12) for this study. Habituate animals to all testing environments and collect baseline data for all behavior tests one week prior to the surgical procedure.
- Perform the behavioral assessments by two observers who are blinded to the experimental groups.
2. Animal Preparation
- Clean the surgical table with 70% ethanol. Place a pre-warmed heating pad on the surgical table. Cover the surgical area with a sterile surgical drape. Place the sterile gauze, cotton swabs, and autoclaved surgical tools on the surface of the surgical drape.
- Turn on a microbead sterilizer for the intersurgery sterilization of surgical tools.
NOTE: An example of the tools used in this experiment is shown in Figure 1. - Anesthetize the rat with an intraperitoneal (i.p.) injection of ketamine (87.7 mg/kg) and xylazine (12.3 mg/kg). Ensure that the proper plane of anesthesia is reached by no response to the toe pinch stimulus. Apply vet ointment to the eyes of the animal to prevent corneal drying during surgery.
- Remove the hair overlying the thoracic vertebrae by shaving (Figure 2A). Remove the shaved fur with a vacuum equipped with a HEPA filter.
- Clean the surgical area with three alternating scrubs of iodine-based scrub and ethanol.
- Cover the animal with a sterile drape with a fenestration over the proposed incision site (Figure 2B). Note; In video, the surgical drape has been omitted for demonstration purposes.
3. Spinal Hemisection
- Touch the 13th rib which is the lowest rib in the rat and a floating rib that does not connect to the sternum. Follow the 13th rib dorsally to identify its connection with the T13 vertebra and then count up to identify the T10 vertebra.
- Use a scalpel blade (#15, Figure 1) to perform a 3 – 4 cm midline skin incision on the back overlying the 8-11th vertebral spinous processes.
- Under a surgical microscope, bluntly dissect and separate the paraspinal muscles laterally from the spinous processes towards the facets of the T9 and T10 vertebrae on both sides using the same scalpel blade.
NOTE: This approach will nicely tease the tissue apart without causing hemorrhage. - Stabilize the spine using a modified stabilizing holder. Make a slit on both sides of the lateral vertebral bone. Slide the stainless steel arms underneath the exposed transverse process facets and tighten the screws to secure stability.
- Use a retractor to retract muscles from the surgical area (Figure 2B) and to expose the T8-11 vertebral laminae and spinous processes (Figure 2C).
NOTE: There is a large gap between the T8 and T9 spinous processes, which are landmarks for identifying T9 (Figure 2C, dorsal view). From the lateral view, the spinous process of the T9 vertebra points caudally, the T10 spinous process points dorsally, and the T11 spinous process points rostrally; so, the 3 spinous processes form a pyramid and the T10 spinous process forms the peak (Figure 2D, lateral view). - Perform a dorsal laminectomy on the T9 vertebra using a rongeur. Snip away the T9 spinous process and remove a small portion of the lamina left to the midline (Figure 3A, dashed line), and the entire right portion of the lamina as laterally as possible (Figure 3A, dashed line). For laminectomy, insert the rongeur gently under the lamina and snip a small piece of bone at a time until a desired region of laminectomy is completed (Figure 3B and Figure 3C).
- Under a surgical microscope, identify the dorsal midline of the spinal cord (Figure 3C). Insert a needle (30 G) vertically through the midline into the spinal cord with the beveled side facing the right side (Figure 4A).
NOTE: The needle should penetrate the entire spinal cord to reach the ventral wall of the vertebral canal. - Stop any bleeding with a small piece of sterile gelfoam.
- Insert one tip of an iridectomy/microsurgical scissors through the midline needle track, and the other tip along the lateral surface of the right hemi-cord, and then make a complete cut on the right hemi-cord with the scissors (Figure 4B).
NOTE: Use a sharp micro-scissors for the spinal cord cut to minimize compression lesion to the spinal cord during the cutting. - Use the lateral edge of the same needle as a knife to cut through the lesion gap to confirm a complete right hemisection. Verify the completeness of the right hemisection by visualizing the bottom of the vertebral canal with the surgical microscope (Figure 4C, cross-sectional view; Figure 4D, lateral view; Figure 4E, dorsal view).
- Place a small piece of gelatin sponge over the lesion site (Figure 4F). Use cement mixture and build a narrow bridge over the sponge and the spinous processes of T8 and T10 (Figure 4G, H).
NOTE: The purpose of using a cement bridge is two-fold: 1) it separates the scar developed at the injury site from the rest of the tissue, and 2) it makes easier to dissect out the spinal cord segment after animal sacrifice. - Suture the muscle and skin layers separately with 4-0 silk thread.
- Inject 0.9% sterile saline subcutaneously to maintain hydration. Inject an analgesic agent Buprenorphine (0.05-2.0mg/kg S) 8-12 h/day subcutaneously for 2 days. Press urinary bladder 2-3 times daily for the first week and 1-2 times in the following weeks till spontaneous bladder voiding returns.
4. Postoperative Animal Care
- Return the animal to its single-housed home cage. Provide moist rodent chow or gel on the bottom of the cage to aid in animal’s ability to eat/hydrate. Place a heating pad below the cage during post-surgery recovery. Make sure that the heating pad only covers half of the cage bottom to avoid overheating.
- Inject 0.9% sterile saline subcutaneously to maintain hydration. Inject an analgesic agent Buprenorphine (0.05-2.0mg/kg S) 8-12 h/day subcutaneously for 2 days. Press urinary bladder 2-3 times daily for the first week and 1-2 times in the following weeks till spontaneous bladder voiding returns.
5. Assess the Unilateral Hemisection Stepping (UHS)
NOTE: The unilateral hemisection stepping (UHS) test is a direct measure of the ability of SCI animals to utilize their ipsilesional hindlimb in the open field. As mentioned in 1.1, the animals were acclimated to an open field environment (diameter 42 inches) 15 twice a day for 7 days. Two observers blinded to the animal groups perform the test. UHS score at both baseline (7 days prior to the T9 HX) and time points after injury will be collected. The steps for assessment are described as followed.
- Place the animal into an open field environment and examine the animal’s locomotion for 4 min.
NOTE: During testing, encouragement may be given to the animal to move actively. - With the form provided in Table 1, assign a value of 1 for Yes and 0 for No for each behavior category and then sum the total value to give a final UHS score of 0 to 8.
NOTE: According to Table 1 0: no observable movement of the hindlimb; 1 – 4: isolated movements of 3 hindlimb joints (hip, knee, and ankle); 5: sweeping with no weight support; 6: placing with no weight support; 7: placing with weight support; and 8: stepping with weight support. - Collect UHS scores at both baseline (7 days prior to the T9 HX) and time points after injury.
NOTE: The scores will be assessed at various time points after the T9 HX.
6. Coupling
- Analyze the CPL (gait coupling) with a video recording an animal walking on a narrow runway device or a simple open-field.
- In the coupling section of Table 1, assign a score of 0 for “No”, 1 for “Irregular/clumsy”, and 2 for “Normal” for each CPL category.
NOTE: The coupling (CPL) test is to evaluate the coordination of alternating movements of limbs, including homologous CPL (front-front/rear-rear limbs, Figure 5A), diagonal CPL (front left-rear right or front right-rear left limbs, Figure 5B), and homolateral CPL (front-rear limbs on the same side, Figure 5C). Following a T9 HX, the deficits of the hindlimb on the ipsilesional side become visible resulting in the alternation of the homologous CPL (Figure 5D), diagonal CPL (Figure 5E), and homolateral CPL (Figure 5F).
7. Contact Placing
NOTE: The hindlimb contact placing test is used to evaluate the motor integration of hindlimb responses to proprioceptive stimuli 16. Proprioception is considered to be intact if the animal steps up with its hindlimb onto the surface after the hindlimb is pulled down below the surface.
- Hold the animal in a vertical position so that both hindlimbs are available for the placing response.
- Brush the dorsal surface of a hindlimb forward lightly towards the edge of a surface (e.g., animal working bench).
- Observe the foot placement onto the surface and assign a hindlimb contact placing score. 0: no placing; 1: placing.
NOTE: The dorsal surface receives stimulation and the foot will subsequently extend and place the foot on the surface if the reflex is intact. The assessment form is also in Table 1.
8. Grid Walking
NOTE: The grid walking test assesses spontaneous motor deficits and limb movements involved in precise stepping, coordination, and accurate paw placement.
- Place a rat on an elevated plastic-coated wire mesh grid (36 × 38 cm with 3 cm2 openings) and allow it to walk freely across the platform for 30 steps.
- Count the total number of footsteps and the number of foot missteps for each limb. Video recordings are made to confirm the counting.
NOTE: Two blinded observers assess paw placement of forelimbs and hindlimbs as animals walk. - Assign the grid walking scores for each hindlimb as follows – 0: missteps greater than 15; 1: missteps less than or equal to 15; 2: missteps less than or equal to 10; and 3: missteps less than or equal to 5.
NOTE: The scoring assessment is based on Table 1. The cutoffs scores are used as measures of severity of motor deficits.
9. Perfusion and Tissue Processing
- After appropriate anesthesia similar in step 2.3, perfuse the animals carefully following the transcardial perfusion protocol 17.
- Dissect and collect the spinal cord samples and post-fix them in 4% PFA overnight.
- The samples can then be transferred to 30% sucrose solution.
- Cut the spinal cord in cross sections and stain the selected sections with an axon marker SMI-31 and astrocytic marker glial fibrillary acidic protein (GFAP) according to standard procedures18.
Representative Results
The surgical procedures described above allow the production of a consistent and reproducible lateral HX at T9. After perfusion and skin removal, the surgical site at T9 could be readily identified by a residual suture (Figure 6A). Further dissection allows the exposure of the cement bridge (Figure 6B), and gelatin sponge (Figure 6C) in layers. The spinal cord is then exposed to the opened vertebral canal and a lateral hemisection on the right side is confirmed (Figure 4D). The level of the injury can be further confirmed by its association with the exposed vertebral bodies and ribs (Figure 6D). Immunofluorescence staining of a cross-section at the injury epicenter shows a complete loss of right hemicord and the preservation of the left hemicord contralateral to the injury. The section stained with an axon marker SMI-31 and astrocytic marker glial fibrillary acidic protein (GFAP) (Figure 6E).
Neurobehaviorally, the CBS-HX system is capable of detecting asymmetric deficits over time following a T9 HX. After HX, the ipsilateral hindlimb lost its ability to step whereas the contralateral hindlimb retained the ability to walk. For each behavior measure, we performed 3 trials and used the mean of the 3 trials for quantification and analysis. We used pre-surgery measure as a baseline which we consider as the most accurate control as compared with using other rats. Scores of the 4 individual measures, i.e. UHS, CPL, contact placing, and grid walking can be analyzed separately (Figure 7A-D) or they can be combined into a composite CBS-HX (Figure 7E). Two-way ANOVA analyses showed significant differences in UHS (F = 23.199, p < 0.001), coupling (F = 8.376, p < 0.01), contact placing (F = 17.672, p < 0.001), grid walking (F = 19.261, p < 0.001), CBS-HX (F = 20.897, p < 0.001) between the ipsilateral and contralateral sides. Figure 7A shows the results of UHS after a T9 HX. In the first 3 days postinjury, rats lost the ability to step and received a score of 0-2 for the ipsilesional hindlimb. Step-like movements began to appear on the ipsilesional side at 7-10 days after injury with most steps being dorsal steps. By 28 days after the T9 HX, the rats could take plantar steps with virtually normal coordination with an assigned UHS score of 8. As a comparison, the contralesional hindlimb was less interrupted and the UHS score dropped within the first 5 days after the T9 HX and returned to the baseline level after day 10 post-injury. For the total CPL (including homolateral, homologous and diagonal coupling) test, both stability and adaptability of coordination after T9 HX were markedly reduced (Figure 7B). At 1-5 days after injury, the HX animals showed no sign of CPL. Over time, CPL of the ipsilateral hindlimb emerged, often clumsy, unsteady, and inappropriately varying in their speed, force, and direction. The contact placing (Figure 7C) and grid walking (Figure 7D) of the ipsilateral hindlimb were also affected by the T9 HX particularly within the first 5 days after the injury, and usually recovered when the animal began to take plantar steps. The composite CBS-HX system includes the UHS, CPL, contact placing, and grid walking tests for a maximum possible score of 18 (Figure 7E). The motor function of the ipsilateral hindlimbs demonstrated a decrease in CBS-HX scores after the T9 lateral HX, which is consistent with the deficits seen in the human Brown-Séquard syndrome. The motor function of the ipsilateral hindlimbs demonstrated a decrease in CBS-HX scores from 1 day to 4 weeks after the T9 lateral HX as compared to the contralateral hindlimbs (Figure 7E).
Thus, the composite CBS-HX system combining the UHS, CPL, contact placing, and grid walking can be used to evaluate the behavioral function of rats after the lateral injury of the thoracic spinal cord for a maximum possible score of 18.
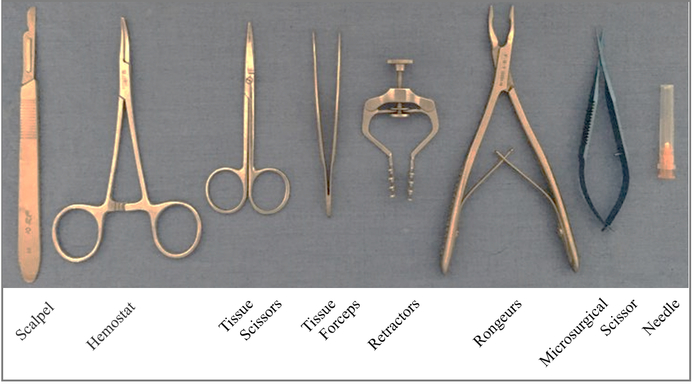
Figure 1. Surgical tools used for producing a T9 right-sided hemisection. Please click here to view a larger version of this figure.
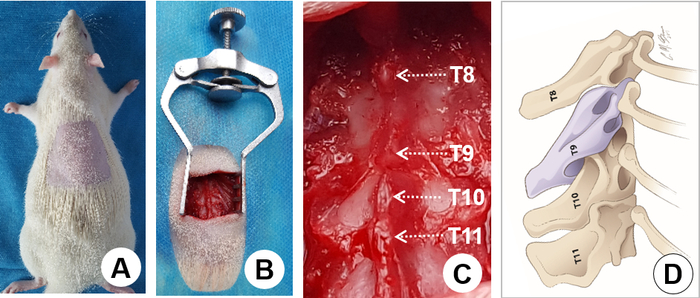
Figure 2. Surgical exposure. A) Shave the hair on the back overlying the surgical region. B) Retract muscles from the surgical area using a retractor. C) Expose the T8-11 vertebral laminae and define individual spinous processes (arrows). Note that there is a large gap between the T8 and T9 spinous processes, which is a landmark for identifying T9. D) The schematic drawing shows the lateral view of the spinous processes. The T9-11 spinous processes form a pyramid with the T10 spinous process being the peak. Again, a large gap between the T8 and T9 spinous processes is clearly seen as a landmark for identifying T9 where a laminectomy is performed. Please click here to view a larger version of this figure.
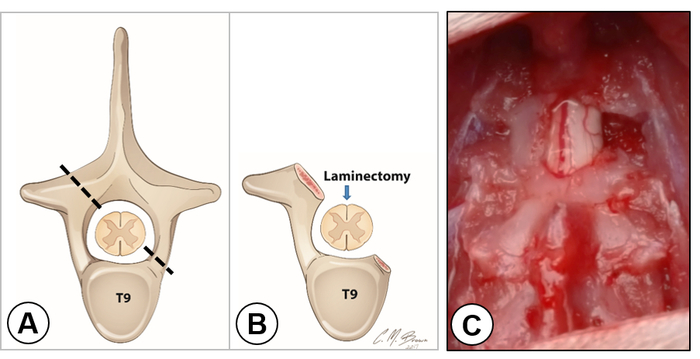
Figure 3. Laminectomy and exposure of the right hemicord. A) The schematic drawing shows the cross section of the spinal cord within the T9 vertebra. The dashed line indicates the extent of laminectomy on each side. B) The schematic drawing shows the removal of a small portion of the lamina on the left side and the entire vertebral arch on the right side. An arrow indicates the dorsal midline of the cord. C) Dorsal view of the exposed spinal cord. Note that the dorsal vein was located in the middle of the spinal cord dividing the left and right hemicords. The right hemicord was completely exposed. Please click here to view a larger version of this figure.
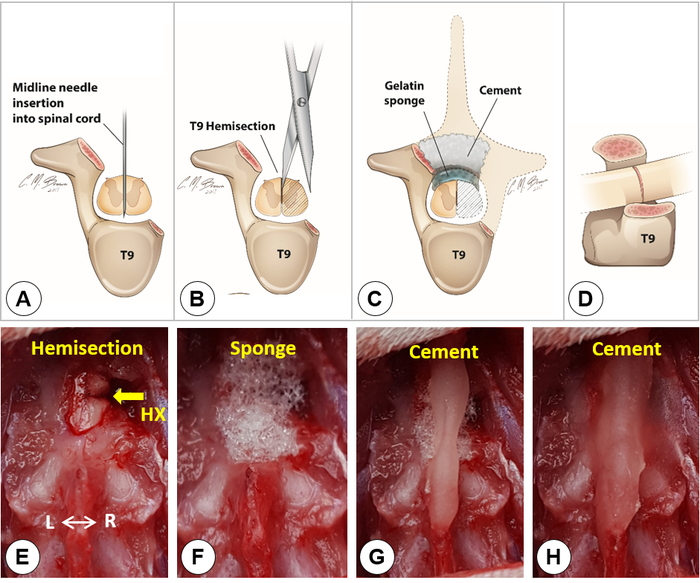
Figure 4. Lateral hemisection. A-D) Schematic drawings show the midline needle insertion into the spinal cord (A), the T9 hemisection (B), the covering of gelatin sponge and cement (C), and the lateral view of a T9 lateral hemisection (D). Dashed lines in C outline the removed T9 vertebral lamina and the right hemicord. E) Dorsal view of a right spinal cord hemisection. F) Placement of a small piece of gelatin sponge over the hemisection site. G-H) A Simplex-P cement bridge built over the sponge and the spinous processes of T8 and T10. Please click here to view a larger version of this figure.
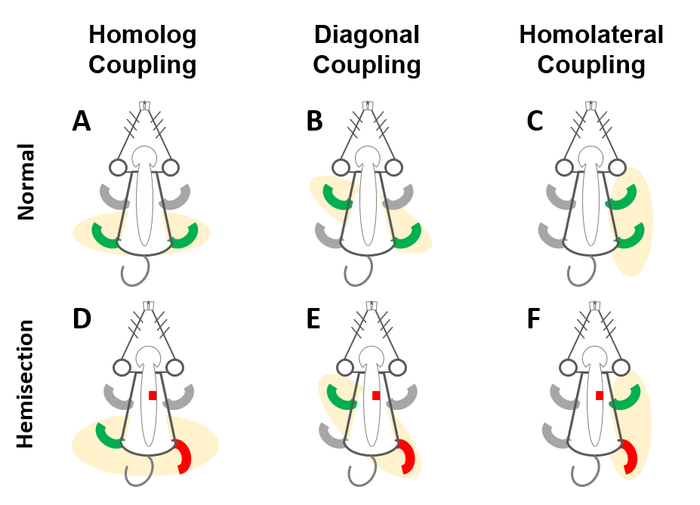
Figure 5. Schematic drawing of the coupling (CPL) test. The CPL test is to evaluate the coordination of alternating movements of limbs, including A) homologous CPL (front-front/rear-rear limbs), B) diagonal CPL (front left-rear right/front right-rear left limbs), and C) homolateral CPL (front-rear limbs on the same side). After T9 HX (red box, D-F), the hindlimb deficit became visible on the ipsilesional side and animals show lack of coordination in homolog (D), diagonal (E), and homolateral (F) CPL. Please click here to view a larger version of this figure.
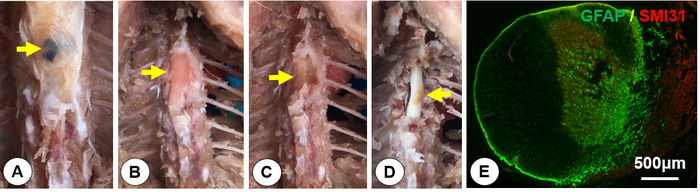
Figure 6. Tissue dissection and histology. After perfusion, tissues were dissected out to expose the spinal cord. Cross sections were processed for double immunofluorescent staining of glial fibrillary acidic protein (GFAP, a marker for astrocytes) and SMI31 (a marker for axons). A) Exposure of the suture as a landmark for the injury site (yellow arrow). B) Exposure of the dental cement (yellow arrow). C) Exposure of the gelatin sponge (yellow arrow). D) Identify the spinal hemisection on the right side (yellow arrow). E) A spinal cord cross section at the injury epicenter immunostained with GFAP (green) and SMI 31 (red). It shows that the right spinal hemicord was completely cut and the left hemicord was well preserved. Please click here to view a larger version of this figure.
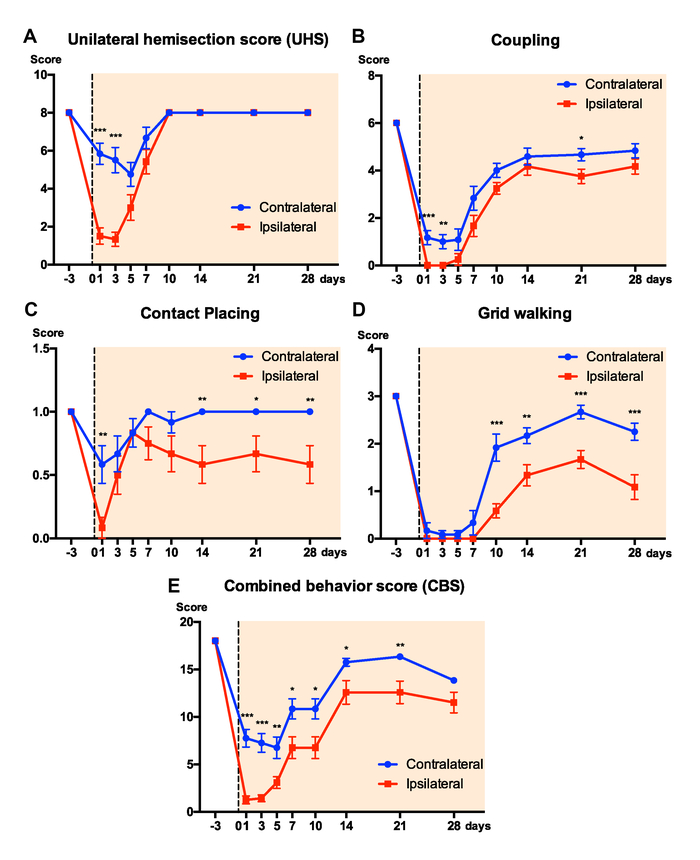
Figure 7. Results of neurobehavioral scores. Graphs show scores of the 5 measures: A, the unilateral hemisection score (UHS); B, coupling (CPL); C, contact placing; D, grid walking, and E, combined behavior score (CBS) on ipsilateral and contralateral hindlimbs after a T9 HX. Data represent mean ± s.e.m. *: p < 0.05, **: p < 0.01, ***: p < 0.001 between ipsilateral and contralateral hindlimbs (Two-way ANOVA, Tukey's multiple comparisons test, n = 12 rats/groups). Please click here to view a larger version of this figure.
| Subscore Name/Range | Description | Score | |
| Unilateral hindlimb stepping | Observable slight hindlimb movement | No | 0 |
| (UHS) | Yes | 1 | |
| (0-8) | Movement of Ankle | No | 0 |
| Yes | 1 | ||
| Movement of Knee | No | 0 | |
| Yes | 1 | ||
| Movement of Hip | No | 0 | |
| Yes | 1 | ||
| Sweeping (no weight support) | No | 0 | |
| Yes | 1 | ||
| Placing (no weight support) | No | 0 | |
| Yes | 1 | ||
| Placing (with weight support) | No | 0 | |
| Yes | 1 | ||
| Stepping | No | 0 | |
| Yes | 1 | ||
| Coupling | Homolateral | No | 0 |
| (0-6) | Irregular/clumsy | 1 | |
| Normal | 2 | ||
| Homologous | No | 0 | |
| Irregular/clumsy | 1 | ||
| Normal | 2 | ||
| Diagonal | No | 0 | |
| Irregular/clumsy | 1 | ||
| Normal | 2 | ||
| Contact placing | No | 0 | |
| (0-1) | Yes | 1 | |
| Grid walking | Miss steps | >15 | 0 |
| (0-3) | ≤15 | 1 | |
| ≤10 | 2 | ||
| ≤5 | 3 | ||
| Total CBS-HX | |||
| (0-18) | |||
Table 1: The combined behavior scores for hemisection (CBS-HX)
Discussion
In this study, we report step-by-step procedures for producing a simple, consistent, and reproducible T9 spinal HX in adult rats that mimics the Brown-Séquard Syndrome in humans. We further introduce a combined behavior score system for hemisection (CBS-HX) which is sensitive to evaluate asymmetrical neurological impairment and progression of recovery, measured by a combination of unilateral hindlimb stepping (UHS), coupling (CPL), placing contact, and grid walking. Although we demonstrate the injury at the T9 level, this procedure can be applied to other regions of the spinal cord including the cervical and lumbar cords in a simple and undemanding way. We hope this model, along with unilateralized behavioral assessments, will be useful for examining injury mechanisms and therapeutic efficacies for such types of SCI.
Since the lateral HX model only lesions the ipsilateral half of the cord, the contralateral side of the cord is largely preserved and can be used as an internal control. Many descending and ascending pathways are unilaterally projected and a lateral hemisection in many circumstances produces damage to an axonal tract at one side and preserves the same tract on the opposite side, allowing comparison of reorganization and functional consequences of these tracts in the same animal. In addition, producing a more localized lesion may allow the targeting of specific pathways. For example, a ventral and ventrolateral lesion may affect reticulospinal and vestibulospinal pathways. A dorsal or dorsolateral lesion may affect the corticospinal and rubrospinal pathways. The hemisection or partial injury model can also be used to study the anatomy and function of other pathways, such as the propriospinal, noradrenergic or serotonergic pathways. Thus, the hemisection model can be uniquely employed to study compensation by sensory afferents, by descending pathways, and by intrinsic spinal circuitry. This model is also suitable for investigating mechanisms of locomotor recovery after HX.
The lateral HX leads to obvious behavioral impairments, which are assessable under motor tasks (e.g., Treadscan or Treadmill) paradigm for the automated gait analysis 19. Also, the conductivity of the axonal tracts on the contralateral side to the lesion could be measured using electrophysiological recordings, and this evaluation provides the possibility to establish a functional reorganization following various treatments. Moreover, unilateral injections of the anatomical tracers into neurons of a particular pathway permit visualization of anterogradely labeled midline crossing fibers and their connection with retrogradely labeled neurons20,21,22,23,24,25.
Although a typical spinal HX surgery takes less than 20 minutes to finish, it requires some practice to achieve a precise and consistent HX. First, it is important that the spinal HX level be consistent from animal to animal. Therefore, it is critical that the appropriate vertebral segment for laminectomy is identified. Second, make sure that the HX is complete. To make a complete HX, one can use a 30-gauge needle inserted vertically through the midline to guide the cutting using microscissors. The needle insertion also avoids damage to the posterior spinal vessels or cord over lesioning. The second function of the 30-gauge needle is that it can serve as a knife to trace the cut to make sure that there is no ambiguity of the lesion. Third, placing gelatin onto the lesion site can minimize the cerebrospinal fluid leakage, and placing the cement on top of the gelatin and bridging the vertebral lamina can strengthen the stability of spinal vertebrae at the lesion site and facilitate wound healing. To avoid the signal interference with the application of electrophysiological recordings, muscles, fascia, and skin should be sutured in layers with 4-0 silk thread. Finally, every effort should be made to minimize the damage to the contralateral spinal cord. Histological verification should be established to confirm a complete lateral hemisection on one side and preservation of the other half of the cord on the other side (as shown in Figure 6E).
To improve locomotion after SCI, previous studies have utilized a wide range of strategies including cell transplantation, axon regeneration 8,18,26,27, and activity-based rehabilitation 28,29,30. Meanwhile, several behavioral tests have been established for functional assessment and to screen for the best treatments following SCI. The BBB locomotor rating scale was designed for locomotor assessment of spinal symmetric injuries such as a midline contusion or transection injuries that affect bilateral hindlimbs 14,31. Certain parameters of BBB, such as coordination and toe clearance, are recorded by observing both hindlimbs. If one hindlimb is intact and the other shows deficits as seen in asymmetric injuries, then the intact hindlimb will confound the score of the affected hindlimb. Since the BBB scoring does not accommodate one hindlimb score from the other after the unilateral injury, it is not ideal for assessing unilateral spinal cord injuries. However, if joint movement and weight support on each side are assessed separately and are not calculated as part of the BBB, then the intact hindlimb (similar to a sham control) will not confound the score of the affected hindlimb. Moreover, the intact side will not bias the overall score of the animal, because the intact hindlimb does not have dramatic deficits in joint movement, weight support, or stepping.
The combined behavior score for hemisection is designed to be a sensitive and easily performed evaluation of behavioral recovery in the rat model of lateral hemisection. It can be used to assess behaviors of both early and late phases of recovery. The early phase is within 7-10 days postinjury. In the first 3-5 days post-HX, the ipsilateral hindlimb activity increased steadily and should be assessed more frequently to record spontaneous or treatment-mediated hindlimb movement recoveries. By 5-7 days post-HX, rats began to make sweeping hindlimb movements without weight support. By 7-10 days, rats typically began to stand and step. During this phase, attention to the stepping pattern should be paid. At the late phase (14-28 days), the ipsilateral hindlimb activity was stable and close to normal.
Close attention should also be paid to coupling (CPL) capacity. The CPL test (gait coupling) can be performed either with a video (e.g., Treadscan/Catwalk) or a filming video during an open-field test. The second option provides flexibility if the researchers do not have access to the gait analysis system. For both video recording sessions, a minimum of two consecutive touchdowns for each foot is required for this test. For the analysis, there are three coupling parameters: homologous, homolateral and diagonal coupling (step 6.2). Each coupling involves a reference foot and the given foot. Take homologous coupling (front left-front right, or hind left-hind right) for example, it is the first touchdown time of the given foot divided by one whole stride time of the reference foot. Since the left and right foot should be out of phase, the perfect coupling should be 0.5. This is the same case in homolateral coupling (left front-left hind, or right front-right hind). However, for diagonal coupling (left front-right hind, or right front-left hind), the perfect coupling should be 0 or 1 since the two feet should be in phase. In step 6.4, we assign a score for each CPL from 0 to 2. In details, a score 0 shall represent the given foot is unable to move to finish a touchdown, hence no CPL; a score 1 represents any irregular or clumsy CPL since the given foot finishes a touchdown but not in the perfect coupling; a score 2 means a perfect coupling of 0.5. The three coupling parameter concepts are well described in the previous publications32,33. CPL can be combined with the assessments of contact placing and grid walking. Individual components of the combined behavior scoring system will be more or less effective in different rat models of SCI. For CPL, the deficits became obviously visible in the rate of alternation and the completeness of the sequence. Proprioceptive hindlimb placing deficits could be clearly revealed after unilateral HX. In our study, all rats showed ipsilesional hindlimb placing deficits while the contralateral hindlimb placing showed no deficits. The grid walking test should be considered when contact placing, which involves the corticospinal tract, begins to recover. To rule out any possible fatigue issues, the sequence of behavioral tests could be randomized at each test.
In conclusion, we report step-by-step procedures to create a reproducible in vivo rat model of the T9 spinal HX that mimics the Brown-Séquard Syndrome in humans. The combined behavior scoring system for hemisection offers a more discriminative measure of individual hindlimb behavioral outcomes for evaluating injury mechanisms and treatments after a unilateral SCI. Although we only provide a visual description of the surgical procedures and behavioral assessments of a thoracic HX, methods described here may be applied to other incomplete SCIs at different injury levels.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Mr. Jeffrey Recchia-Rife for his excellent technical assistance. This work was supported in part by the Foundation of Director of General Hospital of Jinan Military Region of Chines PLA 2016ZD03 and 2014ZX01 (XJL and TBZ). Research in the Xu laboratory is supported by NIH 1R01 100531, 1R01 NS103481, and Merit Review Award I01 BX002356, I01 BX003705, I01 RX002687 from the U.S. Department of Veterans Affairs.
Materials
| Baby-Mixter Hemostat | FST | 13013-14 | Can be any brand of choice |
| Elevated plastic coated wire mesh grid | Any | 36×38 cm with 3 cm2 openings | |
| Gel foam | Moore Medical | 2928 | Can be any brand of choice. |
| Grip cement kit, powder and solvent | Dentsply | 675570 | Can be any brand of choice. |
| Microbead Sterilizer | FST | NA | Can be any brand of choice |
| Pearson Rongeur | FST | 16015-17 | Can be any brand of choice. |
| Retractors | Jinxie surgical tools | 6810 | Can be any brand of choice |
| Scalpel Handle | FST | 10003-12 | Can be any brand of choice |
| Simplex-P cement | Stryker | Can be any brand of choice. | |
| TreadScan automatic gait analysis | CleverSys Inc | NA | Can be any brand of choice |
References
- Center, N. S. C. I. S. Spinal Cord Injury Facts and Figures at a Glance. SCI Data Sheet. , (2018).
- Zhang, X. Y., Yang, Y. M. Scissors stab wound to the cervical spinal cord at the craniocervical junction. Spine Journal. 16 (6), e403-e406 (2016).
- Enicker, B., Gonya, S., Hardcastle, T. C. Spinal stab injury with retained knife blades: 51 Consecutive patients managed at a regional referral unit. Injury. 46 (9), 1726-1733 (2015).
- Witiw, C. D., Shamji, M. F. Brown-Sequard syndrome from herniation of a thoracic disc. Canadian Medical Association Journal. 186 (18), 1395 (2014).
- Webb, A. A., Muir, G. D. Compensatory locomotor adjustments of rats with cervical or thoracic spinal cord hemisections. Journal of Neurotrauma. 19 (2), 239-256 (2002).
- Filli, L., Zorner, B., Weinmann, O., Schwab, M. E. Motor deficits and recovery in rats with unilateral spinal cord hemisection mimic the Brown-Sequard syndrome. Brain. 134 (Pt 8), 2261-2273 (2011).
- Friedli, L., et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Science Translational Medicine. 7 (302), (2015).
- Xu, X. M., Zhang, S. X., Li, H., Aebischer, P., Bunge, M. B. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. European Journal of Neuroscience. 11, 1723-1740 (1999).
- Gulino, R., Dimartino, M., Casabona, A., Lombardo, S. A., Perciavalle, V. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neuroscience Research. 57 (1), 148-156 (2007).
- Xu, X. M., Martin, G. F. The response of rubrospinal neurons to axotomy in the adult opossum, Didelphis virginiana. Experimental Neurology. , 46-54 (1990).
- Wu, W., et al. Axonal and Glial Responses to a Mid-Thoracic Spinal Cord Hemisection in the Macaca fascicularis Monkey. Journal of Neurotrauma. , (2013).
- Shi, F., et al. Glial response and myelin clearance in areas of wallerian degeneration after spinal cord hemisection in the monkey Macaca fascicularis. Journal of Neurotrauma. 26 (11), 2083-2096 (2009).
- Nout, Y. S., et al. Methods for functional assessment after C7 spinal cord hemisection in the rhesus monkey. Neurorehabililation and Neural Repair. 26 (6), 556-569 (2012).
- Basso, D. M., Beattie, M. S., Bresnahan, J. C. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma. 12 (1), 1-21 (1995).
- Liu, N. K., et al. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Annals of Neurology. 75 (5), 644-658 (2014).
- Kunkel-Bagden, E., Dai, H. N., Bregman, B. S. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Experimental Neurology. 116, 40-51 (1992).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of Visualized Experiment. (65), (2012).
- Deng, L. X., et al. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. Journal of Neuroscience. 33 (13), 5655-5667 (2013).
- Liu, J. T., et al. Methotrexate combined with methylprednisolone for the recovery of motor function and differential gene expression in rats with spinal cord injury. Neural Regeneration Research. 12 (9), 1507-1518 (2017).
- Schnell, L., et al. Combined delivery of Nogo-A antibody, neurotrophin-3 and the NMDA-NR2d subunit establishes a functional ‘detour’ in the hemisected spinal cord. European Journal of Neurosciences. 34 (8), 1256-1267 (2011).
- Arvanian, V. L., et al. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Experimental Neurology. 216 (2), 471-480 (2009).
- Hunanyan, A. S., et al. Alterations of action potentials and the localization of Nav1.6 sodium channels in spared axons after hemisection injury of the spinal cord in adult rats. Journal of Neurophysiology. 105 (3), 1033-1044 (2011).
- Garcia-Alias, G., et al. Chondroitinase ABC combined with neurotrophin NT-3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. Journal of Neuroscience. 31 (49), 17788-17799 (2011).
- Petrosyan, H. A., et al. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. Journal of Neuroscience. 33 (9), 4032-4043 (2013).
- Yu, Y. L., et al. Comparison of commonly used retrograde tracers in rat spinal motor neurons. Neural Regeneration Research. 10 (10), 1700-1705 (2015).
- Lu, P., et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 83 (4), 789-796 (2014).
- Teng, Y. D., et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. PNAS. 99, 3024-3029 (2002).
- Wang, H., et al. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Experimental Neurology. 271, 368-378 (2015).
- Courtine, G., et al. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain. 128 (Pt 10), 2338-2358 (2005).
- Ichiyama, R. M., et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. Journal of Neuroscience. 28 (29), 7370-7375 (2008).
- Basso, D. M., Beattie, M. S., Bresnahan, J. C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Experimental Neurology. 139, 244-256 (1996).
- Li, S., et al. Assessing gait impairment after permanent middle cerebral artery occlusion in rats using an automated computer-aided control system. Behavioural Brain Research. 250, 174-191 (2013).
- Bonito-Oliva, A., Masini, D., Fisone, G. A mouse model of non-motor symptoms in Parkinson’s disease: focus on pharmacological interventions targeting affective dysfunctions. Frontiors in Behavioral Neuroscience. 8, 290 (2014).

