Frequent Tail-tip Blood Sampling in Mice for the Assessment of Pulsatile Luteinizing Hormone Secretion
Summary
Here we present a tail-tip blood sampling protocol for frequent sample collection in unrestrained mice. This method is useful for assessing patterns of pulsatile luteinizing hormone secretion and could be adapted for analysis of other circulating factors.
Abstract
In many endocrine systems, circulating factors or hormones are not released continuously, but are secreted as a discrete pulse in response to a releasing factor. Single-point sampling measures are inadequate to fully understand the biological significance of the secretory pattern of pulsatile hormones either under normal physiologic conditions or during conditions of dysregulation. Luteinizing hormone (LH) is synthesized by the anterior pituitary gonadotrope cells and secreted in a pulsatile pattern which requires frequent collection of blood samples for pulse assessment. This has not been possible in mice until recently, due to the development of a high-sensitivity LH assay and advancement in a technique for frequent low-volume sample collection, initially described by Steyn and colleagues.1 Here we describe a protocol for the frequent peripheral blood sample collection from mice with sufficient handling acclimatization to detect pulsatile secretion of LH. The current protocol details an expanded acclimatization period that allows assessment of robust and continuous pulses of LH over multiple hours. In this protocol, the tip of the tail is clipped and blood is collected from the tail using a hand-held pipette. For assessment of pulsatile LH in gonadectomized mice, serial samples are collected every 5-6 min for 90-180 min. Importantly, the collection of blood and measurement of robust pulses of LH can be accomplished in awake, freely behaving mice, given adequate handling acclimatization and effort to minimize environmental stressors. Sufficient acclimatization can be achieved within 4-5 weeks prior to blood collection. This protocol highlights advances in the methodology to ensure collection of whole blood samples for assessment of pulsatile LH secretion patterns over multiple hours in the mouse, a powerful animal model for neuroendocrine research.
Introduction
Gonad function in mammals is dependent upon gonadotropin secretion, luteinizing hormone (LH) and follicle stimulating hormone, from the pituitary gland. The gonadotropins are secreted in either a pulsatile or surge pattern in response to hypothalamic secretion of gonadotropin-releasing hormone (GnRH). The synthesis and secretion of both LH and FSH are regulated via endocrine, paracrine and autocrine action from a variety of molecules including hypothalamic GnRH, gonadal steroid hormones, and the activin-inhibin-follistatin system, as well as a myriad of physiological conditions including stress and energy balance.2
The pulsatile pattern of LH in blood arises from a rather abrupt discharge of LH into peripheral blood, followed by approximately exponential elimination. Important features of the pattern include the frequency of each LH discharge and the amplitude of the LH response, both of which are dictated, in part, by the release of GnRH. Due to the difficulty in collecting hypothalamic-pituitary portal blood for measurement of pulsatile GnRH, the sampling and measurement of LH is used as a proxy for GnRH regulation of the hypothalamic-pituitary-gonadal axis. Therefore, critical information is encoded in the frequency and amplitude of LH pulses, which cannot be determined from a single sample.
Analysis of pulsatile LH secretion has historically been limited to large mammals (humans, primates, and sheep) due to their large blood volume and tolerance for frequent blood sample collection. In rodents, frequent blood sampling was limited to the rat and achieved via indwelling atrial catheter.3,4 The relatively low cost and availability of genetic (e.g. cre-lox, CRISPR) and complex neural circuit (e.g. optogenetics, chemogenetics) manipulations make mice an attractive model organism; however, attainment of frequent blood samples and subsequent analysis of LH concentrations has until recently, proven elusive. This monumental task was pioneered by Steyn and co-workers.1 Since then, several labs have begun to utilize frequent blood sampling and ultra-sensitive LH assays to assess pulsatile LH secretion in a variety of experimental paradigms.5,6,7,8,9 It should be noted that the pursuit of a practical method of collecting multiple blood samples from mice has been in progress for at least 40 years10 with multiple refinements made along the way.11,12
Assessment of LH pulse patterns (i.e. frequency and amplitude) represents a major refinement in monitoring basal gonadotropin secretion in this genetically tractable animal model. Traditionally, LH concentrations in mice were determined in a single blood sample. One weakness of single-point samples is a highly variable data set because LH concentrations are naturally fluctuating during each pulse. Another weakness is that isolated measurements inherently miss critical information conveyed by the patterns of LH pulse secretion. Thus, a method for collecting frequent blood samples in freely behaving, unrestrained mice (except for gentle handling during sampling) will provide enhanced information and prove useful to many laboratories investigating pulsatile hormone regulation.
Here, we describe a protocol for the collection of frequent (every 6 min) blood samples from awake, unrestrained mice. Importantly, we include a handling acclimatization protocol that allows for robust and continuous detection of pulsatile LH secretion in whole blood samples collected over a length of time in which pre- and post-assessment to an acute challenge can be determined, such as the response to the psychosocial stress of immobilization and restraint. An effective assay for LH concentrations from whole blood samples has been described previously;1 this protocol is focused on a method for collecting the blood samples for LH pulse measurement.
Protocol
The method described here is in agreement with the National Institutes of Health Animal Care and Use Guidelines and have been authorized by the Institutional Animal Care and Use Committee at the University of California, San Diego.
1. Acclimatization to Handling
- Handling procedure
- Place mouse cages in the biosafety cabinet. Remove the first mouse from the cage and place on a suitable surface (e.g. a clean cage lid) within the biosafety cabinet.
- Gently restrain the mouse on the work surface by holding the base of the tail with the forefinger and thumb of one hand. Then, firmly stroke the ventral surface of the tail, from the base to the tip of the tail, with the forefinger and thumb of the other hand. Repeat this stroking 6 times (i.e. 1 set) and return the mouse to its cage.
- Wait 6 min and repeat step 1.1.2.
NOTE: In total, step 1.1.2 will be repeated 4 times per mouse (i.e. 4 sets of 6 strokes), so that each mouse will receive a total of 24 tail strokes on each day of handling.
- The frequency of daily handling
- From five until two weeks before the planned blood collection day, handle mice as described in step 1.1, five days per week (mice are not handled on Saturday or Sunday until the final two weeks of acclimatization).
- If experimental treatment during the sampling period will include any additional handling (e.g. scruffing or intraperitoneal injection), acclimatize the mice to this handling activity during each handling session.
Note: For acclimatization to injection procedures, use a blunted needle to perform a sham injection. - During the two weeks before the planned blood collection day, handle mice as described in 1.1, seven days per week.
- At least two weeks before the planned blood collection day, house mice in pairs to minimize disruptions to the mice during sampling.
2. Preparation of Blood Collection Tubes
- Prepare ultrasensitive LH assay buffer in a glass bottle: 0.2% bovine serum albumin and 0.05% Tween-20 in PBS [0.2 g KCl, 8 g NaCl, 1.44 g Na2HPO4 (anhydrous), 0.24 g KH2PO4, ultrapure water to 1 L, pH 7.4, filter and autoclave], store at 4°C.1
NOTE: Volume of ultrasensitive LH assay buffer to be prepared can be calculated based on the number of samples and the volume per sample as indicated below (2.2 & discussion) - Prepare blood collection tubes (0.6mL microcentrifuge tubes). Label tubes and aliquot assay buffer (57 µL of buffer per tube; note, this volume may vary depending on the volume of blood collected or the ratio of the blood to the buffer desired). Tubes can be prepared days ahead of sampling, store at 4°C.
3. Blood Collection
- Preparation of blood collection materials.
- Prepare biosafety cabinet for use, and arrange the following materials in the hood or on a cart nearby: 10 µL pipette (set to 3 µL or desired blood collection volume), pipette tips, waste bin for used pipette tips, timer, printout with animal numbers and sample numbers (for notes regarding sampling or animal issues), ice bucket with blood collection tubes, scissors, and small gauze (2" x 2").
NOTE: The "count up" function on a laboratory timer is useful because it does not have an audible alarm that could disturb the mice - Use a permanent marker to mark the tail of each mouse (near the base) to assist with rapid identification of the correct mouse during sampling.
- Prepare biosafety cabinet for use, and arrange the following materials in the hood or on a cart nearby: 10 µL pipette (set to 3 µL or desired blood collection volume), pipette tips, waste bin for used pipette tips, timer, printout with animal numbers and sample numbers (for notes regarding sampling or animal issues), ice bucket with blood collection tubes, scissors, and small gauze (2" x 2").
- Clipping of the tail and pre-blood collection preparation.
- At 45 min before the start of the blood collection, remove the first mouse from its cage and place on a suitable surface (a clean cage lid works well) within the biosafety cabinet.
- Use sharp, sterile scissors to remove 1-2 mm of the tip of the mouse tail, following aseptic preparation (i.e. scrub with alcohol and betadine).
NOTE: This can be accomplished in awake animals with gentle restraint or under isoflurane anesthesia. Additionally, analgesia may be provided following the recommendation of the local IACUC. - Carefully stroke the tail of the mouse as described above (1.1.2; 1-2 strokes are generally sufficient) until the droplet of blood forms on the tail tip.
- Use a pipette with a clean tip to collect 3 µL of blood from the tail tip and discard the pipette tip with blood.
- Before returning the mouse to its cage, ensure that blood flow has stopped. If necessary, apply gentle pressure with sterile gauze.
- Repeat steps 3.2.1 – 3.2.5 for all mice. Subsequent blood samples can be collected by firmly stroking the mouse's tail until a droplet of blood forms at the tip of the tail.
NOTE: If a blood droplet does not form after firm strokes, the tail can be dabbed with a gauze pad soaked in saline. Clots are more likely to form during less frequent sampling (i.e. >15 min intervals). Once the clot is cleared from the vein, sampling can continue. - Continue to collect 3 µL of blood from each mouse as described above every 6 min for approximately 45 min during this pre-blood collection preparation period. Discard the blood and pipette tip.
- Blood sampling
- Collect 3 µL of blood from the tail tip as described above. Take caution to ensure that the entire 3 µL sample is taken into the pipette tip (without bubbles or bedding contamination). Eject the blood sample into the assay buffer in a labeled tube. Mix by inversion and return the tube to ice.
- Before returning the mouse to its cage, ensure that the blood flow has stopped. If necessary, apply gentle pressure with sterile gauze.
- Repeat steps 3.3.1 – 3.3.2 for each mouse, every 6 min, for the predetermined sampling duration. Monitor each animal for signs of distress (e.g. partially closed eyelids, hunched position [not caused by other treatment] or lack of response to handling) and terminate sampling, if necessary.
NOTE: Behavioral distress or other complications that require termination of sampling occur extremely rarely in our laboratory. - If animals are not euthanized following blood sampling, ensure that the blood flow from the tail-tip has completely stopped. Wipe any spilled blood from the inside of each animal's cage (or change cages) and return the animal cages to the housing rack.
4. Sample Processing and Analysis
- Store blood samples at -20 °C until analysis.
NOTE: LH is assayed in whole blood samples diluted in assay buffer; no separation of sera or plasma is required prior to storage or analysis. - Assay blood samples by ultrasensitive ELISA as described previously1 and report values in ng/mL of whole blood following correction for dilution of the sample volume in the assay buffer.
- Identify LH pulses in the data set. Calculate and compare mean pulse amplitude and pulse frequency (or inter-pulse interval) as appropriate for the data set.
NOTE: Published criteria13,14 and programs,15,16 for detecting LH pulses are available.
Representative Results
Representative LH pulse patterns from 4 mice are shown in Figure 1 (data reprinted from Yang et al., 2017). LH was measured in frequent blood samples to determine the response to an acute psychosocial stress challenge. Adult female C57/Bl6 mice were ovariectomized and handled as detailed in this protocol for frequent blood collection. Blood samples were collected for the first 90 min in all animals to establish a pre-treatment baseline of LH pulsatile secretion. During the second 90 min of sampling, control (non-stressed) animals remained in their cages and restraint stress animals were placed into rodent restraint devices and isolated to new cages. Control animals showed clear, unambiguous LH pulses throughout the entire sampling period. In contrast, the psychosocial stress of restraint rapidly and unambiguously suppressed pulsatile LH secretion.
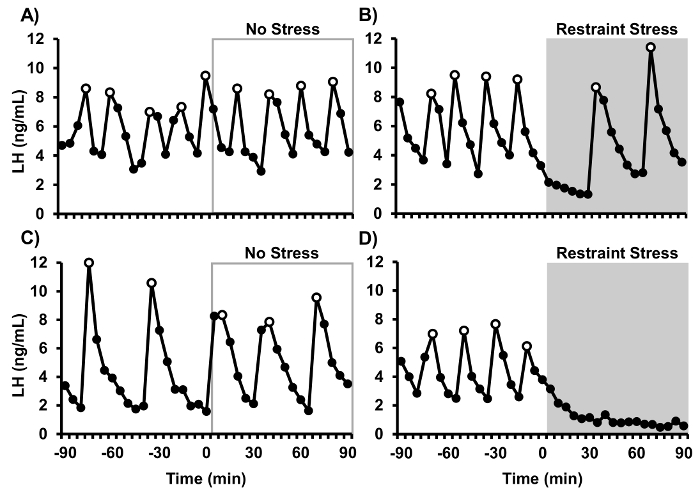
Figure 1: Representative LH pulse patterns from ovariectomized mice. (A, C) Control non-stressed mice. (B, D) Stressed mice in which restraint was imposed from 0-90 min (grey shaded region) and LH was assayed. Open data points represent pulses identified by DynPeak.15 This figure has been modified from Yang et al., Endocrinology 158(11): 3716-3723, 2017 with permission of The Endocrine Society, Copyright 2017. Please click here to view a larger version of this figure.
Discussion
Here we describe a protocol for frequent blood collection of whole blood tail-tip samples for assessment of pulsatile LH secretion in mice. This protocol enables the collection of samples for detection of acute changes in pulsatile LH secretion following exposure to psychosocial stress and is well-suited for assessment of LH pulses under other acute or chronic manipulations.
A critical component of this protocol for achieving reliable and robust profiles of LH pulses is the handling acclimatization period. The idea that handling stress can impair hormone secretion has been speculated upon in the literature,17 but not explicitly tested. However, analysis of the representative LH pulse patterns depicted in recent publications clearly demonstrates an advantage to thorough acclimatization to handling. In early studies, utilizing 10 days of handling, LH pulses occurred at irregular intervals with prolonged periods in which no LH pulses were detected.1 Subsequent to that seminal publication, there has been a trend in the literature for longer handling periods (i.e. 3 or 4 weeks of daily handling).5,6,8 The protocol described here involves a total of 5 weeks of handling but omits handling on the first 3 weekends, thereby, achieving a similar number of handling sessions and minimizing weekend workloads for experimentalists. Although we cannot provide a validated threshold number of handling sessions required for successful detection of LH pulses, we would encourage new practitioners of this procedure to consider an extended acclimatization period. Under certain experimental paradigms (e.g. studying LH pulses in young animals), several weeks of handling acclimatization may not be practical. For these experiments, it is critical that sufficient handling acclimatization is achieved; perhaps multiple handling sessions per day would be sufficient.
Minimizing environmental stress during the blood collection period is also critical to the success of this protocol. In pursuit of this goal, we house our mice in a quiet room within the vivarium during the entire acclimatization period. Placement of a "Quiet Please" sign on the outside of the animal room door on the day of sampling may help to eliminate unintended interruptions. During the blood collection period, a cloth can be used in the biosafety cabinet to provide a place to rest the pipette, pipette tips and pens on, thereby, minimizing noise caused by manipulating these items. Finally, it is advised that one person should handle the mice and collect all the blood samples on the experimental day. This individual should be diligent in handling animals with care, and at a consistent time of day (which should be similar to the sampling time), since circadian inputs are known to influence stress responses.18 If LH pulses are infrequent or undetectable, sources of extraneous stress should be identified and minimized. Another procedural detail related to minimizing stress during the blood collection period is the amount of time that elapses between clipping of the tail and collecting samples to be assayed for LH. We clip the tail of each mouse 45 min before collecting samples for LH assay. This delay serves to temporally separate the stress associated with tail clipping from any additional treatments imposed during sampling, as the tail clipping alone has been demonstrated to elevate circulating corticosterone concentrations.19 Blood samples are withdrawn (but discarded) during this 45-min window to further acclimatize the mice to the handling procedure and prevent blood clot formation during this delay.
A final consideration when employing this protocol for frequent blood collection in mice is that the small blood volume of this species will limit the number and volume of each sample collected. According to the USDA Animal Inspection Guide, mice have 72 mL of blood per kg of body weight.20 For survival experiments, 7.5% of the blood volume can be collected in a single sampling period given a 1-week recovery period and 10% of the blood volume can be collected with a 2-week recovery period. We routinely collect <7.5% of the estimated blood volume of the mouse using the current frequent blood collection protocol (including representative results shown here), without any adverse effect on LH secretory patterns. In our vivarium, adult C57/Bl6 ovariectomized mice weigh approximately 22 g (theoretical blood volume of 1.58 mL), allowing for 118.5 µL of blood to be collected. In addition to 7 pre-collection handling samples (which are not assayed or analyzed), 30 samples are collected over 180 min for a total of 111 µL of blood (3 µL per sample), which is below the 7.5% blood volume limit. It should be noted that the volume of the blood sample may need to be adjusted to detect LH in certain conditions when LH concentrations are expected to be low. In gonadectomized mice, 3 µL of whole blood is sufficient to detect LH. Collecting a larger blood volume should be considered when LH concentrations are low and therefore expected to be closer to the sensitivity of the LH assay. Thus, despite the small blood volume of a mouse, sufficient blood samples can be collected to assess an uncompromised profile of pulsatile LH secretion over a 180-min period.
In conclusion, this frequent blood sampling protocol highlights an extended handling acclimatization period to ensure assessment of pulsatile LH secretion in mice over an extended period of time. We have demonstrated that this protocol is sufficient to characterize robust and regular pulses of LH that can be monitored prior to and following exposure to psychosocial stress. This protocol can be adapted to assess the response of LH to other types of stress or other treatments that may alter reproductive hormone secretion. Furthermore, this blood collection protocol could be adapted for the measurement of other circulating factors that vary in concentration over time, as has been reported for growth hormone.21 It is a remarkable contribution to the field of neuroendocrinology that the secretion pattern of LH can now be monitored in a species where complex genetic and in vivo molecular approaches to study LH pulse regulation are available and practical.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Drs. Jennifer Yang and Alexander (Sasha) Kauffman for technical assistance with this technique as well as numerous helpful and critical discussions. Serum hormone assays were performed by Yang et al., 2017 at The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934.
Sources of Research Support: R01 NICHD 86100 (KMB), RBM was supported by T32 NICHD 007203.
Materials
| Biosaftey cabinet | Lab Products Inc | L/F-B | |
| Bovine serum albumin | Sigma | A5403 | |
| Tween-20 | Sigma | P2287 | |
| KCl | Sigma | P9333 | |
| NaCl | Sigma | S7653 | |
| Na2HPO4 (anhydrous) | Sigma | S7907 | |
| KH2PO4 | Sigma | P5655 | |
| Ultrapure water | Millipore | Purified and filtered water | |
| Broome Rodent Restraint Device | Harvard Apparatus | 52-0460 | Not necessary for blood collection, but were used in the collection of representative data. |
| DynPeak | n/a | n/a | http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0039001 |
References
- Steyn, F. J., et al. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 154 (12), 4939-4945 (2013).
- McArdle, C. A., Roberson, M. S., Plant, T. M., Zeleznik, A. J. . Knobil and Neill’s Physiology of Reproduction. , (2015).
- Parasuraman, S., Raveendran, R., Kesavan, R. Blood sample collection in small laboratory animals. Journal of Pharmacology and Pharmacotherapy. 1 (2), 87-93 (2010).
- Steiner, R. A., Bremner, W. J., Clifton, D. K. Regulation of luteinizing hormone pulse frequency and amplitude by testosterone in the adult male rat. Endocrinology. 111 (6), 2055-2061 (1982).
- Moore, A. M., Prescott, M., Marshall, C. J., Yip, S. H., Campbell, R. E. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proceedings of National Academy of Science U S A. 112 (2), 596-601 (2015).
- Czieselsky, K., et al. Pulse and Surge Profiles of Luteinizing Hormone Secretion in the Mouse. Endocrinology. 157 (12), 4794-4802 (2016).
- Campos, P., Herbison, A. E. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proceedings of National Academy of Science U S A. 111 (51), 18387-18392 (2014).
- Yang, J. A., et al. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology. 158 (11), 3716-3723 (2017).
- Clarkson, J., et al. Definition of the hypothalamic GnRH pulse generator in mice. Proceedings of National Academy of Science U S A. 114 (47), E10216-E10223 (2017).
- Lewis, V. J., Thacker, W. L., Mitchell, S. H., Baer, G. M. A new technic for obtaining blood from mice. Laboratory Animal Science. 26 (2 Pt 1), 211-213 (1976).
- Abatan, O. I., Welch, K. B., Nemzek, J. A. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. Journal of the American Association of Laboratory Animal Science. 47 (3), 8-15 (2008).
- Durschlag, M., Wurbel, H., Stauffacher, M., Von Holst, D. Repeated blood collection in the laboratory mouse by tail incision–modification of an old technique. Physiology and Behavior. 60 (6), 1565-1568 (1996).
- Goodman, R. L., Karsch, F. J. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 107 (5), 1286-1290 (1980).
- Clarke, I. J., Cummins, J. T. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology. 116 (6), 2376-2383 (1985).
- Vidal, A., Zhang, Q., Medigue, C., Fabre, S., Clement, F. DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One. 7 (7), e39001 (2012).
- Merriam, G. R., Wachter, K. W. Algorithms for the study of episodic hormone secretion. American Journal of Physiology. 243 (4), E310-E318 (1982).
- Xie, T. Y., et al. Effect of Deletion of Ghrelin-O-Acyltransferase on the Pulsatile Release of Growth Hormone in Mice. Journal of Neuroendocrinology. 27 (12), 872-886 (2015).
- Koch, C. E., Leinweber, B., Drengberg, B. C., Blaum, C., Oster, H. Interaction between circadian rhythms and stress. Neurobiology of Stress. 6, 57-67 (2017).
- Tuli, J. S., Smith, J. A., Morton, D. B. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Laboratory Animal. 29 (1), 90-95 (1995).
- . Animal Welfare Inspection Guide Available from: https://www.aphis.usda.gov/animal_welfare/downloads/Animal-Care-Inspection-Guide.pdf (2017)
- Steyn, F. J., et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 152 (8), 3165-3171 (2011).

