A Standardized Method for Measurement of Elbow Kinesthesia
Summary
Here, we present a standardized method for measurement of elbow passive kinesthesia using the threshold to detection of passive movement (TDPM) that is appropriate for a research setting.
Abstract
Proprioception is an important component of controlled movement. The threshold to detection of passive movement (TDPM) is a commonly used method for quantifying the proprioceptive submodality of kinesthesia in research settings. The TDPM paradigm has been found to be valid and reliable; however, the equipment and methods used for TDPM vary between studies. In particular, the research laboratory apparatuses for producing passive movement of an extremity are often custom designed by individual laboratories or inaccessible due to high cost. There is a need for a standardized, valid, and reliable method for measuring TDPM using readily available equipment. The purpose of this protocol is to provide a standardized method for measurement of TDPM at the elbow that is economical, easy to administer, and that produces quantitative results for measurement purposes in research-based settings. This method was tested on 20 healthy adults without neurological impairment, and eight adults with chronic stroke. The results obtained suggest this method is a reliable way to quantify elbow TDPM in healthy adults, and provides initial support for validity. Researchers seeking a balance between equipment affordability and measurement precision are most likely to find this protocol of benefit.
Introduction
Proprioceptive information is an important contributor to the control of human movement. Proprioceptive deficits accompany a wide range of neurologic conditions such as stroke1,2,3,4,5,6, Parkinson’s disease7, and sensory neuropathies8. Orthopedic injuries such as ligament and muscle tears have also been shown to reduce proprioceptive function9. The construct of proprioception is often tested in clinical outcome measures via detection of provider-applied small alterations in finger or toe position10,11,12,13,14. Such measures produce relatively coarse measurements: “absent”, “impaired”, “normal”12. While sufficient for detection of gross proprioceptive impairments, laboratory mechanical testing methods are required to precisely measure subtle proprioceptive impairments14,15,16.
Researchers and clinicians often divide proprioception into submodalities for measurement. The most commonly investigated submodalities of proprioception are joint position sense (JPS) and kinesthesia, typically defined as the sense of movement3,16,17. Joint position sense is often tested via active matching tasks, where individuals replicate a reference joint angle18,19. Kinesthesia is commonly measured using the threshold to detection of passive movement (TDPM), whereby a participant’s limb is passively moved slowly, with the participant indicating the point at which movement is first detected16,17,19. Measurement of TDPM typically requires use of specialized equipment to provide the slow passive movement and denote the point of detection17.
Valid and reliable results have been found at different joints using TDPM methods9,16,19,20,21,22. However, there is considerable variation in TDPM equipment and methods, creating a challenge for comparison of findings across studies16,17. Laboratories often develop their own limb movement and measurement devices, or use expensive commercial devices and software16. Passive movement speeds also vary; movement speed is known to affect detection thresholds7,16,23. A standardized, easily reproducible method capable of quantifying TDPM across a range of impairment levels is needed. Because the anatomy and physiology of each joint differs, protocols should be joint specific19. The protocol outlined here is specific to the elbow joint. However, the methods of this protocol may be useful to establish protocols for other joints.
To increase generalizability across sensorimotor research laboratories, the preferred apparatus for providing the passive movement for elbow TDPM testing would be commercially available at an affordable cost. To this end, an elbow continuous passive movement (CPM) machine (available speed range 0.23°/s – 2.83°/s) was chosen to produce the motorized, consistent motion. CPM machines are commonly found in rehabilitation hospitals and medical supply stores and can be rented or borrowed to reduce research costs. Additional equipment requirements include items commonly found in sensorimotor laboratories (i.e., electrogoniometer and electromyography (EMG) sensors), and hardware stores (e.g., PVC pipe, string and tape).
Two different groups were tested to explore the measurement properties of this TDPM protocol: healthy adults and adults with chronic stroke. For the adults with chronic stroke, the ipsilesional (i.e., less affected) arm was tested. Kinesthetic sense in the ipsilesional elbow in adults with chronic stroke may appear normal with clinical testing, but impaired when evaluated using quantitative laboratory methods5,15. This example illustrates the importance of developing and using sensitive and precise measures of somatosensory impairment and makes this a useful population for testing purposes. For validation of this protocol, we used the known groups method24. We compared TDPM to another quantitative measure of kinesthesia, the Brief Kinesthesia Test (BKT). The BKT has been shown to be sensitive to ipsilesional upper limb impairment post stroke25. The tablet-based version (tBKT) was used in this study because it is the same test as the BKT, administered on a tablet with more trials. The tBKT has been shown to be stable in one-week test-retest measurement and sensitive to proprioceptive knockdown26. It was hypothesized that the elbow TDPM and tBKT outcomes would be correlated as sensorimotor control of the elbow contributes to BKT performance26.
The purpose of this paper is to outline a standardized method of measuring elbow TDPM that is reproducible using common equipment. Data is presented regarding reliability and initial validity testing of the method, as well as feasibility of use for persons with no known pathology, and those who were hypothesized to have mild somatosensory impairment.
Protocol
The Institutional Review Board at The College of St. Scholastica has approved the study under which this protocol was developed and tested.
1. Fabrication of the visual screen
- Cut the ¾ inch (1.9 cm) diameter PVC pipe into various lengths: two 30 inch (76.2 cm) pieces (screen base); two 8 inch (20.3 cm) pieces (screen base); one 44 inch (111.8 cm) piece (vertical screen support); and one 32 inch (81.3 cm) piece (screen fabric holder).
- Place an end cap on one end of each 30 inch (76.2 cm) piece, and a 90° PVC elbow on the other end. Insert 8 inch (20.3 cm) pieces into the remaining open ends of both elbows. Connect open ends of the two 8 inch (20.3 cm) pieces with the PVC tee to create a screen base.
- Insert the 44 inch (111.8 cm) PVC piece into the vertical portion of the PVC tee to create a vertical support for screen. Place the 45° PVC elbow on the open end of the 44 inch (111.8 cm) piece. Insert the 32 inch (81.3 cm) piece into the open end of the 45° PVC elbow to create a screen fabric holder. Place an end cap on the open end of the 32 inch (81.3 cm) piece.
- Place dishtowels on top of one another to ensure fabric opacity. Secure to the 32 inch (81.3 cm) piece with athletic tape. The fully assembled screen can be seen in Figure 1.
2. Preparation of the testing equipment
- Calibrate electrogoniometer and electromyography (EMG) sensors according to the manufacturers’ instructions.
- Turn on the continuous passive motion (CPM) machine and activate Extension/Flexion mode. Program the CPM machine to move through 90° to 130° of elbow extension at a speed of 0.23°/s.
3. Preparation of the participant for TDPM testing
- Seat the participant in a standard height chair (18 inch/45.7 cm), ensuring sitting with a straight back and feet flat on floor.
- Verbally prepare the participant for the EMG sensor and the electrogoniometer placement using a standardized script: “To begin, I am going to prepare your skin to attach sensors. They will help record movement and ensure your muscles are relaxed during the test. I’m going to mark landmarks on your arm and start attaching the sensors, so you can just relax in the position I place you in.”
- Attach the biceps brachii and the triceps brachii EMG sensors.
- Manually resist elbow flexion to locate the biceps brachii muscle belly and mark the central point of the muscle belly with a small dot of washable marker to denote the location for the EMG sensor placement. Prepare the skin by removing the dead skin cells followed by scrubbing with an alcohol swab, and then attach the EMG sensor.
- Manually resist elbow extension to locate the muscle belly of the lateral head of the triceps brachii and mark the central point in the bulk of the muscle belly with a small dot of washable marker to denote the location for the EMG sensor placement. Prepare the skin by removing dead skin cells followed by scrubbing with an alcohol swab, and then attach the EMG sensor.
- Test the EMG function by evoking an isometric biceps brachii contraction, followed by an isometric triceps brachii contraction, and observing for EMG activation.
- Attach the electrogoniometer to the participant.
- Determine the midpoint of the dorsal aspect of the wrist and mark with a washable marker.
- Palpate the most prominent aspect of the lateral epicondyle and mark with a washable marker.
- Palpate the greater tubercle of the humerus and mark with a washable marker. Verify the greater tubercle location by passively moving the testing arm through internal and external rotation of the humerus as needed.
- Attach one end of the string to the lateral epicondyle mark using paper tape. Pull the string taut, connecting it with the dorsal wrist midpoint mark.
- Trace a line along the proximal forearm in line with the string using a washable marker.
- Move the free end of the string to the greater tubercle mark and pull the string taut.
- Trace a line along the distal humerus in line with the string using a washable marker, and then remove the string.
- Place the distal paddle of the electrogoniometer along the path of the traced line, 1.5 inches (3.8 cm) distally from the lateral epicondyle mark.
- Place the proximal paddle of electrogoniometer along the path of the traced line, 1.5 inches (3.8 cm) proximally from the lateral epicondyle mark. Secure the remaining components of the electrogoniometer to the skin using paper tape.
- Position the participant’s upper extremity comfortably in the CPM machine.
- Adjust the height and orientation of the CPM machine to achieve a position of 90° sagittal plane shoulder flexion, 90° elbow flexion, and a neutral forearm. Align the participant’s lateral epicondyle with the rotational axis of the CPM machine.
- Adjust the CPM machine hand support to fit comfortably with the palm of the participant’s hand and secure the forearm via a wrist strap. Figure 1 shows the final participant setup for TDPM testing.
4. Administration of the TDPM test
- Inform the participant of the testing procedure with the following standardized verbal information: “During this test, the machine is going to move very slowly to either straighten or bend your elbow. We will say “begin” at the start of each trial, there will be eight trials. When I say begin, the machine may or may not move your arm. Please press the button as soon as you feel your arm move, but only when you feel movement. If you don’t feel movement, we will stop the trial after a period of time; try to pay attention until we stop the trial. This is the button you’ll be using. Please press the button right now to test it.”
- Hand the participant the electrogoniometer event marking trigger switch and test the switch.
- Inform the participant of additional aspects of the procedure: “In between each trial, whether your arm moved or not, we will take your arm out of the machine and straighten it, and then place it back in the machine. Please remain relaxed. Do you have any questions about the test? We will be using this curtain to block your vision during this test and place this hearing protection over your ears to minimize any sounds you might hear during the testing.”
- Occlude visual input by blocking the view of the arm being tested and the CPM machine using a visual screen. Drape screen material at the participant’s shoulder to avoid sensory input during arm movement. Diminish auditory input by placing noise-cancelling headphones on the participant (see Figure 1).
- Loudly state “begin,” and wait the corresponding amount of time per trial before initiating movement of the CPM machine to decrease participant guessing when movement will begin19. Standardized delay times are shown in Table 1.
| Trial Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Delay (s) | 1 | Catch | 3 | 1 | 2 | Catch | 3 | 1 |
Table 1: Standardized time delays and catch trial locations. Varied trial start time delays are included to prevent participant attempts to guess when movement will begin. Catch trials are included to test whether or not participant is actually detecting movement19,31.
- Observe for activation of biceps brachii and triceps brachii muscles by monitoring EMG sensor feedback readings to ensure that the participant does not attempt to use active movement to assist in movement detection.
- If muscle activation is noted, stop the trial and use the following standardized script: “Your muscles are activating. Please try to keep your arm relaxed during the test.” This trial should be noted for exclusion from data analysis, with the researcher proceeding with resetting the participant and CPM to start the next trial (protocol step 4.7).
- Between each trial, remove the participant’s testing arm from the CPM machine and return the CPM machine to a 90° start position. Passively move the participant’s elbow through full extension and then back to 90° flexion to standardize the muscle spindle movement history27,28. Place the arm back in the CPM machine for the next trial.
- Complete eight trials, including two “catch” trials where the participant’s arm is not moved19. Terminate each trial (catch and non-catch) when the participant depresses the trigger switch, or after 15 seconds if the trigger switch is not depressed.
- If during a catch trial a participant verbally reports they cannot feel movement, or depresses the trigger switch, use the following standardized response: “Your arm did not actually move during that trial. I know it’s hard to feel, the machine moves very slowly; try to concentrate and push the button as soon as you feel your arm move or that your arm position has changed.”
5. Calculation of participant’s TDPM score
- Using the electrogoniometer tracing, identify the electrogoniometer angle measurement for the point at which the CPM machine movement started, and for the point at which the participant depressed the trigger switch indicating movement was felt. See Figure 2 for a representative example.
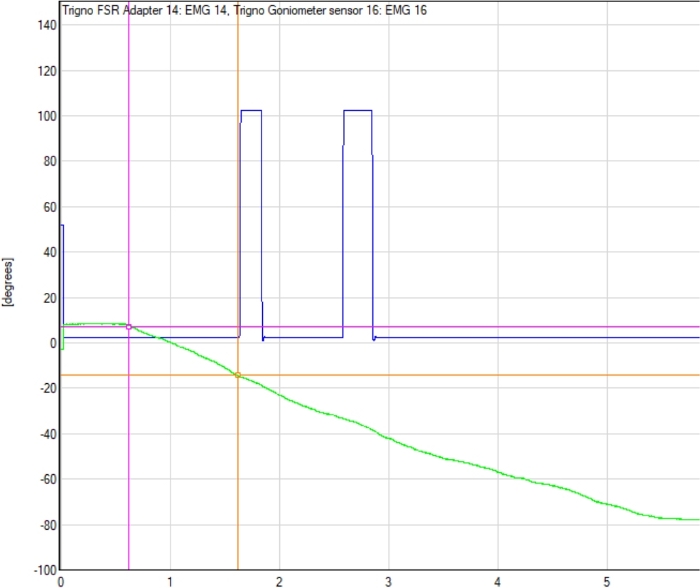
Figure 2: Example electrogoniometer tracing with detection point. The electrogoniometer line tracing (green line), start point of the continuous passive motion (CPM) machine movement, and the point at which participant indicated movement was detected (first blue peak) are shown. The difference between electrogoniometer readings at the start of the trial (pink circle) and at detection point (orange circle) determines the TDPM value for that trial. Please click here to view a larger version of this figure.
- Subtract the starting angle from the final angle, thus identifying the number of degrees the CPM moved; this is the participant’s elbow TDPM value for that trial.
- To determine participant’s overall TDPM score, remove the smallest and largest TDPM values from the six non-catch trials, and then average the remaining four trial scores29.
Representative Results
Participants:
Using the protocol presented here, elbow TDPM was measured in an academic research laboratory for two different groups of individuals: 20 healthy adults, and eight adults with chronic stroke. Participants for both groups were recruited from the community using fliers, emails, and word-of-mouth. The healthy adults (14 females, six males; mean age (SD) = 28 (7.9) years; 19 right- and one left-handed) were tested to generate representative results for an unimpaired population. Inclusion criteria were: age of 18 to 85 years; ability to follow two-step directions as determined by screening at initial meeting. Exclusion criteria were: history of disease or conditions affecting neuromuscular function of the upper limbs based upon self-report; reported allergy to metal or latex. Handedness was assessed using the Edinburgh Handedness Inventory30. Half of the healthy adult participants had TDPM of their right elbow tested, and half had their left elbow tested (block randomization). To determine the test-retest reliability of this protocol, healthy adult participant elbow TDPM was measured twice, one week apart. The tBKT was completed on Day 1 following TDPM testing. No adverse events occurred for any participant in the healthy participants group.
The elbow of the ipsilesional (i.e., less affected) upper limb of the individuals with chronic stroke (five males, three females; mean age (SD) = 69 (11.3) years; five right hemisphere stroke, three left hemisphere stroke) was tested to represent the protocol’s capability for detecting and quantitatively discriminating TDPM in individuals with suspected mild proprioceptive impairment. Inclusion criteria for this group were the same as for the healthy adult group, with the addition of: history of stroke occurring more than six months prior that impacted upper extremity function. Exclusion criteria were: any history of ipsilesional upper extremity pain or musculoskeletal injury; reported allergy to metal or latex. Participants with chronic stroke completed one elbow TDPM testing session. The tBKT was completed following elbow TDPM testing. One participant with stroke reported mild irritation from the EMG sensor adhesive; no other adverse events occurred.
Results:
No statistical difference was found between right and left elbow TDPM scores for healthy adults (p = 0.86, two-tailed); the data was combined for subsequent analyses. The average elbow TDPM for healthy adult participants (n = 20) was 1.19 (±1.02) degrees. The Spearman correlation and intraclass correlation coefficient (ICC) were calculated to evaluate test-retest reliability of the TDPM; a positive and statistically significant relationship was found (rs = 0.72, p < 0.001), (ICC 2,4 = 0.84), suggesting moderate to good reliability of the measure among healthy adult participants24 (Figure 3).
The average ipsilesional elbow TDPM for participants with chronic stroke (n = 8) was 8.24 (±4.53) degrees (Table 2). Participants with chronic stroke were more variable than healthy adult participants (Figure 4A). Using a two-tailed t-test, the TDPM of the healthy adult and chronic stroke groups were found to be statistically different, with the adults with chronic stroke requiring a greater elbow extension excursion prior to movement being detected (t = 4.4, p = 0.003, two-tailed) (Table 2). Spearman correlation between elbow TDPM and error in targeted reaching as measured by the tBKT showed a moderate relationship between these two measures (rs = 0.63, p < 0.001) (Figure 5). Participant tBKT scores are shown in Figure 4B.
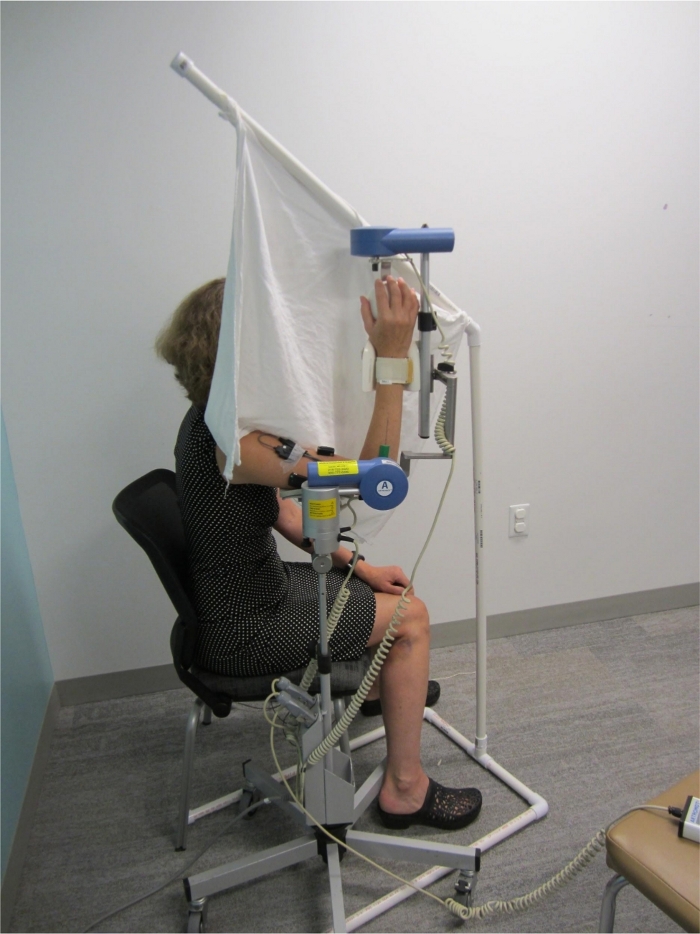
Figure 1: Participant setup for elbow threshold to detection of passive movement (TDPM) testing. The continuous passive motion (CPM) machine extended the participant’s elbow at a constant speed of 0.23°/s. Note the visual screen placed to occlude vision of the testing arm. Not visible are hearing occlusion headphones, and a trigger switch for participant indication of movement detection. Please click here to view a larger version of this figure.
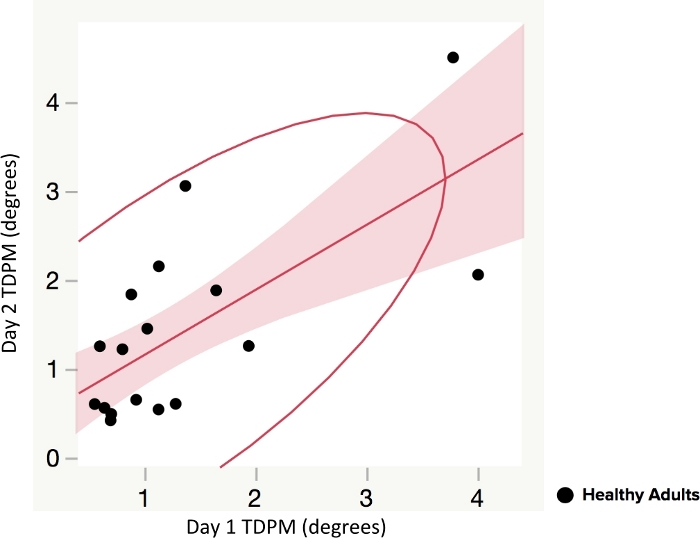
Figure 3: Test-retest reliability of elbow threshold to detection of passive movement (TDPM) method in healthy adults. Spearman correlation and intraclass correlation coefficient (ICC) of Day 1 and Day 2 (tested one week apart) were used to compare TDPM scores. Figures show line of fit with 95% confidence interval (shaded area) and a density ellipse. A positive and statistically significant relationship was found (rs = 0.72, p < 0.001). Please click here to view a larger version of this figure.
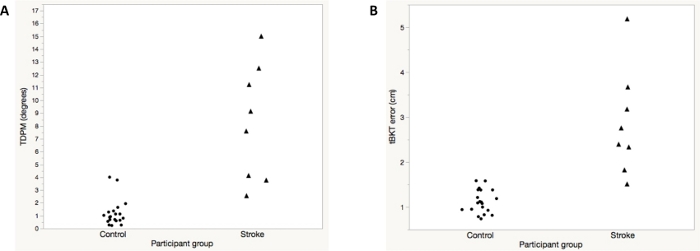
Figure 4: Representative results for elbow threshold to detection of passive movement (TDPM) (A) and the tablet version of Brief Kinesthesia Test (B) for healthy adult control subjects versus participants with chronic stroke. Note that one individual with chronic stroke was unable to detect movement on any trial; the maximum specified TDPM value of 15° was assigned. This same individual had the greatest amount of error during tBKT testing. Please click here to view a larger version of this figure.
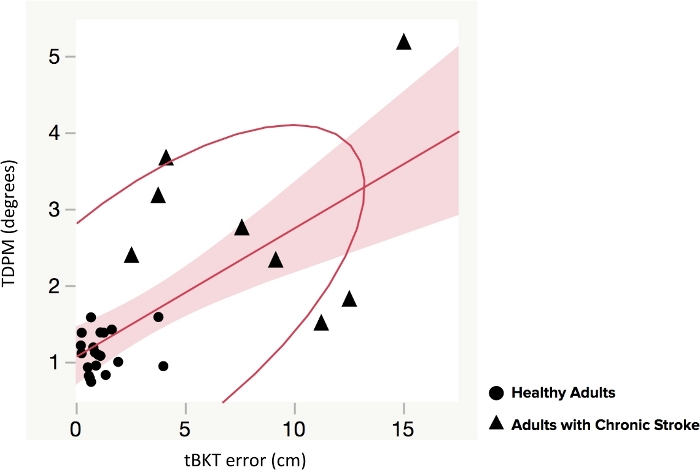
Figure 5: Elbow threshold to detection of passive movement (TDPM) scores compared to tablet version of the Brief Kinesthesia Test (tBKT) scores in healthy adults and adults with chronic stroke. Spearman correlation between elbow TDPM and error in targeted reaching as measured by the tablet version of the Brief Kinesthesia Test (tBKT) is shown. There was a moderate positive relationship (rs = 0.63, p < 0.001). Please click here to view a larger version of this figure.
| Age | Sex | Stroke Chronicity | Handedness | Fugl-Meyer | TDPM | tBKT Error | |
| Mean(SD) in years | Mean(SD) in months | Mean (SD) of shoulder-elbow subscore/36 | Mean (SD) in degrees | Mean (SD) in cm | |||
| Healthy adults (control) n = 20 | 28(7.9) | 14 F; 6 M | NA | 19 – R 1- L | NA | 1.19 (1.02) | 1.12 (0.26) |
| Adults with chronic stroke n = 8 | 69(11.3) | 3 F; 5 M | 33(19) | 7 – R 1- L | 23.9(8.5) 5 – R CVA 3 – L CVA | 8.24 (4.53) | 2.85 (1.16) |
| SD = Standard deviation; F = Female; M = Male; R = Right; L = Left; CVA = Cerebrovascular Accident; NA = Not applicable; cm = centimeter | t = 4.4, p = 0.003 (two-tailed) | t = 4.15, p = 0.004 | |||||
Table 2: Participant description, average elbow threshold to detection of passive movement (TDPM) scores (degrees), and average tablet version of the Brief Kinesthesia Test (tBKT) scores. A significant difference was found in average elbow TDPM between healthy controls and adults with chronic stroke, as well as in the average tBKT scores.
Discussion
The presented protocol describes how to measure elbow TDPM in a standardized fashion using a common CPM machine to provide the passive movement. Across 20 healthy participants the average elbow TDPM measurement was found to be similar to the average value identified in previous studies using other TDPM measuring setups7,19,32, and produced reliable results across testing sessions. TDPM of the ipsilesional elbow among the eight participants with chronic stroke on average differed significantly, and perhaps clinically meaningfully, from the healthy adult population as has been previously shown5,15. It is likely a portion of the difference in TDPM between groups can be attributed to age differences21,33,34,35 and to potential reaction time differences1. Regardless, the findings indicate this method is able to discriminate between groups that have subtle differences in performance.
Selecting the CPM machine movement speed is a critical protocol step that will affect TDPM scores (protocol step 2.2). Previous studies have shown that TDPM increases with decreasing passive motion velocity7,16,23. The speed selected for this protocol, 0.23°/s, is similar to values tested in prior studies7,22,28, and is near the inflection point where TDPM exponentially increases in difficulty for healthy subjects7. As noted in the representative results, one participant with chronic stroke was unable to feel movement in any trial, suggesting the CPM machine movement speed of 0.23°/s has a potential floor effect and may need to be increased for testing of individuals with more severe kinesthetic impairments. The range of available speeds differs across CPM machine manufacturers; researchers should select a model that will meet their study needs. Providing clear participant instruction with verification of understanding is also a critical protocol element to support accurate performance of the TDPM task.
All participants with chronic stroke were able to depress the trigger switch with their more affected upper extremity; alternative methods of indicating when movement is felt may be needed for participants who are unable to do so. It is possible a larger style of switch could be used. Additional modifications to the protocol may include elimination of the biceps and triceps EMG sensors. EMG use was incorporated into the protocol to confirm muscle contraction did not occur during trials, as active muscle contraction and muscle contraction history have been shown to impact proprioceptive thresholds due to the thixotropic properties of muscle fibers and spindles27,28. However, muscle activation was not noted during any trial for any participant, suggesting EMG monitoring may be unnecessary.
A possible limitation of this protocol is the testing position of 90° of shoulder and elbow flexion, as some individuals may be unable to achieve or tolerate this position. Modification of the testing position is known to change kinesthesia36. An aspect of the TDPM paradigm that is not unique to this protocol is the high attentional demand of the task, which limits the appropriateness of this measurement method for individuals with attention deficits. To reduce error due to inattention or fatigue19, we intentionally designed this protocol to take no more than 15 minutes per limb. This protocol does not control for potential differences in reaction time between participants, which is a potential limitation. The slow passive movement speed used in this protocol decreases the proportional contribution of reaction time error to a participant’s TDPM score.
This detailed elbow TDPM protocol provides sensorimotor researchers a sensitive and precise measure of kinesthesia. The data suggest the TDPM’s resolution is high affording the possibility of detecting mild impairment or perhaps being sensitive to change if used in a study of recovery of function. Future research could be conducted to determine the minimal clinically important difference in TDPM. Adaptation of this protocol to other joints may also be appropriate.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Dr. Jon Nelson for technical support of EMG and electrogoniometer equipment used here.
Materials
| 3/4 inch diameter PVC pipe | Charlotte Pipe | Pipe to be cut into lengths of: 30 inches/76.2 cm (x2); 8 inches/20.3 cm (x2); 44 inches/111.8 cm (x1); 32 inches/81.3 cm (x1). | |
| 3/4 inch diameter PVC pipe end caps (x3) | Charlotte Pipe | ||
| 45° PVC elbow (x1) | Charlotte Pipe | ||
| 90° PVC elbows (x2) | Charlotte Pipe | ||
| Athletic tape | 3M | ||
| Delsys acquisition software (EMGworks) | Delsys | ||
| Double-sided tape | 3M | ||
| Duct tape | 3M | Used to assist in removal of dead skin cells on participant's skin prior to EMG sensor placement. | |
| Elbow Continuous Passive Motion (CPM) Machine | Artromot | Chattanooga Artromot E2 Compact Elbow CPM; Model 2038 | |
| Electrogoniometer | Biometrics, Ltd | ||
| Flour sack dishcloths (x2) | Room Essentials | Fabric used for creation of visual screen. | |
| Handheld external trigger switch | Qualisys | Trigger switch used for electrogoniometer event marking. | |
| Hearing occlusion headphones | Coby | ||
| Isopropyl alcohol | Mountain Falls | ||
| Paper tape | 3M | ||
| Ruler with inch markings | Westcott | ||
| Standard height chair | KI | ||
| String | Quality Park | Approximately 15 inches of string needed. String used for standardization of electrogoniometer placement. | |
| Trigno Goniometer Adapter | Delsys | ||
| Trigno Wireless Electromyography Sensors | Delsys | ||
| Washable marker | Crayola | ||
| Washcloth | Aramark | Used in combination with isopropyl alcohol for cleaning participant's skin prior to EMG sensor placement. |
References
- Coderre, A. M., et al. Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabilitation and Neural Repair. 24 (6), 528-541 (2010).
- Dukelow, S. P., et al. Quantitative assessment of limb position sense following stroke. Neurorehabilitation and Neural Repair. 24 (2), 178-187 (2010).
- Semrau, J. A., Herter, T. M., Scott, S. H., Dukelow, S. P. Robotic identification of kinesthetic deficits after stroke. Stroke. 44 (12), 3414-3421 (2013).
- Meyer, S., Karttunen, A. H., Thijs, V., Feys, H., Verheyden, G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Physical Therapy. 94 (9), 1220-1231 (2014).
- Desrosiers, J., Bourbonnais, D., Bravo, G., Roy, P. M., Guay, M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 27 (9), 1564-1570 (1996).
- Carey, L. M., Matyas, T. A. Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. Journal of Rehabilitation Medicine. 43 (3), 257-263 (2011).
- Konczak, J., Krawczewski, K., Tuite, P., Maschke, M. The perception of passive motion in Parkinson’s disease. Journal of Neurology. 254 (5), 655 (2007).
- Van Deursen, R. W. M., Simoneau, G. G. Foot and ankle sensory neuropathy, proprioception, and postural stability. Journal of Orthopaedic & Sports Physical Therapy. 29 (12), 718-726 (1999).
- Reider, B., et al. Proprioception of the knee before and after anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 19 (1), 2-12 (2003).
- Hizli Sayar, G., Unubol, H. Assessing Proprioception. The Journal of Neurobehavioral Sciences. 4 (1), 31-35 (2017).
- Fugl-Meyer, A. R., Jääskö, L., Leyman, I., Olsson, S., Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of Rehabilitation Medicine. 7 (1), 13-31 (1975).
- Stolk-Hornsveld, F., Crow, J. L., Hendriks, E., Van Der Baan, R., Harmeling-van Der Wel, B. The Erasmus MC modifications to the (revised) Nottingham Sensory Assessment: a reliable somatosensory assessment measure for patients with intracranial disorders. Clinical Rehabilitation. 20 (2), 160-172 (2006).
- Winward, C. E., Halligan, P. W., Wade, D. T. The Rivermead Assessment of Somatosensory Performance (RASP): standardization and reliability data. Clinical Rehabilitation. 16 (5), 523-533 (2002).
- Lincoln, N. B., et al. The unreliability of sensory assessments. Clinical rehabilitation. 5 (4), 273-282 (1991).
- Sartor-Glittenberg, C. Quantitative measurement of kinesthesia following cerebral vascular accident. Physiotherapy Canada. 45, 179-186 (1993).
- Hillier, S., Immink, M., Thewlis, D. Assessing proprioception: a systematic review of possibilities. Neurorehabilitation and Neural Repair. 29 (10), 933-949 (2015).
- Han, J., Waddington, G., Adams, R., Anson, J., Liu, Y. Assessing proprioception: a critical review of methods. Journal of Sport and Health Science. 5 (1), 80-90 (2016).
- Goble, D. J. Proprioceptive acuity assessment via joint position matching: from basic science to general practice. Physical Therapy. 90 (8), 1176-1184 (2010).
- Juul-Kristensen, B., et al. Test-retest reliability of joint position and kinesthetic sense in the elbow of healthy subjects. Physiotherapy Theory and Practice. 24 (1), 65-72 (2008).
- Deshpande, N., Connelly, D. M., Culham, E. G., Costigan, P. A. Reliability and validity of ankle proprioceptive measures. Archives of Physical Medicine and Rehabilitation. 84 (6), 883-889 (2003).
- Boerboom, A., et al. Validation of a method to measure the proprioception of the knee. Gait & Posture. 28 (4), 610-614 (2008).
- Nagai, T., Sell, T. C., Abt, J. P., Lephart, S. M. Reliability, precision, and gender differences in knee internal/external rotation proprioception measurements. Physical Therapy in Sport. 13 (4), 233-237 (2012).
- Refshauge, K. M., Chan, R., Taylor, J. L., McCloskey, D. Detection of movements imposed on human hip, knee, ankle and toe joints. The Journal of Physiology. 488 (1), 231-241 (1995).
- Portney, L. G., Watkins, M. P. . Foundations of Clinical Research: Applications to Practice. 892, (2009).
- Borstad, A., Nichols-Larsen, D. S. The Brief Kinesthesia test is feasible and sensitive: a study in stroke. Brazilian Journal of Physical Therapy. 20 (1), 81-86 (2016).
- Vernoski, J. L. J., Bjorkland, J. R., Kramer, T. J., Oczak, S. T., Borstad, A. L. A Simple Non-invasive Method for Temporary Knockdown of Upper Limb Proprioception. Journal of Visualized Experiments. (133), e57218 (2018).
- Proske, U., Tsay, A., Allen, T. Muscle thixotropy as a tool in the study of proprioception. Experimental Brain Research. 232 (11), 3397-3412 (2014).
- Wise, A. K., Gregory, J. E., Proske, U. Detection of movements of the human forearm during and after co-contractions of muscles acting at the elbow joint. The Journal of Physiology. 508, 325 (1998).
- Wilcox, R. R., Granger, D. A., Clark, F. Modern robust statistical methods: Basics with illustrations using psychobiological data. Universal Journal of Psychology. 1 (2), 21-31 (2013).
- Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9 (1), 97-113 (1971).
- Piriyaprasarth, P., Morris, M. E., Delany, C., Winter, A., Finch, S. Trials needed to assess knee proprioception following stroke. Physiotherapy Research International. 14 (1), 6-16 (2009).
- Juul-Kristensen, B., et al. Poorer elbow proprioception in patients with lateral epicondylitis than in healthy controls: a cross-sectional study. Journal of Shoulder and Elbow Surgery. 17 (1), 72-81 (2008).
- Skinner, H. B., Barrack, R. L., Cook, S. D. Age-related decline in proprioception. Clinical Orthopaedics and Related Research. (184), 208-211 (1984).
- Pai, Y. C., Rymer, W. Z., Chang, R. W., Sharma, L. Effect of age and osteoarthritis on knee proprioception. Arthritis & Rheumatism. 40 (12), 2260-2265 (1997).
- Dunn, W., et al. Measuring change in somatosensation across the lifespan. American Journal of Occupational Therapy. 69 (3), (2015).
- Alghadir, A., Zafar, H., Iqbal, Z., Al-Eisa, E. Effect of sitting postures and shoulder position on the cervicocephalic kinesthesia in healthy young males. Somatosensory & Motor Research. 33 (2), 93-98 (2016).

