Measuring Phosphorus Release in Laboratory Microcosms for Water Quality Assessment
Summary
Accurate quantification of phosphorus (P) desorption potential in saturated soils and sediments is important for P modeling and transport mitigation efforts. To better account for in situ soil-water redox dynamics and P mobilization under prolonged saturation, a simple approach was developed based on repeated sampling of laboratory microcosms.
Abstract
Phosphorus (P) is a critical limiting nutrient in agroecosystems requiring careful management to reduce transport risk to aquatic environments. Routine laboratory measures of P bioavailability are based on chemical extractions performed on dried samples under oxidizing conditions. While useful, these tests are limited with respect to characterizing P release under prolonged water saturation. Labile orthophosphate bound to oxidized iron and other metals can rapidly desorb to solution in reducing environments, increasing P mobilization risk to surface runoff and groundwater. To better quantify P desorption potential and mobility during extended saturation, a laboratory microcosm method was developed based on repeated sampling of porewater and overlying floodwater over time. The method is useful for quantifying P release potential from soils and sediments varying in physicochemical properties and can improve site-specific P mitigation efforts by better characterizing P release risk in hydrologically active areas. Advantages of the method include its ability to simulate in situ dynamics, simplicity, low cost, and flexibility.
Introduction
Phosphorus (P) is a critical limiting nutrient for both crop and aquatic biomass productivity. Surface water hydrology is a main driver of P fate and transport, as it controls the physical transport of sediment and P while also affecting remobilization potential during runoff and flooding/ponding events. Various laboratory-based extraction methods are typically used to estimate P release at the field scale under oxidizing conditions. While different mechanisms can contribute to P release, reductive dissolution of iron-phosphates is a well-established reaction mechanism that can lead to large orthophosphate-P fluxes to water1,2,3,4. In a review of mechanisms controlling P biogeochemistry in wetlands, redox status was hypothesized to be the main variable controlling P release to soils and shallow groundwater5. As such, traditional P tests may not be reliable predictors of P release under prolonged saturation.
Given the importance of water residence time and redox status on P fate and transport, laboratory approaches designed to better simulate in situ conditions could lead to improved P transport risk indices for agricultural and wetland ecosystems subject to variable saturation. Since orthophosphate is immediately bioavailable, the rate and extent of desorption during saturation can be used as an index of nonpoint source P pollution risk. Our method was designed to quantify P desorption to porewater (PW) and mobilization to overlying floodwater (FW), a typical condition in areas with variable source area hydrology (e.g., flooded agricultural fields, wetlands, drainage ditches, and riparian/near-stream zones). The method was originally developed to characterize P release potential in seasonally flooded soils from northern New York (USA) and recently applied to quantify P desorption potential of riparian soils from northwestern Vermont’s Lake Champlain Basin6. Here, we provide a protocol for the laboratory microcosm method and highlight results from a recently published study demonstrating its ability to quantify P desorption potential. We also demonstrate the relationship between P release potential and the reliability of routine soil tests (labile extractable P, pH) to predict release across sites.
Carrying out the method requires access to an analytical laboratory with adequate climate control, ventilation, water, and a proper acid waste disposal system. The method presumes access to routine chemical reagents and laboratory equipment (sinks, hoods, glassware, etc.). Beyond routine laboratory needs, a membrane filtration (≤ 0.45 µm) system is required and a UV spectrophotometer to measure P. A pH meter or multiparameter water quality probe are also recommended but not required. Laboratory temperature is an important factor and should be kept constant unless temperature itself is being investigated as an experimental factor (20 °C is recommended). Unhindered access to an adequate analytical laboratory with proper equipment is a prerequisite to perform the method properly and generate meaningful results.
Protocol
1. Sample collection
- Collect approximately 4 L of soil (or sediment) from desired sites. Collection areas should be relatively small to limit spatial variation in P and soil properties.
- Sieve samples through a coarse (20 mm) screen followed a 2 mm screen. Thoroughly hand-mix samples after sieving.
- Weigh out 100 g of field-moist soil or sediment. Dry in an oven at 105 °C for 24 h and calculate gravimetric water content (soil water mass/dry soil mass).
- Take a 500 mL subsample for chemical analysis.
NOTE: Soil pH, organic matter content and labile inorganic P (Pi) concentration are recommended soil tests. Here, labile soil Pi availability was assessed by: 1) Pi extracted by 1.25 mol L-1 ammonium-acetate (pH = 4.8; hereafter referred to as modified Morgan extractable P) measured colorimetrically7,8, 2) Pi extracted by distilled water, and 3) P extracted by 1.25 mol L-1 ammonium-acetate (pH = 4.8) measured by inductively coupled plasma-optical emission spectroscopy (ICP)8. - Use remaining sieved soil for microcosm studies or store in polyethylene bags at 5 °C for later use.
NOTE: Soils dry out when refrigerated for long periods (>30 days) and will require remoistening. Do not freeze soil samples as it affects microbial integrity and P release potential.
2. Microcosm construction
- Use one-liter (1 L) graduated polypropylene or other non-reactive plastic beakers as individual experimental units (microcosms). Wash beakers in 10% hydrochloric acid and triple rinse with distilled water.
- Measure 2 cm up from the bottom and place a mark next to beaker graduations. Drill a 1.25 cm diameter hole for drainage ports.
- Place a small bead of silicone around the inside edge of hose barb and outer circumference of the bore hole. Carefully insert the drainage port into the hole.
NOTE: Allow air-drying for at least 24 h before proceeding to step 2.4. - Trace the outside circumference of hose barbs onto nylon mesh filter screen and cut out with scissors. Apply a thin bead of silicone around the circumference of each filter on the outside edge and press filters onto hose barb inlets. Allow at least 24 h of drying time before using.
NOTE: A 100 µm pore size is recommended for most applications; however, finer-textured soils may require a larger filter pore size to avoid excessively long PW sample collection time. - Fit a short piece of 0.625 cm diameter latex hose to hose barb ends. Attached a 3.3 cm wide paper binder clip to the hose to prevent flow.
3. Conducting a phosphorus release trial
- Place 500 mL of sample into duplicate microcosms and gently apply distilled water along beaker walls until FW reaches the 1 L mark.
NOTE: Allow microcosms to equilibrate for 24−48 h before taking initial samples. - Unclip paper binders to induce PW flow through drainage port. Collect samples by placing clean 30 mL beakers directly beneath PW drainage ports. Allow several mL of PW to drain, discard and use the next 10 mL as a representative sample volume.
- Filter PW samples through 0.45 µm membrane filters and immediately analyze for soluble reactive P (SRP). Record absorbance values and time of measurements.
NOTE: SRP is generally assumed to be orthophosphate; however, molybdate-reactive P can also form complexes with colloids and/or nanoparticles that pass through 0.45 µm filters4. - Take initial FW sample by inserting a 10 mL bulb syringe pipette halfway down the water column and withdraw a sample using a circular motion. Empty into beakers, filter and analyze immediately for SRP.
- Replace sampled water by refilling beakers to the 1 L level with distilled water.
NOTE: Evaporative losses will vary. The goal is to consistently maintain a total volume (flooded soil + water column) of 1 L in all microcosms. Replacing evaporative water losses has negligible dilution effects on SRP. - Repeat steps 3.2 through 3.5 based on the desired number of P release time points for analysis.
NOTE: The number of samples taken over time depends on experimenter goals. Sampling one to three times per week is sufficient for many applications assuming incubations are close to 20 °C. Incubating at higher temperatures increases SRP release rates and will require more frequent sampling. The intent here is to show the utility of the microcosm method rather than focusing on data analysis from experiments. Both kinetically-based and empirical models to fit P desorption/sorption data are presented elsewhere9,10. Since the microcosm method relies on a repeated measures design and accommodates replication and differing treatments, generalized linear mixed modeling approaches are also appropriate11.
Representative Results
Results from a recent study focused on P release potential of riparian areas are highlighted to demonstrate the method’s ability to characterize site-level P release dynamics6. While some soils showed minimal changes in SRP over time, others had large increases in PW- and FW-SRP concentrations (Figure 1). Two sites with contrasting trends are shown in Figure 1. Soil 7 is a riparian site with low soil pH and characterized by nearly continuous SRP sorption from PW (Figure 1A). Soil 14 was sampled from an adjoining maize production field with elevated labile soil Pi (soil 14) and demonstrated nearly a 7-fold increase in PW-SRP over the first month of inundation (Figure 1B).
In contrast to PW-SRP concentrations, FW-SRP tended to decrease over time (Figure 1). Porewater ferrous Fe (Fe2+) was also measured as a proxy for redox status. In all but one soil, PW-Fe2+ increased substantially after approximately 3 weeks, indicating reducing conditions. Since soil drying alters organic carbon and Pi solubility, two sites were also dried prior to flooding. Flooding dry soil substantially increased Pi desorption to PW and subsequent mobilization to overlying water compared to flooding the same soil in a field-moist state (Figure 1C,D).
Select soil P tests were also performed to determine their reliability to predict average SRP concentrations. Distilled water and modified Morgan extractable P (measured by molybdate colorimetry) were among the best predictors of average PW- and FW-SRP concentrations (Figure 2A,C). Modified Morgan extractable P measured by ICP was not as good of a predictor compared to modified Morgan extractable P measured by molybdate colorimetry or distilled water (Figure 2C). The ratio of PW-SRP:FW-SRP increased linearly as a function of soil pH (Figure 2D).
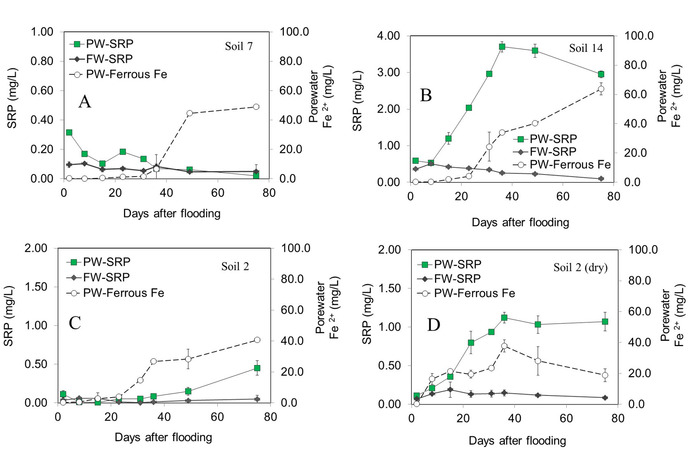
Figure 1: Soluble reactive phosphorus (SRP) concentrations in soil porewater (PW) and overlying floodwater (FW) over a 75-day incubation for a riparian soil with low pH and labile Pi (A), a soil from a maize production field with high labile Pi (B) and a riparian soil flooded field-moist (C) vs. flooding after drying (D). Error bars represent the standard deviation of duplicate microcosm measurements. Data were modified from Young and Ross6 with permission. Please click here to view a larger version of this figure.
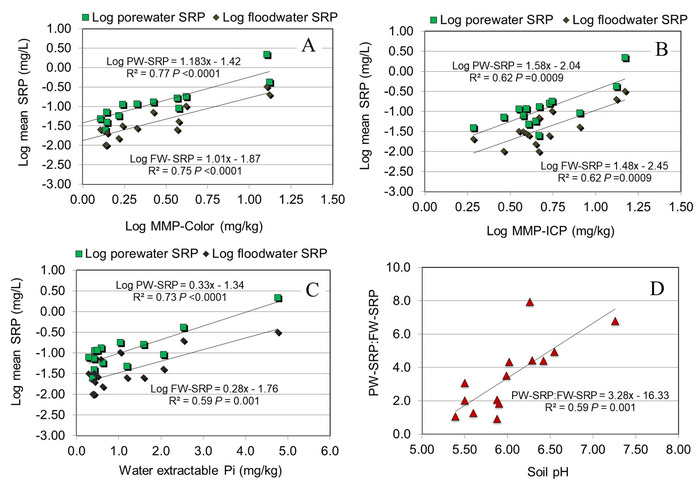
Figure 2: Mean porewater (PW) and floodwater (FW) soluble reactive P (SRP) concentrations over the experiment as a function of modified Morgan extractable P measured by molybdate colorimetry (A), modified Morgan extractable P (Pi + organic P) measured by inductively coupled plasma emission optical emission spectroscopy (B), and distilled water (C). (D) Relationship between mean PW-SRP:FW-SRP for the study as a function of soil pH. Data were modified from Young and Ross6 with permission. Please click here to view a larger version of this figure.
Discussion
A main technical advantage of the microcosm approach is its ability to simulate in-situ conditions whereby saturated soil or sediment is immediately overlain by FW that may substantially differ in redox and P status. Landscapes with variable source area hydrology such as drainage ditches, flooded cropland, wetlands, and riparian/near-stream zones are all examples of where reduced PW is periodically overlain by more oxidized water with lower Pi concentrations. These redox gradients can strongly affect Pi sorption/desorption and therefore mobility to surface and groundwater1,2,3,4,5,6,12,13,14. Unlike more routine extractions or Pi sorption isotherms, the microcosm method naturally simulates reducing conditions as microbial respiration consumes dissolved PW oxygen. At the same time, FW remains open to ambient air allowing diffusion of oxygen into FW, similar to natural conditions in the field. To the extent that overlying water remains oxidizing, dissolved metals such as Fe2+ and Mn2+ may diffuse upward from PW and resorb SRP upon oxidation at aerobic interfaces, helping to prevent SRP mobilization to FW2,3,6,14,15. This particular Pi sorption mechanism is important in wetlands, lake sediments, and flooded agricultural soils. The ability of the microcosm method to capture these essential natural system dynamics offers an advantage over more traditional methods.
Our results also highlight the importance of routine soil/sediment chemical measurements as potential predictors of PW- and FW-SRP release. For example, both distilled water and modified Morgan extractable Pi provided reliable estimates of average SRP concentrations over the incubation, indicating that previously sorbed labile Pi is an important constraint on the magnitude of Pi release. The quantity of extractable Pi is an important variable for managing agricultural P in addition to being an input for empirical and process-based water quality models16. The ratio of PW-SRP:FW-SRP was linearly related to soil pH, indicating a higher fraction of PW-SRP mobilized to FW at higher pH. This effect is probably related to the fact that solubility of strongly P-sorbing metal cations such as Al3+ and Fe3+ increases at lower pH and therefore more readily forms bonds with SRP in solution (note it is also well established that orthophosphate availability in soils tends to be maximized at a pH close to neutrality due to the same mechanism). Results also demonstrated that flooding dry soil substantially increased Pi release. Enhanced Pi solubility after drying has also been reported by others17,18,19 and is worthy of additional research to refine current P cycling models. It is clear that interactions among soil properties (labile Pi status, soil pH, mineralogy) and redox fluctuations can strongly influence Pi release and mobility. The microcosm method facilitates the isolation and interaction of these and other factors and allows experimentation under controlled conditions while simulating in situ environments.
The microcosm approach readily accommodates modifications that may be of interest to P researchers. In addition to variation in basic chemical and physical properties affecting Pi release, addition of soil amendments (i.e., livestock manure/fertilizer, biosolids, composts, and P-sorbing materials) and other management aspects will remain important considerations. Since changes in temperature strongly affect Pi release/sorption kinetics9,20 and redox reactions9,15,20, experiments designed to isolate temperature effects on Pi release may also be beneficial. In addition, Pi sorption capacity experiments can be readily done by adding known amounts of Pi to FW and measuring disappearance over time3; the quantity of P sorbed can then be related to soil properties to predict Pi retention in wetland ecosystems. Given the method’s simplicity, low cost and flexibility, other design modifications are also possible depending on objectives.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding was made available by the Vermont Water Resources and Lake Studies Center through an agreement with the U.S. Geological Survey. Conclusions and opinions are those of the authors and not the Vermont Water Resources and Lake Studies Center or the USGS.
Materials
| 1.25 cm plastic hose barbs | numerous | NA | |
| Chemical reagents for phosphorus determination | numerous | NA | P analysis capability is assumed; refer to cited references for details on method |
| Chordless or electric drill with 1.25 cm bit | numerous | NA | |
| Graduated plastic beakers (1L) | numerous | NA | |
| Laboratory with fume hoods, temperature control, and acid waste disposal system | NA | NA | |
| Nylon mesh filter screen (100um) | numerous | NA | |
| Silicone | numerous | NA | |
| UV Spectrophotometer | numerous | NA |
References
- Patrick, W. H., Khalid, R. A. Phosphate release and sorption by soils and sediments: Effect of aerobic and anaerobic conditions. Science. 186 (4158), 53-55 (1974).
- Moore, P. A., Reddy, K. R. Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida. Journal of Environmental Quality. 23, 955-964 (1994).
- Young, E. O., Ross, D. S. Phosphate release from seasonally flooded soils: a laboratory microcosm study. Journal of Environmental Quality. 30 (1), 91-101 (2001).
- Henderson, R., et al. Anoxia-induced release of colloid- and nanoparticle-bound phosphorus in grassland soils. Environmental Science & Technology. 46 (21), 11727-11734 (2012).
- Vidon, F., et al. Hot spots and hot moments in riparian zones: potential for improved water quality management. Journal of the American Water Resources Association. 46 (2), 278-298 (2010).
- Young, E. O., Ross, D. S. Phosphorus mobilization in flooded riparian soils from the Lake Champlain Basin, VT, USA. Frontiers in Environmental Science. 6 (120), 1-12 (2018).
- McIntosh, J. L. Bray and Morgan soil extractants modified for testing acid soils from different parent materials. Agronomy Journal. 61 (2), 259-265 (1969).
- Young, E. O., Ross, D. S., Cade-Menun, B. J., Liu, C. Phosphorus speciation in riparian soils: a phosphorus-31 nuclear magnetic resonance and enzyme hydrolysis study. Soil Science Society of America Journal. 77 (5), 1636-1647 (2013).
- McGechan, M. B., Lewis, D. R. Sorption of phosphorus by soil: Part 1. Principles, equations, and models. Biosystems Engineering. 82 (1), 1-24 (2002).
- Cabrera, M. L., Radcliffe, D. E., Cabrera, M. L. Modeling phosphorus in runoff: Basic approaches. Modeling Phosphorus in the Environment. , 65-81 (2007).
- Gbur, E. E., et al. . Analysis of Generalized Linear Mixed Models in the Agricultural and Natural Resources Sciences. , (2012).
- Moore, P. A., Reddy, K. R., Fisher, M. M. Phosphorus flux between sediment and overlying water in Lake Okeechobee, Florida: Spatial and temporal variations. Journal of Environmental Quality. 27 (6), 1428-1439 (1998).
- Young, E. O., Briggs, R. D. Phosphorus concentrations in soil and subsurface water: A field study among cropland and riparian Buffers. Journal of Environmental Quality. 37 (1), 69-78 (2008).
- Hoffmann, C. C., Kjaergaard, C., Uusi-Kämppä, J., Hansen, H. C. B., Kronvang, B. Phosphorus retention in riparian buffers: review of their efficiency. Journal of Environmental Quality. 38 (5), 1942-1955 (2009).
- Bartlett, R. J., Ross, D. S., Tabatabai, M. A., Sparks, D. L. Chemistry of Redox Processes in Soils. Chemical Processes in Soils, SSSA Book Ser. 8. , 461-487 (2005).
- Radcliffe, D. E., Freer, J., Schoumans, O. Diffuse phosphorus models in the United States and Europe: Their usages, scales, and uncertainties. Journal of Environmental Quality. 38 (5), 1956-1967 (2009).
- Bartlett, R. J., James, B. R. Studying dried, stored soil samples—some pitfalls. Soil Science Society of America Journal. 44 (4), 721-724 (1980).
- Turner, B. L., McKelvie, I. D., Haygarth, P. M. Characterization of water extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biology & Biochemistry. 34 (1), 27-35 (2002).
- Turner, B. L., Haygarth, P. M. Phosphorus solubilization in rewetted soils. Nature. 411, 258 (2001).
- Sparks, D. L., Sparks, D. L. Kinetics of reactions in pure and mixed systems. Soil Physical Chemistry. , (1986).

