マウスの主なオリゴデンドロ サイトの特異的・迅速免疫分離
Summary
体外培養の細胞の迅速かつ特定の分離を可能にするプライマリ マウス オリゴデンドロ サイトの免疫隔離について述べる。
Abstract
効率的で堅牢な分離とプライマリ オリゴデンドロ サイト (OLs) の文化はオリゴデンドログリアの開発だけでなく、多発性硬化症などの疾患を脱髄の生物学の生体外で研究のための貴重なツールとPelizaeus メルツバッハー様疾患 (PMLD)。ここでは、単純な効率的な選択方法の免疫隔離のためステージ 3 O4+ preoligodendrocytes 細胞新生仔マウスの子犬。未熟な OL が、80% 以上の生後 7 日目で齧歯動物脳白質 (P7) この分離方法だけでなく細胞高利回りを保証も既に別個の系統にコミット OLs の特定の分離を構成するので減少、アストロ サイトなど汚染細胞とマウスの脳から他の細胞を分離することの可能性。このメソッドは、以前、報告方法の変更です、オリゴデンドロ サイト準備純度約 4 時間で 80% 以上を提供します。
Introduction
オリゴデンドロ サイト (OLs) は、中枢神経系 (CNS)1の髄の細胞です。分離と規制の厳しい環境でプライマリ オリゴデンドロ サイトの文化はオリゴデンドログリアの開発だけでなく、脱髄疾患多発性硬化症2 などの生物の生体外で研究のための貴重なツール.これは、効率的で堅牢なオリゴデンドロ サイト分離と培養法3を必要があります。本研究では迅速かつ具体的変更された分離手法を実装する、特徴的なオリゴデンドロ サイト細胞の表面マーカーの発現を利用をしました。
成熟オリゴデンドロ サイトの 4 つの明瞭な段階を識別されている、各各発育ステージ (図 1) 特徴的な細胞表面マーカーの発現が特徴します。これらの細胞の表面のマーカー抗体4、5、によって認識することができます、特定の段階で最小二乗法を分離する使用ことができます。最初の段階では、オリゴデンドロ サイト前駆細胞 (Opc) は増殖、移行、および具体的にはエクスプレス ・血小板由来成長因子受容体 (PDGF-Rα)6, ガングリオシド A2B5, プロテオグリカン NG27,8 能力を持っています。、ポリシアル酸神経細胞接着分子9および脂肪酸酸結合タンパク質 7 (FABP7)10。Opc 神経前駆細胞11の特徴である細胞体の反対の極から発せられるいくつかの短いプロセスとバイポーラの形態があります。
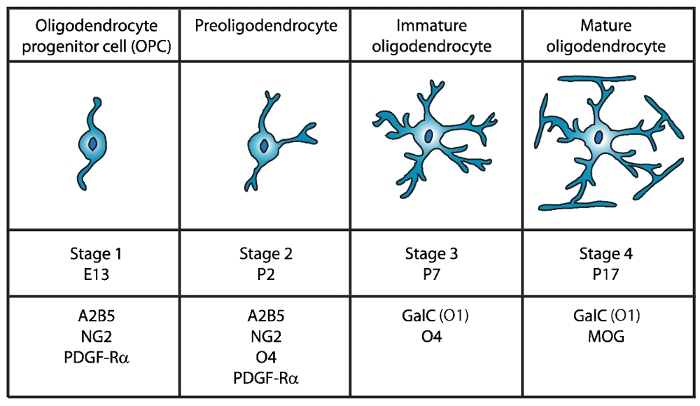
図 1: マウス オリゴデンドロ サイト開発時にセル表面のマーカーの表現。A2B5、GalC (O1)、NG2、O4、PDGF Rα は具体的には特定の抗体を使用して、特定の発達段階でオリゴデンドロ サイトを分離する使用ことができますよう、OLs セル表面のマーカーです。 この図の拡大版を表示するのにはここをクリックしてください。
第二段階で Opc preoligodendrocytes に上昇を与えるし、細胞膜で OPC マーカーだけでなく、O4 抗体12,13、14GPR17 蛋白質によって認識されるスルファチド (硫酸化 galactolipid)未熟なオリゴデンドロ サイト (OL) 段階まで主張します。この段階では、preoligodendrocytes は、多極短いプロセスを拡張します。Preoligodendrocytes 生後 2 (P2) で主要な段階の OL は未熟な最小二乗法15のマイナーな人口を持つラットおよびマウスの大脳白質します。
第 3 段階では、未熟な OLs 続行 O4 を表現し A2B5 と NG2 マーカーの発現を失う、galactocerebroside C16を表現し始めます。この段階で OLs は別個の系統にコミットしているし、長い分岐し枝17,18と分裂後の細胞になります。未熟な OL が P7 で齧歯動物の白質の 80% 以上を構成して、この時点で最初の MBP+細胞は15,19,20,21が観察されます。したがって、P7 で OLs の分離は、高細胞収量を確保できます。
OL 開発の最後、第 4 段階で成熟した OLs エクスプレス髄蛋白質 (ミエリン塩基性タンパク質 (MBP)、ミエリンプロテオリピドタンパク質 (PLP)、関連付けられているミエリン糖タンパク質 (MAG) とミエリン オリゴデンドロ サイト糖タンパク質 (モグ)22,23 ,24,25,26。この段階で成熟した最小二乗法は、軸索の周り鞘を enwrapping フォーム最適化される膜を拡張し、脳内を活性化することができます。これは、ラットやマウスの脳で MBP+細胞なる P1419,20,21ますます豊富な観察と一致します。
Immunopanning28,29,30, を含む Fewster と 1967年27で同僚のオリゴデンドロ サイトの最初の分離後 OLs の齧歯動物の中枢神経系からの分離のためのいくつかの方法を実施しています蛍光活性化細胞は、細胞表面抗原28,31, 微分勾配遠心法32,33,34,35 を利用 (FACS) を選別と異なる中枢神経系グリア36,37の差分の遵守に基づく振動法。ただし、現行の培養法には、純度、収量、38の手順の実行に要する時間の観点から特に制限があります。したがって、オリゴデンドロ サイトの効率的分離法が必要です。
本稿で提案する簡単な効率的な選択方法の免疫隔離のためステージ 3 O4+ preoligodendrocytes 細胞新生仔マウスの子犬。このメソッドは、エメリーらによって報告される技術の変更39と Dincmanら40し約 4 時間で 80% 以上のオリゴデンドロ サイト準備純度を提供します。
Protocol
Representative Results
Discussion
この通信は、高純度の未熟なマウス オリゴデンドロ サイト文化の効率的な分離法を提案する.以前に公開されたプロトコル39,40に比べると、このメソッドは、GFAP 陽性アストロ サイトの非常に低いレベルと他の非特徴の細胞の割合が非常に低い高い純度を得られました。オリゴデンドロ サイトの血統に既にコミット未熟な最小二乗法であることを?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
本研究は、国民の多数硬化の社会 (RG4591A1/2) および健康の国民の協会 (R03NS06740402) からの助成金によって支えられました。著者は、実験スペース、機器やアドバイスを提供するため博士イバン エルナンデスと彼の研究室のメンバーをありがとうございます。
Materials
| 10ml serological pipets | Fisher Scientific | 13-676-10J | |
| 10ml syringe Luer-Loc tip | BD, Becton Dickinson | 309604 | |
| 15ml conical tubes | Falcon | 352097 | |
| 24-well tissue culture plates | Falcon | 353935 | |
| 40µm cell strainer | Fisher Scientific | 22368547 | |
| 50ml conical tubes | Falcon | 352098 | |
| 5ml serological pipets | Fisher Scientific | 13-676-10H | |
| 60mm tissue culture plates | Falcon | 353002 | |
| 70µm cell strainer | Fisher Scientific | 22363548 | |
| Alexa Fluor 488 goat anti-mouse IgG (H+L) secondary antibody | Invitrogen | A11001 | |
| Alexa Fluor 488 goat anti-rabbit IgM (H+L) secondary antibody | Invitrogen | A21042 | |
| Alexa Fluor 488 goat anti-rabbit IgM (H+L) secondary antibody | Invitrogen | A11008 | |
| Alexa Fluor 594 goat anti-chicken IgG (H+L) secondary antibody | Invitrogen | A11042 | |
| Anti-O4 beads- Anti-O4MicroBeads | Miltenyi Biotec | 130-094-543 | |
| Apo-Transferrin human | Sigma | T1147 | |
| Autofil complete bottle top filter assembly, 0.22um filter, 250ml | USA Scientific | 6032-1101 | |
| Autofil complete bottle top filter assembly, 0.22um filter, 250ml | USA Scientific | 6032-1102 | |
| B27 Supplement | Invitrogen | 17504-044 | |
| Boric acid | Sigma | B7660 | |
| Bovine Growth Serum (BGS) | GE Healthcare Life Sciences | SH30541.03 | |
| BSA | Fisher Scientific | BP-1600-100 | |
| CNTF | Peprotech | 450-50 | |
| d-Biotin | Sigma | B4639 | |
| Desoxyribonuclease I (DNAse I) | Worthington | LS002007 | |
| EDTA | Fisher Scientific | S311 | |
| Epifluorescence microscope with an Olympus DP70 camera | Olympus | Bx51 | |
| Feather disposable scalpels | Andwin Scientific | EF7281C | |
| Forskolin | Sigma | F6886 | |
| German glass coverslips, #1 thickness, 12mm diameter round | NeuVitro | GG-12-oz | |
| GFAP antibody | Aves | GFAP | |
| Glucose | Fisher Scientific | D16-1 | |
| GlutaMAX | Invitrogen | 35050-61 | |
| Insulin | Invitrogen | 12585-014 | |
| Magnetic separator stand – MACS multistand | Miltenyi Biotec | 130-042-303 | |
| Magnetic separator-MiniMACS separator | Miltenyi Biotec | 130-042-302 | |
| Millex PES 0.22µm filter unit | Millipore | SLG033RS | |
| Mounting media- Prolong Gold with DAPI | Thermo Fisher | P36930 | |
| N-acetyl-cysteine (NAC) | Sigma | A8199 | |
| Natural mouse laminin | Invitrogen | 23017-015 | |
| Neurobasal Medium A | Invitrogen | 10888-022 | |
| Neurotrophin-3 (NT-3) | Peprotech | 450-03 | |
| NG2 antibody | Millipore | AB5320 | |
| Papain | Worthington | LS003126 | |
| PBS without Ca2+ and Mg2+ | Sigma | D5652 | |
| PDGF | Peprotech | 100-13A | |
| Petri dishes | Falcon | 351029 | |
| Poly-D-Lysine | Sigma | P6407 | |
| Primocin | Invivogen | ant-pm-2 | |
| Progesterone | Sigma | P8783 | |
| Putrescine | Sigma | P5780 | |
| Selection column-LS columns | Miltenyi Biotec | 130-042-401 | |
| Sodium Selenite | Sigma | S5261 | |
| Trace elements B | Corning | 25-000-CI | |
| Triiodothyronine (T3) | Sigma | T6397 | |
| Triton-X | Sigma | T8787 | |
| Trypan Blue Solution | Corning | 25-900-CI | |
| Tween 20 | Sigma | P1379 | |
| B27NBMA | 487.75 mL Neurobasal Medium A; 10 mL B27 Supplement; 1 mL Primocin; 1.25 mL Glutamax; Filter sterilize and store at 4 °C until use. | ||
| B27NBMA + 10% BGS | 27 mL B27NBMA; 3 mL Bovine growth serum | ||
| CNTF solution stock (10 µg/ml; 1000X) | Order from Peprotech (450-50). Make up at 0.1 to 1 mg/ml according to Manufacturer’s instruction (may vary from lot to lot) in buffer (e.g. DPBS + 0.2% BSA). Store at -80 °C. Working solution (10 µg/ml, 1000X) 1. Make on 0.2% BSA (Fisher scientific BP-1600-100) in DPBS solution and filter sterilize. 2. Dilute master stock aliquot to 10µg/ml in sterile, chilled 0.2% BSA/DPBS. 3. Aliquot (20µl/tube) and snap freeze in liquid nitrogen. 4. Store aliquots at -80 °C. |
||
| d-Biotin stock solution (50 µg/ml; 5000X) | Resuspend d-Biotin (Sigma-B4639) in double-distilled H2O at 50 µg/ml (e.g. 2.5 mg in 50 ml of ddH2O). Resuspension might take fair amount of agitation/vortexing, or mild warming briefly at 37°C. If the d-Biotin still will not solubilize, it is fine to make up a less concentrated (e.g. 10µg/ml), and to add a higher volume to the B27NBMA (1/1000), instead of 1/5000). Store at 4°C. | ||
| DNase I stock solution | 1. Dissolve at 12,500 U Deoxyribonuclease I / ml in HBSS chilled on ice. 2. Filter sterilize on ice 3. Aliquot at 200 µl and freeze overnight at -20°C. 4. Store aliquots at -20 to -30°C. |
||
| Dulbecco’s Phosphate Buffered Saline (w/o Ca2+ and Mg2+) | Dissolve pouch in 1 Liter of water to yield 1 liter of medium at 9.6 grams of powder per liter of medium. Store at 2-8 °C. | ||
| Forskolin stock solution (4.2 mg/ml; 1000X) | Add 1 ml of sterile DMSO to 50 mg Forskolin in bottle (Sigma-F6886) and pipette until resuspended. Transfer to a 15 ml centrifuge tube and add 11 ml of sterile DMSO to bring to 4.2 mg/ml. Aliquot (e.g. 20 µl) and store at -20°C. | ||
| Hank’s balanced salts (HBSS) (Sigma | 1. Measure 900 ml of water (temperature 15-20 °C) in a cylinder and stir gently. 2. Add the power and stir until dissolved. 3. Rinse original package with a small amount of water to remove all traces of the powder. 4. Add to the solution in step 2. 5. Add 0.35 gr of sodium bicarbonate (7.5% w/v) for each liter of final volume. 6. Keep stirring until dissolved. 7. Adjust the pH of the buffer while stirring to 0.1-0.3 units below pH= 7.4 since it may rise during filtration. The use of 1N HCl or 1N NaOH is recommended to adjust the pH. 8. Add additional water to bring the final volume to 1L. 9. Sterilize by filtration using a membrane with a porosity of 0.22 microns. 10. Store at 2-8 °C. |
||
| Insulin stock solution (4000 µg/ml) | Thaw the bottle and aliquot 25 µl per microcentrifuge tube and store at -20°C. | ||
| Laminin solution | Slowly thaw laminin in the cold (2°C to 8°C) to avoid gel formation. Then, aliquot into polypropylene tubes. Store at 5° C to -20° C in aliquots (e.g. 20 µl) and do not freeze/thaw repeatedly. Laminin may be stored at these temperatures for up to six months. | ||
| Magnetic Cell Sorting (MCS) Buffer | Prepare the solution containing phosphate-buffered saline (PBS), pH 7.2, and 0.5% bovine serum albumin (BSA), 0.5 mM EDTA, 5µg/ml Insulin, 1 g/L Glucose. Sterilize and degas by filtration the buffer by passing it through a 0.22 µm Millex filter. Store the buffer at 4°C until use | ||
| N-Acetyl-L-cysteine (NAC) stock solution (5mg/ml; 1000X) | Dissolve N-Acetyl-L-cysteine (Sigma-A8199) at 5 mg/ml in DMEM (e.g. 50 mg NAC in 10 ml B27NBMA). Filter sterilize and aliquot (e.g. 20 µl). Store at -20°C. | ||
| NT3 stock solution (1 µg/ml; 1000X) | Master stock: Order from Peprotech (450-03). Make up at 0.1 to 1 mg/ml according to manufacturer’s instructions (may vary from lot to lot), in buffer (e.g. DPBS + 0.2% BSA). Store at -80°C. Working stock (1µg/ml; 1000X): 1. Make on 0.2% BSA in DPBS solution and filter sterilize. 2. Dilute master stock aliquot to 1 µg/ml in sterile, chilled 0.2% BSA/DPBS. 3. Aliquot (e.g. 20µl/tube) and snap freeze in liquid nitrogen. 4. Store aliquots at -80°C. |
||
| PDGF stock solution (10 µg/ml; 1000X) | Master stock: Order from Peprotech (100-13A). Make up at 0.1 to 1 mg/ml according to manufacturer’s instructions (may vary from lot to lot) in buffer (e.g. DPBS) + 0.2% BSA). Store at -80°C. Working stock (1µg/ml; 1000X): 1. Make on 0.2% BSA in DPBS solution and filter sterilize. 2. Dilute master stock aliquot to 1µg/ml in sterile, chilled 0.2% BSA/DPBS. 3. Aliquot (e.g. 20µl/tube) and snap freeze in liquid nitrogen. 4. Store aliquots at -80°C. |
||
| Poly-D-lysine (1mg/ml; 100X) | Resuspend poly-D-lysine, molecular weight 70-150 kD (Sigma P6407) at 0.5mg/ml in 0.15M boric acid pH 8.4 (e.g. 50mg in 50ml borate buffer). Filter sterilize and aliquot (e.g. 100µl/tube). Store at -20°C. Prior to use, dilute the 100X stock (1mg/ml) to 50 µg/ml in sterile water. | ||
| Oligodendrocyte proliferation media | see Supplementary Table 1 | ||
| Oligodendrocyte differentiation media | see Supplementary Table 1 | ||
| Sato supplement (100X) | see Supplementary Table 1 | ||
| References: the list of reagents and recipes were adopted from the protocols previously described by Emery et. al. 2013 (Emery, B. & Dugas, J. C. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb Protoc. 2013 (9), 854-868, doi:10.1101/pdb.prot073973, (2013)) and Dincman et. al. (Dincman, T. A., Beare, J. E., Ohri, S. S. & Whittemore, S. R. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 209 (1), 219-226, doi:10.1016/j.jneumeth.2012.06.017, (2012)) |
References
- Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science. 330 (6005), 779-782 (2010).
- Yang, Z., Watanabe, M., Nishiyama, A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 149 (1), 50-56 (2005).
- Niu, J., et al. An efficient and economical culture approach for the enrichment of purified oligodendrocyte progenitor cells. J Neurosci Methods. 209 (1), 241-249 (2012).
- Zhang, S. C. Defining glial cells during CNS development. Nat Rev Neurosci. 2 (11), 840-843 (2001).
- Pfeiffer, S. E., Warrington, A. E., Bansal, R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3 (6), 191-197 (1993).
- Hart, I. K., Richardson, W. D., Heldin, C. H., Westermark, B., Raff, M. C. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development. 105 (3), 595-603 (1989).
- Nishiyama, A., Lin, X. H., Giese, N., Heldin, C. H., Stallcup, W. B. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 43 (3), 315-330 (1996).
- Pringle, N. P., Mudhar, H. S., Collarini, E. J., Richardson, W. D. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 115 (2), 535-551 (1992).
- Grinspan, J. B., Franceschini, B. Platelet-derived growth factor is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitor cells. J Neurosci Res. 41 (4), 540-551 (1995).
- Sharifi, K., et al. Differential expression and regulatory roles of FABP5 and FABP7 in oligodendrocyte lineage cells. Cell Tissue Res. 354 (3), 683-695 (2013).
- Chittajallu, R., Aguirre, A., Gallo, V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 561 (Pt 1), 109-122 (2004).
- Bansal, R., Warrington, A. E., Gard, A. L., Ranscht, B., Pfeiffer, S. E. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 24 (4), 548-557 (1989).
- Sommer, I., Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 83 (2), 311-327 (1981).
- Boda, E., et al. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia. 59 (12), 1958-1973 (2011).
- Dean, J. M., et al. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci. 33 (3-4), 251-260 (2011).
- Yu, W. P., Collarini, E. J., Pringle, N. P., Richardson, W. D. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 12 (6), 1353-1362 (1994).
- Armstrong, R. C., Dorn, H. H., Kufta, C. V., Friedman, E., Dubois-Dalcq, M. E. Pre-oligodendrocytes from adult human CNS. J Neurosci. 12 (4), 1538-1547 (1992).
- Gard, A. L., Pfeiffer, S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 106 (1), 119-132 (1989).
- Bjelke, B., Seiger, A. Morphological distribution of MBP-like immunoreactivity in the brain during development. Int J Dev Neurosci. 7 (2), 145-164 (1989).
- Hardy, R. J., Friedrich, V. L. Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis. Dev Neurosci. 18 (4), 243-254 (1996).
- Hartman, B. K., Agrawal, H. C., Kalmbach, S., Shearer, W. T. A comparative study of the immunohistochemical localization of basic protein to myelin and oligodendrocytes in rat and chicken brain. J Comp Neurol. 188 (2), 273-290 (1979).
- Wei, Q., Miskimins, W. K., Miskimins, R. Stage-specific expression of myelin basic protein in oligodendrocytes involves Nkx2.2-mediated repression that is relieved by the Sp1 transcription factor. J Biol Chem. 280 (16), 16284-16294 (2005).
- Stolt, C. C., et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16 (2), 165-170 (2002).
- Emery, B., et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 138 (1), 172-185 (2009).
- Reynolds, R., Wilkin, G. P. Development of macroglial cells in rat cerebellum. II. An in situ immunohistochemical study of oligodendroglial lineage from precursor to mature myelinating cell. Development. 102 (2), 409-425 (1988).
- Scolding, N. J., et al. Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J Neuroimmunol. 22 (3), 169-176 (1989).
- Fewster, M. E., Scheibel, A. B., Mead, J. F. The preparation of isolated glial cells from rat and bovine white matter. Brain Res. 6 (3), 401-408 (1967).
- Gard, A. L., Williams, W. C., Burrell, M. R. Oligodendroblasts distinguished from O-2A glial progenitors by surface phenotype (O4+GalC-) and response to cytokines using signal transducer LIFR beta. Dev Biol. 167 (2), 596-608 (1995).
- Gard, A. L., Pfeiffer, S. E. Glial cell mitogens bFGF and PDGF differentially regulate development of O4+GalC- oligodendrocyte progenitors. Dev Biol. 159 (2), 618-630 (1993).
- Barres, B. A., Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 361 (6409), 258-260 (1993).
- Behar, T., McMorris, F. A., Novotny, E. A., Barker, J. L., Dubois-Dalcq, M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 21 (2-4), 168-180 (1988).
- Vitry, S., Avellana-Adalid, V., Lachapelle, F., Baron-Van Evercooren, A. Migration and multipotentiality of PSA-NCAM+ neural precursors transplanted in the developing brain. Mol Cell Neurosci. 17 (6), 983-1000 (2001).
- Duncan, I. D., Paino, C., Archer, D. R., Wood, P. M. Functional capacities of transplanted cell-sorted adult oligodendrocytes. Dev Neurosci. 14 (2), 114-122 (1992).
- Goldman, J. E., Geier, S. S., Hirano, M. Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture. J Neurosci. 6 (1), 52-60 (1986).
- Althaus, H. H., Montz, H., Neuhoff, V., Schwartz, P. Isolation and cultivation of mature oligodendroglial cells. Naturwissenschaften. 71 (6), 309-315 (1984).
- McCarthy, K. D., de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 85 (3), 890-902 (1980).
- Szuchet, S., Yim, S. H. Characterization of a subset of oligodendrocytes separated on the basis of selective adherence properties. J Neurosci Res. 11 (2), 131-144 (1984).
- Chew, L. J., DeBoy, C. A., Senatorov, V. V. Finding degrees of separation: experimental approaches for astroglial and oligodendroglial cell isolation and genetic targeting. J Neurosci Methods. 236, 125-147 (2014).
- Emery, B., Dugas, J. C. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb Protoc. 2013 (9), 854-868 (2013).
- Dincman, T. A., Beare, J. E., Ohri, S. S., Whittemore, S. R. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 209 (1), 219-226 (2012).
- Buttery, P. C., ffrench-Constant, C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 14 (3), 199-212 (1999).
- Chun, S. J., Rasband, M. N., Sidman, R. L., Habib, A. A., Vartanian, T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 163 (2), 397-408 (2003).
- Colognato, H., Ramachandrappa, S., Olsen, I. M., ffrench-Constant, C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 167 (2), 365-375 (2004).
- ffrench-Constant, C., Colognato, H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 14 (12), 678-686 (2004).
- Oh, L. Y., Yong, V. W. Astrocytes promote process outgrowth by adult human oligodendrocytes in vitro through interaction between bFGF and astrocyte extracellular matrix. Glia. 17 (3), 237-253 (1996).
- Besnard, F., Perraud, F., Sensenbrenner, M., Labourdette, G. Effects of acidic and basic fibroblast growth factors on proliferation and maturation of cultured rat oligodendrocytes. Int J Dev Neurosci. 7 (4), 401-409 (1989).
- Armstrong, R., Friedrich, V. L., Holmes, K. V., Dubois-Dalcq, M. In vitro analysis of the oligodendrocyte lineage in mice during demyelination and remyelination. J Cell Biol. 111 (3), 1183-1195 (1990).
- Grinspan, J. B., Stern, J. L., Franceschini, B., Pleasure, D. Trophic effects of basic fibroblast growth factor (bFGF) on differentiated oligodendroglia: a mechanism for regeneration of the oligodendroglial lineage. J Neurosci Res. 36 (6), 672-680 (1993).
- Mason, J. L., Goldman, J. E. A2B5+ and O4+ Cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 20 (1), 30-42 (2002).
- Schildge, S., Bohrer, C., Beck, K., Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J Vis Exp. (71), (2013).

