Isolation and Culture of Primary Neurons and Glia from Adult Rat Urinary Bladder
Summary
This protocol attempts to establish a repeatable protocol for primary neurons and glia isolation from rat bladder for further cellular experiments.
Abstract
The lower urinary tract has two main functions, namely, periodic urine storage and micturition; these functions are mediated through central and peripheral neuroregulation. Although extensive research on the lower urinary tract nervous system has been conducted, most studies have focused on primary culture. This protocol introduces a method for the isolation and culture of bladder neurons and glia from Sprague–Dawley rats. In this method, the neurons and glia were incubated in a 37 °C, 5% CO2 incubator for 5–7 days. As a result, they grew into mature shapes suitable for related subsequent immunofluorescence experiments. Cells were morphologically observed using an optical microscope. Neurons, synaptic vesicles, and glia were identified by β-III-tubulin and MAP-2, Synapsin-1, and GFAP staining, respectively. Meanwhile, immunocytochemistry was performed on several neurotransmitter-related proteins, such as choline acetyltransferase, DYNLL2, and SLC17A9.
Introduction
The lower urinary tract has two main functions: periodic urine storage and micturition1. The lower urinary tract nervous system (LUTNS) controls these functions and is delicate and susceptible to many neuropathies, which can be innate (porphyria), acquired (Lyme disease), secondary to disease states (diabetic cystopathy), drug induced (hemorrhagic cystitis), surgery caused (abdominoperineal resection), or injury caused (traumatic spinal cord injury)2,3,4,5,6,7. In physiological/pathological studies, in vivo and in vitro experiments are equally important. While in vivo research on LUTNS has been conducted at organ, cellular, and molecular levels for some time, in vitro research on primary neurons from the urinary bladder is almost nonexistent8,9. Although the present study is limited, we hope to pioneer research in this area so that other researchers could improve it. In this manner, this co-culture may lead to a cellular understanding of physiological dysfunction in phenotypes, such as bladder neuron dysfunction.
In contrast to enteric muscles with a clear directionality of the muscle cells into discrete layers, the muscles of the bladder are unorganized10. Therefore, instead of peeling off the outer layer of the bladder, this method proposes digesting the entire bladder to reduce the difficulty of operation and shorten the preprocessing time for a high cell survival rate.
Following this method, we can obtain a mixed culture of neurons and other cells. The other cells are indispensable because their presence mimics an in vivo environment11. In addition, such cells provide the substances that are unavailable in the medium.
This method involves two steps for digestion. First, collagenase type II is used to hydrolyze collagen, followed by trypsin, to dissociate the tissue into cells10. In this manner, bladder tissues are dispersed into single cells and then grow relatively independent. When the culture of neurons matures, the neurons can be used for imaging or functional assays.
Protocol
All experimental protocols and animal procedures complied with the ethical principle guidelines of the National Research Council.
1. Preparation of materials
- Sterilize all instruments and ddH2O using an autoclave before performing the experiment. Instruments include but are not limited to surgical scissors, ophthalmic scissors, forceps, spoons nucleus divider, glass dishes (60–100 mm in diameter), and glass breakers.
- Prepare the Krebs solution as follows (Table 1): Dissolve all the chemicals together with ddH2O prior to use. Dissolve CaCI2 separately and add slowly into the mixed solution while stirring to avoid sediment.
- Prepare the Rinse media, which consists of F12 media with 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic (100x). Add 5 mL of FBS and 0.5 mL of antibiotic/antimycotic to 44.5 mL of F12 media.
- Prepare neuron media A, which comprises neurobasal A media with 2% B-27, 1% FBS, 1% L-glutamine, 1% antibiotic/antimycotic (100x), and 0.1% glia-derived neurotrophic factor (GDNF). Add 200 µL of B-27, 100 µL of FBS, 100 µL of L-glutamine, 100 µL of antibiotic/antimycotic (100x), and 10 µL of GDNF (10 µg/mL) to 9.5 mL of neurobasal A media.
NOTE: Prepare neuron media A within one week of usage to ensure freshness of B-27, L-glutamine and GDNF. - Prepare neuron media B using the same procedure as the preparation of neuron media A but without adding FBS.
- Prepare digestion solution 1 by dissolving 20 mg of collagenase type II, 6 mg of bovine serum albumin, and 200 µL of antibiotic/antimycotic (100x) in 10 mL of oxygen-stable Krebs solution.
- Prepare digestion solution 2 by diluting 1 mL of 0.25% trypsin with 4 mL of Hank’s balanced salt solution.
- Prepare a plate with coated coverslips.
- Use forceps in a laminar flow bench when placing glass coverslips into a 48-well culture plate.
- Dilute poly-D-lysine with ddH2O to a concentration of 0.1 mg/mL as poly-D-lysine stock. Store the stock at –20 °C, and thaw before use.
- Add 40 µL of poly-D-lysine stock on top of each coverslip, and incubate the solution at room temperature for 10 min.
NOTE: For different plates with different basal areas, adjust the concentration to 4 µg/cm2. For example, if the basal area of one well from a 24-well plate is 2 cm2, then we should transfer 80 µL of poly-D-lysine stock in a well. Each cm2 would contain 4 µg of poly-D-lysine. - Remove poly-D-lysine in the 48-well plate, and rinse coverslips with ddH2O thrice.
- Dry the plate in a laminar flow hood for at least 30 min to ensure that the plate is anhydrous.
- Store the plate at 4 °C before laminin coating for 1 day at most. Storing the plate at −20 °C is preferable for long-term storage.
- Thaw laminin at 4 °C. Dilute laminin with ddH2O to a concentration of 50 µg/mL as laminin stock. Store the stock at –20 °C, and thaw at 4 °C before use.
- Use a pipette to transfer 100 µL of diluted laminin to the top of each coverslip and incubate laminin at 4 °C for 1 h.
NOTE: For different plates with different basal areas, adjust the concentration to 5 µg/cm2. For example, if the basal area of one well from a 24-well plate is 2 cm2, then transfer 200 µL of diluted laminin in a well. Each cm2 would contain 5 µg of laminin. - Remove the laminin solution, and rinse coverslips with ddH2O once. Perform this operation on the edge of the coverslip to avoid scraping.
NOTE: Coated coverslips could be stored at 4 °C for 2 weeks at most.
2. Bladder harvest
- Obtain five-week-old Sprague–Dawley rats.
- Infuse carbogen (95% oxygen, 5% CO2) into the Krebs solution for at least 30 min in an ice bath to reach a stable oxygen status and pH level.
- After euthanasia via cervical dislocation, soak the rats in 75% ethanol for 30 s for sterilization.
- Place rats on a sterilized surgical towel and expose their abdomen. Open the abdominal cavity, and reveal the bladder with a set of scissors and forceps.
- Lift the bladder gently and cut the bladder from the bladder neck with another set of scissors and forceps to avoid cross-contamination. Place the bladder rapidly in cold oxygen-stable Krebs solution to improve cell survival.
NOTE: Once the bladder is removed, perform the following operations rapidly to improve the prospect of neuron survival. - Add Krebs solution into three glass dishes and glass breakers.
- Prepare glass dishes and glass breakers with Krebs solution in an ice bath for precooling.
- Mark these containers with numbers 1–3 correspondingly to prevent confusion.
- Pair each glass dish with forceps and a spoons nucleus divider.
- In glass dish 1, cut open the bladder with ophthalmic scissors, and unfold it with forceps and a spoons nucleus divider.
- Rinse the bladder in glass breaker 1, and place it in glass dish 2.
- Eliminate adherent fat on the tissue surface using the forceps and ophthalmic scissors in glass dish 2.
- Rinse the bladder in glass breaker 2, and place it in glass dish 3.
- Gently scrape the bladder using the forceps and the spoons nucleus divider onto glass dish 3 to remove exogenous attachments.
- Rinse the bladder in glass breaker 3, transfer the bladder to a 15 mL centrifuge tube with 14 mL of cold Krebs solution, and spin the sample for 1 min at 356 x g and 4 °C.
- Repeat the previous step twice in two other tubes with Krebs solution to reduce contamination.
3. Two-step bladder digestion
- Transfer the bladder from the centrifuge tube to a 2 mL vial containing 1 mL of digestion solution 1. Use ophthalmic scissors to cut the bladder into small pieces (smaller than 1 mm) in solution.
- Mix the bladder solution with 9 mL of digestion solution 1 in a sterile cell culture dish (100 mm in diameter). Perform step 1 digestion in a shaking incubator for 1 h under 5% CO2, 37 °C, and 200 rpm.
- After step 1 digestion, centrifuge the solution at 356 x g at 4 °C for 8 min.
- Place digestion solution 2 in a 37 °C water bath for preheating.
- After centrifugation, remove the supernatant that contains digestion solution 1, and harvest the cell sediment. Some remaining liquid is allowed. Complete removal of the solution may promote cell loss.
- Mix cell sediment with warm digestion solution 2 in a 15 mL centrifuge tube, and shake the mixture while digesting in a 37 °C water bath for 5 min. Do not exceed 7 min of step 2 digestion or neurons will perish.
- After digestion, immediately deactivate trypsin in the mixture with 10 mL of cold rinse media.
NOTE: Perform the following steps at 0 °C–4 °C. An ice bath could provide such condition. - Harvest the cell sediment after centrifugation at 356 x g at 4 °C for 8 min. Remove as much media as possible because the remaining trypsin is harmful to cell growth.
- Resuspend sediment with 3 mL of neuron media gently. Ensure that air bubbles are not generated in the solution containing cells for a high survival rate.
- Filter the mixture media through a 70 µm cell strainer into a 50 mL centrifuge tube.
- Keep the filtrate on a shaker at 30 rpm in an ice bath for 30 min. This step is not necessary but recommended.
- Collect cells by centrifugation at 356 x g at 4 °C for 8 min, and gently resuspend the cell pellets in 1 mL of neuron media A.
- Add 500 µL of cell mixture into each well of the prepared 48-well plate.
- Culture cells in an incubator at 37 °C and 5% CO2.
- Replace all the media with neuron media B in 1 h to provide a serum-free culture.
- Change half of the neuron media B every 3 days.
NOTE: Neurons are ready for immunocytochemical experiments after 5–7 days of culture.
Representative Results
In the process of primary cell culture, the cells acquired were round with bright and clear boundaries before the attached state. As the neurons grew, dendrites and axons started to be distinct. After 5–7 days of culture, the neurons reached a mature form with long projections, which were ideal for imaging or function studies. Although most of the impurities and cell debris could be removed due to changing media, certain residuals attached to poly-D-lysine and laminin coating were visible (Figure 1).
After proper culture, neurons could be identified via typical β-III-tubulin and MAP-2 immunostaining10,12. In addition, glia was specifically identified via GFAP immunostaining10. Mature neurons developed synaptic spines, which were close to the presynaptic specializations identified by the immunostaining of the synaptic protein maker, synapsin-1 (Figure 2)12. These results indicated that mature cells with well-developed synapses were obtained through this method. This result suggests its important role in future function studies.
Meanwhile, several neuron subtypes were recognized through immunocytochemistry experiments (Figure 3). Peptidergic neurons, which contain various neuropeptides, were immunostained with substance P13. Purinergic neurons with expressed vesicular nucleotide transporters were identified via SLC17A9 staining14. Nitrergic neurons were visualized with DYNLL-2, which connects nNOS with motor proteins in neurons15. Cholinergic neurons were immunoreactive with choline acetyltransferase16.
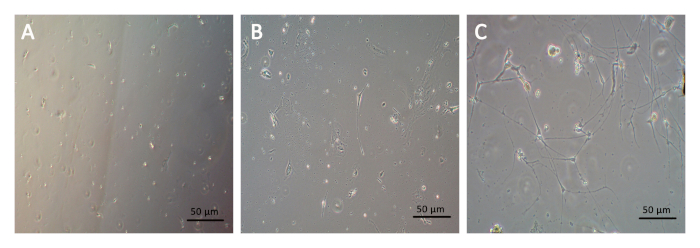
Figure 1. Phase-contrast images of primary cells isolated from the rat bladder culture taken at 1, 3, and 7 days after plating (A, B, C, respectively). Scale bar: 50 μm. Please click here to view a larger version of this figure.
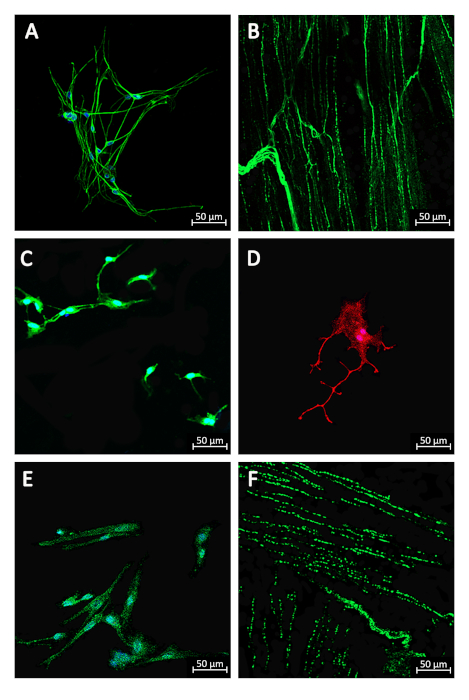
Figure 2. Immunofluorescence images of primary cells isolated from the rat bladder. Confocal microscopy analysis showed neuron cytoskeleton protein staining (β-III-tubulin, RRID: AB_2827688, 1:200) in primary culture neurons (A) and in whole mount bladder preparation (B). In primary culture neurons, neuronal phosphoprotein immunostaining (MAP 2, RRID:AB_2827689, 1:200) was also visualized (C). Glia were identified via glial fibrillary acidic protein staining (D; RRID: AB_627673, 1:50). Synapsins were visualized via synapsin protein staining (Synapsin-1, RRID: AB_2798146, 1:200) in cellular (E) and tissue (F) levels. The secondary antibodies used were as follows: Alexa Fluor 488 (green, goat anti-rabbit lgG, 1:200), Alexa Fluor 555 (red, goat anti-mouse lgG, 1:200). The nucleus was visualized using Hoechst 33342 (A, C, D, E; blue, 1 μg/mL). Scale bar: 50 μm. Please click here to view a larger version of this figure.
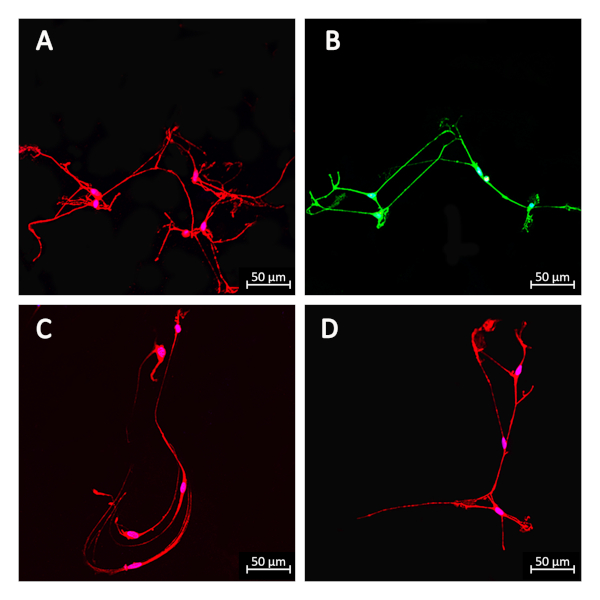
Figure 3. Immunofluorescence images of several neuron subtypes of primary neurons. Peptidergic neurons were immunostained with substance P (A; RRID: AB_785913, 1:50). Purinergic neurons were identified via SLC17A9 staining (B; RRID: AB_10597575, 1:200). Nitrergic neurons were visualized via DYNLL-2 staining (C; RRID: AB_654147, 1:50). Cholinergic neurons were immunoreactive with choline acetyltransferase (D; RRID: AB_2244867, 1:100). The secondary antibodies used were as follows: Alexa Fluor 488 (green, goat anti-rabbit lgG, 1:200), Alexa Fluor 555 (red, goat anti-mouse lgG, 1:200). The nucleus was visualized using Hoechst 33342 (A, B, C, D, blue, 1 μg/mL). Scale bar: 50 μm. Please click here to view a larger version of this figure.
| Ingredients | Molarity (mM) |
| NaCI | 120 |
| KCI | 5.9 |
| NaHCO3 | 25 |
| Na2HPO4·12H2O | 1.2 |
| MgCI2·6H2O | 1.2 |
| CaCI2 | 2.5 |
| Glucose | 11.5 |
Table 1. Krebs solution composition
Discussion
Plate Preparation
The use of glass coverslips in 6-, 12-, or 48-well culture plates for immunofluorescent or calcium imaging experiments is an economical and sample-sparing operation. Cells grow well in plates without coverslips during the preparation of primary cell cultures. Therefore, coverslips are dispensable in experiments, such as Western blot or polymerase chain reaction. Furthermore, coating is a necessary step before plating cells, with or without coverslips. Laminin and poly-D-lysine are common choices in coating neurons, particularly laminin, which is essential for neuron growth17.
Media Preparation
After first medium replacement, cell isolation requires media without serum because serum stimulates cell division and leads to a limited neuron-growing space18. Thus, neuron growth factors are crucial. The quality of B27 and GDNF can vary largely from different batches and cause major effects on neuron growth19. Therefore, checking the lot number of the media is recommended when neuron yield is poor. Meanwhile, fresh media stock is crucial; the required amount should be calculated and prepared in advance each time before media are replaced.
Animals
Sprague–Dawley rats are used in this method. C57BL/6 mice are also acceptable in this experiment. Therefore, other strains of rats or mice may also be adopted for this method despite a few variations in morphology and neuronal circuitry. In terms of different animal models, researchers should develop an optimized and targeted protocol. Furthermore, young animals should always be considered prior to the application of this method.
Tissue Treatment
During the experiments, except for the digestive process, keeping tissues at a low temperature is essential to increase cell viability, which can reduce cell metabolism and avoid energy deficit. Oxygen levels, nutrition, and pH can also affect cell yield11. Moreover, for other tissues, we suggest that researchers perform this method with adjusted digestion condition.
Cell Culture
One remarkable characteristic of neurons when inoculated is their quick adherence to coated plates20. In this case, changing media after 1 h of culture is recommended to gain a high proportion of neurons. Moreover, when most of the cells start to grow pseudopodium, the frequency of changing media can be reduced appropriately depending on the color of the media and the cellular state. Primary cell culture in a good state displays black soma with a bright border.
Most nerve cells isolated from the bladder are bladder intramural ganglia, which consist of afferent and autonomic efferent innervations of the bladder13. Moreover, no major pelvic ganglia are present in the harvested tissue. It distributes below the bladder neck21.
Limitation
This is a preliminary research to isolate and culture neurons and glia. Many attempts had been done, like cytarabine treatment or density gradient centrifugation. However, the proportion of desired cells was still not ideal, and even more cell loss appeared. Moreover, traditional digestive conditions in this protocol, such as 37 °C, are likely to kill off some sensitive neuron types, and cause potential gene expression artifacts22.
In conclusion, this protocol offers a method to culture neurons and glia from rat bladder. The isolation is easy to repeat, time efficient, and involves minimal microbial contamination. Although improvement evoking the purity of neurons is necessary, we hope this method contributes to LUTNS research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant no. 81673676) and Dongguan Science and Technology Bureau (Grant no. 2019622101002). The authors thank Dr. Maryrose Sullivan (Assistant Professor in Surgery, Harvard Medical School) for technical consulting.
Materials
| 0.25% trypsin | Gibico | 15050065 | Enzyme digestion |
| 48-well culture plate | Corning | 3548 | Coating dish |
| antibiotic/antimycotic | Gibico | 15240062 | Culture media/Rinse media |
| Anti-Glial Fibrillary Acidic Protein Antibody | Santa Cruz | sc-33673 | ICC |
| B-27 | Gibico | 17504044 | Culture media |
| BSA Fraction V | Gibico | 332 | Enzyme digestion |
| Choline Acetyltransferase Antibody | Abcam | ab18736 | ICC |
| CO2 Incubator | Heraeus | B16UU | Cells culture |
| Collagenase type II | Sigma | 2593923 | Enzyme digestion |
| DMEM/F-12 | Gibico | 11330032 | Rinse media |
| DYNLL2 Antibody | Santa Cruz | sc-13969 | ICC |
| Fetal Bovine Serum | Gibico | 10100147 | Culture media/Rinse media |
| Forceps | Shanghai Jin Zhong Medical Devices | 1383 | 10 cm; Sterile operation |
| Glass breakers | Huan Qiu Medical Devices | 1101 | 50 ml; Sterile operation |
| Glass coverslips | WHB Scientific | WHB-48-CS | Coating dish |
| Glass dishes | Huan Qiu Medical Devices | 1177 | 100 mm; Sterile operation |
| Goat Anti-Rat IgG(H+L), Mouse ads-Alexa Fluor 488 | Southernbiotech | 3050-30 | ICC |
| Goat Anti-Rat IgG(H+L), Mouse ads-Alexa Fluor 555 | Southernbiotech | 3050-30 | ICC |
| Hoechst 33342 | BD | 561908 | ICC |
| Laminar flow bench | Su Jie Medical Devices | CB 1400V | Sterile operation |
| Laminin | Sigma | L2020 | Coating dish |
| L-glutamine | Gibico | 25030081 | Culture media |
| MAP-2 Antibody | Affinity | AF5156 | ICC |
| Murine GDNF | Peprotech | AF45044 | Culture media |
| Neurobasal-A Medium | Gibico | 10888022 | Culture media |
| Ophthalmic scissors | Shanghai Jin Zhong Medical Devices | J21010 | 12.5 cm; Sterile operation |
| Pipettes | Eppendorf | 3120000240 | 100-1000 ul; Reagent and sample pipetting |
| Pipettes | Eppendorf | 3120000267 | 10-100 ul; Reagent and sample pipetting |
| Poly-D-lysine | Sigma | P7280 | Coating dish |
| Refrigerated centrifuge | Ping Fan Instrument | TGL-16A | Enzyme digestion |
| Shaking incubator | Haimen Kylin-Bell Lab Instruments | T8-1 | Enzyme digestion |
| SLC17A9 Antibody | MBL International | BMP079 | ICC |
| Spoons nucleus divider | Shanghai Jin Zhong Medical Devices | YZR030 | 12 cm; Sterile operation |
| Substance P Antibody | Santa Cruz | sc-58591 | ICC |
| Surgical scissors | Shanghai Jin Zhong Medical Devices | J21130 | 16 cm; Sterile operation |
| Surgical towel | Fu Kang Medical Devices | 5002 | 40 x 50 cm; Sterile operation |
| Synapsin-1 Antibody | CST | 5297T | ICC |
| Tubulin beta Antibody(β-III-tubulin) | Affinity | AF7011 | ICC |
References
- Fowler, C. J., Griffiths, D., de Groat, W. C. The neural control of micturition. Nature Reviews Neuroscience. 9 (6), 453-466 (2008).
- Golbidi, S., Laher, I. Bladder dysfunction in diabetes mellitus. Frontiers in Pharmacology. 1, 136 (2010).
- Iwasawa, E., et al. Long-term Effects of Intravenous Cyclophosphamide in Combination with Mesna Provided Intravenously and via Bladder Perfusion in a Patient with Severe Multifocal Motor Neuropathy. Internal Medicine. 56 (14), 1893-1896 (2017).
- Lange, M. M., van de Velde, C. J. Urinary and sexual dysfunction after rectal cancer treatment. Nature Reviews. Urology. 8 (1), 51-57 (2011).
- Lin, C. S., et al. Nerve function and dysfunction in acute intermittent porphyria. Brain. 131, 2510-2519 (2008).
- Rantell, A., et al. What is the utility of urodynamics, including ambulatory, and 24 h monitoring, in predicting upper urinary tract damage in neuro-urological patients and other lower urinary tract dysfunction? ICI-RS 2017. Neurourology and Urodynamics. 37, 25-31 (2018).
- Halperin, J. J. Diagnosis and management of Lyme neuroborreliosis. Expert Review of Anti-Infective Therapy. 16 (1), 5-11 (2018).
- Walter, M., et al. Reliability of supraspinal correlates to lower urinary tract stimulation in healthy participants – A fMRI study. Neuroimage. 191, 481-492 (2019).
- Leitner, L., et al. A novel infusion-drainage device to assess lower urinary tract function in neuro-imaging. BJU International. 119 (2), 305-316 (2017).
- Smith, T. H., Ngwainmbi, J., Grider, J. R., Dewey, W. L., Akbarali, H. I. An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. Journal of Visualized Experiments. (78), e50688 (2013).
- Gordon, J., Amini, S., White, M. K. General overview of neuronal cell culture. Methods in Molecular Biology. 1078, 1-8 (2013).
- Roppongi, R. T., Champagne-Jorgensen, K. P., Siddiqui, T. J. Low-Density Primary Hippocampal Neuron Culture. Journal of Visualized Experiments. (122), e55000 (2017).
- Arms, L., Vizzard, M. A. Neuropeptides in lower urinary tract function. Handbook of Experimental Pharmacology. (202), 395-423 (2011).
- Moriyama, Y., Hiasa, M., Sakamoto, S., Omote, H., Nomura, M. Vesicular nucleotide transporter (VNUT): appearance of an actress on the stage of purinergic signaling. Purinergic Signal. 13 (3), 387-404 (2017).
- Chaudhury, A., He, X. D., Goyal, R. K. Myosin Va plays a key role in nitrergic neurotransmission by transporting nNOSα to enteric varicosity membrane. American journal of physiology. Gastrointestinal and Liver Physiology. 301 (3), 498-507 (2011).
- Le Berre-Scoul, C., et al. A novel enteric neuron-glia coculture system reveals the role of glia in neuronal development. The Journal of Physiology. 595 (2), 583-598 (2017).
- Hayashi, H., Yamada, M., Kumai, J., Takagi, N., Nomizu, M. Biological activities of laminin-111-derived peptide-chitosan matrices in a primary culture of rat cortical neurons. Archives of Biochemistry and Biophysics. 648, 53-59 (2018).
- Bottenstein, J. E., Sato, G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proceedings of the National Academy of Sciences of the United States of America. 76 (1), 514-517 (1979).
- Brewer, G. J., Torricelli, J. R., Evege, E. K., Price, P. J. Optimized Survival of Hippocampal Neurons in B27-Supplemented Neurobasalm, a New Serum-free Medium Combination. Journal of Neuroscience Research. 35 (5), 567-576 (1993).
- Kaech, S., Banker, G. Culturing hippocampal neurons. Nature Protocols. 1 (5), 2406-2415 (2006).
- Georgas, K. M., et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 142 (10), 1893-1908 (2015).
- Adam, M., Potter, A. S., Potter, S. S. Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development. Development. 144 (19), 3625-3632 (2017).

