Applying Dynamic Strain on Thin Oxide Films Immobilized on a Pseudoelastic Nickel-Titanium Alloy
Summary
Dynamic, tensile strain is applied on TiO2 thin films to study the effects of strain on electrocatalysis, specifically proton reduction and water oxidation. TiO2 films are prepared by thermal treatment of the pseudo-elastic NiTi alloy (Nitinol).
Abstract
Direct alteration of material structure/function through strain is a growing area of research that has allowed for novel properties of materials to emerge. Tuning material structure can be achieved by controlling an external force imposed on materials and inducing stress-strain responses (i.e., applying dynamic strain). Electroactive thin films are typically deposited on shape or volume tunable elastic substrates, where mechanical loading (i.e., compression or tension) can affect film structure and function through imposed strain. Here, we summarize methods for straining n-type doped titanium dioxide (TiO2) films prepared by a thermal treatment of a pseudo-elastic nickel-titanium alloy (Nitinol). The main purpose of the described methods is to study how strain affects electrocatalytic activities of metal oxide, specifically hydrogen evolution and oxygen evolution reactions. The same system can be adapted to study the effect of strain more broadly. Strain engineering can be applied for optimization of a material function, as well as for design of adjustable, multifunctional (photo)electrocatalytic materials under external stress control.
Introduction
The ability to alter the surface reactivity of catalytic materials by introducing strain has been widely recognized1,2,3. Effects of strain in crystalline materials can be introduced either by adjusting material architecture (static strain) or by applying a variable external force (dynamic strain). In crystalline materials, static strain can be introduced by doping4, de-alloying5,6, annealing7, epitaxial growth on a mismatched crystal lattice2 or size confinement2,3. In polycrystalline materials, strain can occur within grain boundaries due to crystal twinning8. Determining the optimal degree of static strain with material architectures requires designing a new sample for each discrete level of strain, which can be time consuming and expensive. Furthermore, introducing static strain often introduces chemical or ligand effects9,10, making it difficult to isolate the strain contribution. Applying a dynamic strain precisely controlled by an external force allows systematic tuning of a material’s structure/function relationship in order to explore a dynamic range over the strain space without introducing other effects.
To study the effects of dynamic strain on electrocatalysis, metals or metal oxides are deposited on elastic shape or volume tunable substrates, such as organic polymers11,12,13,14,15 or alloys16,17. Applications of mechanical, thermal or electrical loading results in bending, compression, elongation or expansion of an elastic substrate, further inducing a stress-strain response on the deposited catalytic material. So far, catalyst engineering through dynamic strain has been exploited to tune electrocatalytic activities of various metallic and semiconducting materials. Examples include i) the hydrogen evolution reaction (HER) on MoS2, Au, Pt, Ni, Cu, WC11,12,13,14, ii) the oxygen evolution reaction (OER) on NiOx16, nickel-iron alloys18 and iii) the oxygen reduction reaction (ORR) on Pt, Pd12,15,19,20. In most of these reports, organic polymers, such as polymethyl methacrylate (PMMA), were used as elastic substrates. We previously demonstrated the application of elastic metallic substrates, such as stainless steel16 and a superelastic/shape-memory NiTi alloy (Nitinol17,21) for strain studies. Nitinol has also been used as an elastic substrate for deposition of platinum films for ORR19 and deposition of battery cathode materials for energy storage22,23. Due to its shape memory and pseudoelastic properties, NiTi alloys can be deformed by applying moderate heat19 or mechanical strain17, respectively. In contrast to organic elastic substrates, metallic substrates typically do not require deposition of adhesion promoters, are highly conductive and can easily be functionalized. Nitinol is used as a more elastic alternative to stainless steel (SS). While SS can be reversibly strained up to 0.2%, nitinol can be reversibly strained up to 7%. Nitinol owes its unique properties to a martensitic solid state crystal transformation that allows for large elastic deformations24,25. Both materials are commercially available in different geometries (e.g., foils, wires, and springs). When shaped into elastic springs, metallic substrates can be used to study effects of dynamic strain on electrocatalysis without the need for expensive instrumentation16; however, defining the stress-strain response is more challenging than for other geometries.
In previous experimental studies with transition metal catalysts, changes in activities of catalytic surfaces under strain have been attributed to changes in the energetics of the d orbitals colloquially known as d-band theory26. In contrast, the effects of strain on metal oxides is significantly more complex, as it can effect bandgap, carrier mobility, diffusion and distribution of defects and even direct/indirect transitions21,27,28,29,30,31. Herein we provide detailed protocols for the preparation and characterization of n-type doped TiO2 thin films, as well as protocols to study electrocatalytic activities of these films under tunable, tensile strain. The equivalent system can be applied to study electrocatalytic activities of different materials as a function of dynamic strain.
Protocol
1. Preparation of NiTi/TiO2 electrodes
- Chemical and mechanical polishing of NiTi substrates
- Cut the superelastic NiTi foil (0.05-mm thickness) into 1 cm x 5 cm strips.
- Polish sample using 320-, 600- and 1200-grit sandpaper, and then rinse with ultrapure water (18.2 MΩ).
- Polish sample with 1 μm diamond, 0.25 μm diamond, and 0.05 μm alumina polish.
- After polishing, sonicate for 5 min in sequential baths of ultrapure water (18.2 MΩ), isopropanol, ethanol, ultrapure water (18.2 MΩ), and then dry under nitrogen (used organic solvents were reagent grade).
CAUTION: Organic solvents are flammable, can irritate skin and eyes, poisonous if ingested. Use with caution in well ventilated areas.
NOTE: Foils should be treated gently. Repeated bending or twisting can result in nano-to-micro sized fissures, which will affect its elastic properties decreasing the effects of strain on the electrocatalytic activities.
- Preparation of TiO2 films
- Oxidize NiTi foils by placing foils in a 500 °C oven under aerobic conditions (Figure 1).
- For preparation of 50 nm thick rutile TiO2 films, heat NiTi foils for 30 min at 500 °C. Longer heating will result in thicker TiO2 films. Heating will cause a change in the surface color from gray to blue/purple (Figure 2).
- Applying tensile stress on NiTi/TiO2
- Gently clamp foil (1 cm x 5 cm strip) in a mechanical tester (Table of Materials) with 1 cm of foil exposed at each end.
- Strain the NiTi/TiO2 samples at a rate of 2 mm/min. Keep the strain at desired level (0-3%).
NOTE: Extension of the available 3 cm NiTi/TiO2 lengthwise from 0.0 to 2.1 mm is considered straining from 0 to 7%, which can be calculated by simple equation strain=(l-l0)/l0 , where l0 is initial and l final length of foil exposed to tensile strain. Typical stress-strain curve is shown in Figure 3.
- To start electrochemical measurements, pre-strain the foil to 5 N (taken as 0% strain).
NOTE: The slight pre-straining of the foil leads to more reproducible results.
2. Conducting electrochemical measurements under strain
- Applying tensile stress on working electrode
- To conduct electrochemical experiments under applied strain, assemble the custom-made electrochemical cell (Figure 4 and Figure 5) loosely around the NiTi/TiO2 foil. Ensure that the center of the NiTi/TiO2 foil is exposed by carefully positioning the cell in the middle (Figure 5).
- Tighten the cell gently onto the sample to create a solution-tight cell for the electrochemical measurements.
- Fill up with an electrolyte and purge the solution gently with nitrogen.
- Increase strain to specific levels, typically 0 to 3% in 0.5% increments and conduct electrochemical experiments for each discrete strain value.
- Before each strain adjustment, loosen the electrochemical cell around NiTi/TiO2 foil, so that the sample can move freely. Then realign the cell by gently tightening back onto the sample and refill the electrolyte for the next electrochemical measurements.
NOTE: Tightening and untightening the cell around the NiTi/TiO2 foil is obviously more laborious and time consuming than working with a continuously tightened cell through the experiments. Nevertheless, this approach minimizes possible wrinkling of NiTi/TiO2 foil leading to the most reproducible results and the highest effects of strain.
- Electrochemical characterization of strained working electrode
- As an initial experiment, conduct cyclic voltammetry (CV) or linear sweep voltammetry (LSV) measurements (Figure 6A). Further characterization could include impedance, electrolysis, chronoamperometry, etc.
- Collect electrochemical measurements with samples exposed to discrete, increasing levels of strain (e.g., from 0 to 3% in 0.5% increments), followed by gradual decreasing of applied strain (e.g., from 3 to 0% in 0.5% increments).
- Collect data for multiple experimental cycles (0%→3%→0%) to test the system mechanical stability and data reproducibility.
- Alternatively, keep the foil strained at a discrete amount of strain for prolonged time periods (e.g., hours or days) and conduct electrochemical experiments periodically (e.g., voltammetry) or continuously (e.g. electrolysis).
- HER experiments
- Use 0.5 M sulfuric acid as the electrolyte, Ag/AgCl (1 M NaCl) as the reference electrode, and a coiled platinum wire (0.5 mm diameter by ~10 cm length) as the counter electrode.
CAUTION: Sulfuric acid causes severe skin burns and eye damage. Do not breathe mist, vapors, or spray. Wear protective gloves, protective clothing, eye protection, and face protection. Immediately wash exposed skin with copious amounts of water if exposed. - Scan the potentials between the open-circuit voltage (OCV) to -0.8 V vs RHE, starting with the highest potential value with scan rate 5-50 mV/s (Figure 6A).
- Use 0.5 M sulfuric acid as the electrolyte, Ag/AgCl (1 M NaCl) as the reference electrode, and a coiled platinum wire (0.5 mm diameter by ~10 cm length) as the counter electrode.
- OER experiments
- Use 1 M sodium hydroxide as the electrolyte, Hg/HgO (1 M NaOH) as the reference electrode, and a coiled platinum wire (0.5 mm diameter by ~10 cm length) as the counter electrode.
CAUTION: 1 M sodium hydroxide can cause skin burns and eye damage Do not breathe mist, vapors, or spray. Wear protective gloves, protective clothing, eye protection, and face protection. Immediately wash exposed skin with copious amounts of water if exposed. - For OER experiments, scan the potential between OCV to 2 V vs RHE, starting with the lowest potential value, with scan rate 5-50 mV/s (Figure 6B).
- Use 1 M sodium hydroxide as the electrolyte, Hg/HgO (1 M NaOH) as the reference electrode, and a coiled platinum wire (0.5 mm diameter by ~10 cm length) as the counter electrode.
- Impedance
- Carry out electrochemical impedance spectroscopy (EIS) measurements at frequencies ranging from 1 Hz-100 kHz at a potential where no Faradaic process is observed (OCV) (Figure 6C).
- Analyzing time profile, system stability and products
- To test the stability of the system and measure products (e.g., H2 and O2), conduct electrolysis experiments.
- For amperometric i-t measurements, choose the most suitable potential based on CV or LSV results (e.g., -0.25 V vs RHE for HER).
- Alternatively, for chronopotentiometry experiments, choose the most suitable current density based on CV results.
- If gas chromatograph is available, measure in-line hydrogen (from HER) or oxygen (from OER) gas produced electrochemically (Figure 4B).
NOTE: These are examples of electrochemical analyses. Electrochemical characterization can be tailored for a specific study.
3. Controls
- Capacitance measurements
- To determine if increases in HER activities are simply due to increases in electroactive surface, conduct capacitance measurements at different strain values.
- Run CV experiments at different scan rates (e.g., 1 and 500 mV/s) at a potential range where Faradic currents are negligible, so that currents represent only the charge/discharge of the electric double layer (e.g., 0 to 0.1 V vs RHE).
- Plot scan rates versus currents (Figure 7A).
- Compare increases in capacitance with strain with increases in electrocatalytic activities (e.g., HER or OER) with strain (Figure 7A).
NOTE: If increases in electrocatalytic activities are higher than increases in capacitance, it can be concluded that simple increase in grain separation and electroactive surface is not the only contributor to the increase in electrocatalytic activities.
- Characterization of cracked films
- Purposely crack NiTi/TiO2 foil by keeping the foil strained at 7% for 30 min or longer for 50 nm TiO2 films (Figure 8). Thicker TiO2 films (100 nm) can be cracked at lower strains (3% strain).
- Analyze the surface for cracking by scanning electrochemical microscopy (SEM), or other surface analysis methods, as described below.
- Conduct electrochemical measurements as described above with pristine and purposely cracked TiO2 films at different incrementally increased and then decreased strain values from 0%→3%→0% (Figure 6D). NiTi/TiO2 foils with 50 nm thick TiO2 films that were never strained pass 3% are considered pristine, elastic.
NOTE: Determine the specific “elastic limit”: the maximum stress that can be applied on a material before the onset of an irreversible deformation (e.g., grain rearrangement or even film cracking). Elastic range depends on film type, thickness and deposition method. For example, we show that 100 nm thick TiO2 films crack at lower strains than 50 nm thick TiO2 films.
- Characterization of NiTi foils (i.e., unoxidized foils)
- Polish NiTi folis as described in step 1.1, but do not thermally treat them.
- Run all the electrochemical experiments, as described above, with NiTi foils that were not thermally treated as a control.
4. Surface characterization
- Sample preparation
- Cut and pretreat NiTi/TiO2 as described in steps 1.1 and 1.2.
NOTE: The size of the sample foil depends on the size of the sample holder, which depends on a specific instrumentation used for the surface characterization. - Wash samples with water to remove any residual salt if used in electrochemical experiments before the characterization.
- Assemble NiTi/TiO2 foil in the tensile stretcher and strain to a desired level as described in section 1.3.
- Assemble the custom-made sample holders around the strained sample and gently tighten the screws (Figure 9).
- Cut and pretreat NiTi/TiO2 as described in steps 1.1 and 1.2.
- Surface characterization
- To check film quality and changes in film topology with strain, collect scanning electrochemical microscopy (SEM) images.
- Use other available surface analysis methods to monitor changes in surface chemical composition, grain rearrangements and exposed crystal lattices (e.g., Raman spectroscopy, XPS or XRD experiments) (Figure 10).
- To check if a sample holder kept constant strain during the surface characterization experiments untighten the sample from the sample holder and look for any curl in the sample between the strained portion under the clamp and the unrestrained portion that was previously in the tensile tester.
Representative Results
Pre-treated NiTi foils are oxidized at 500 °C under aerobic conditions (Figure 1). Due to the oxophilic nature of titanium, calcination at elevated temperatures results in a surface layer of rutile TiO2. The thickness of the layer and degree of n-type doping are affected by annealing time and temperature, which is reflected in color change from gray (untreated sample) to uniform blue/purple after 20 min heating (Figure 2). Longer heating time results in thicker TiO2 films (60 min for 100 nm films) and is accompanied by gradual loss of blue/purple color. Thicker TiO2 films show analogous electrochemistry but are more prone to surface fissuring and therefore loss in film elasticity.
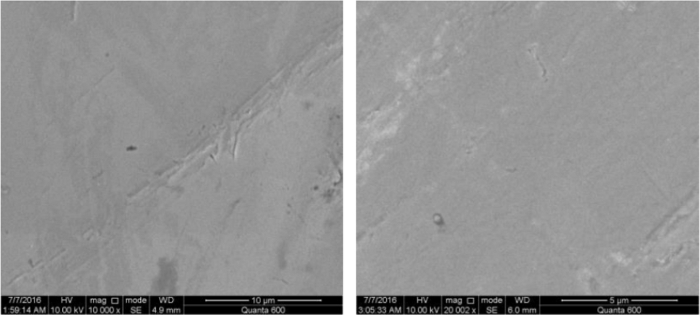
Figure 1: Scanning electrochemical microscopy images of polished (left) and oxidized (right) NiTi films. Please click here to view a larger version of this figure.
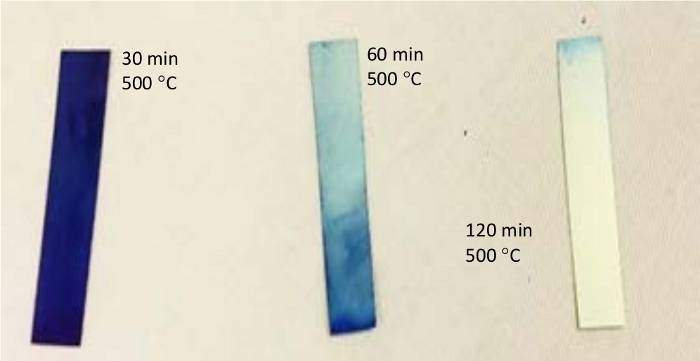
Figure 2: NiTi foil heated at 500 °C in air for different time periods. Figure shows characteristic color changes. Please click here to view a larger version of this figure.
Nitinol behavior under thermal and mechanical stress reflects reversible solid-state phase transformation known as a martensitic transformation, between two different martensite crystal phases, making it a pseudo-elastic rather than an elastic material. A typical stress-strain curve of NiTi/TiO2 samples is given in Figure 3. Note that the shape of the foil is rectangular and not specifically shaped for mechanical testing, which could result in nonuniform stress distribution from the center of the sample to the clamped sample section. Nevertheless, electrochemical characterization of strained foils is conducted with only a small section of NiTi/TiO2 foil positioned in the middle (see further text). An assumption is made that within this small surface applied stress is uniform.
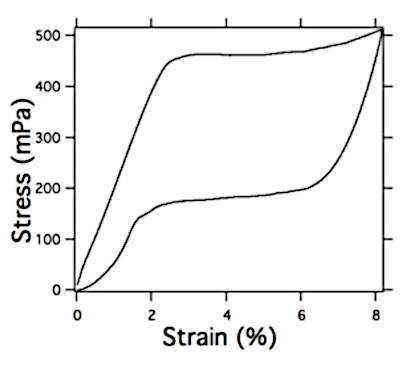
Figure 3: Typical stress-strain curve for NiTi/TiO2 foil (1 cm x 5 cmstrip). Please click here to view a larger version of this figure.
For measuring effects of strain on electrocatalytic properties of different materials, single or double compartment electrochemical cells are custom-built. Figure 4 shows the electrochemical cell with both the cathode and anode compartment. If focus is only on the electrochemical characterization rather than the product (H2 and/or O2) collection, double compartment cells and membrane separation are not necessary for HER and OER experiments. The size of the cathode is limited by an opening in the electrochemical cell (Figure 5) that allows exposure of NiTi/TiO2 foil to the electrolyte. Therefore, even though a large fraction of NiTi/TiO2 foil is exposed to strain, only a small circle (i.e., 5 mm diameter) in the middle of the foil undergoes electrocatalysis. The working electrode volume should be kept relatively small relative to the surface of a counter electrode to minimize the effects of solvent resistance.
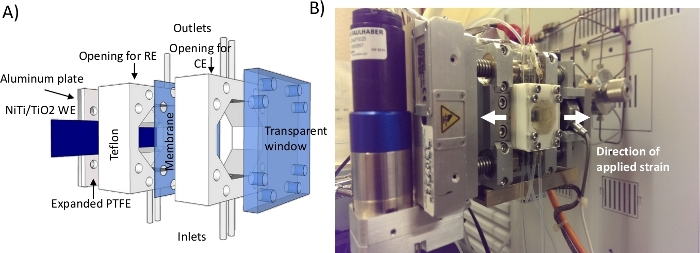
Figure 4: Two compartment cell. (A) The scheme showing the individual components. (B) The cell assembled into the tester for applying tensile strain. The cell was installed in proximity to the gas chromatograph for analysis of gaseous products. This figure illustrates how the tester can be easily assembled to work in conjunction with other instrumentation. Please click here to view a larger version of this figure.
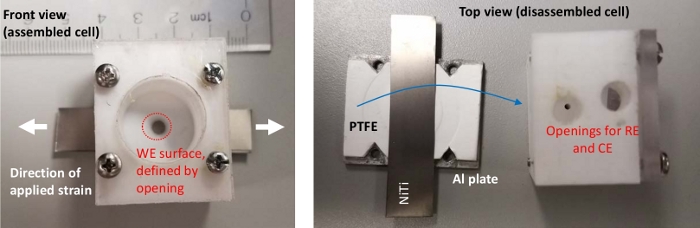
Figure 5: The single compartment cell used for HER and OER experiments. Please click here to view a larger version of this figure.
Typically, first experiments include CV or LSV (Figure 6A,B). These experiments are important for understanding the electrochemical system, such as Faradic versus non-Faradic ranges. Further electrochemical characterization can include electrochemical impedance to study changes in electrode surface reactivities with strain (Figure 6C). Amperometry or chronoamperometry can be used to study system stability and accumulated products. Gas chromatography can be used to detect produced H2 (cathode) or O2 (anode).
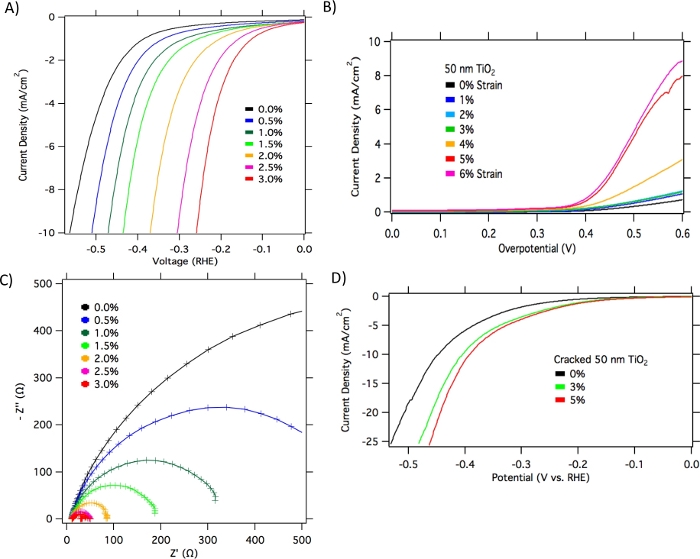
Figure 6: Representative LSV and EIS data. (A) LSV experiments showing HER on NiTi/TiO2 films in 0.5 M sulfuric acid at a scan rate of 50 mV/s. (B) LSV experiments showing OER on NiTi/TiO2 films in 1 M sodium hydroxide at a scan rate of 50 mV/s. (C) Electrochemical impedance at −0.38 V vs. RHE from 1 Hz to 100 kHz (Nyquist plots). (D) LSV experiments in 0.5 M sulfuric acid at a scan rate of 50 mV/s with the purposely cracked TiO2 films. This figure has been modified from Benson et al17. Please click here to view a larger version of this figure.
Applying mechanical stress that exceeds a material elastic limit leads to grain rearrangements and cracking of the material’s surface, which could increase electrocatalytic activities simply by increasing the overall electroactive surface or by exposing more catalytically active crystal facets or defects32. In these cases, dynamic strain would only affect grain rearrangement, which is different than actual changes at atomic or nanoscale material architecture. To rule out nonelastic effects on electrocatalytic activities, various control experiments are performed. First, to determine if increases in HER and OER activities are simply due to increases in electroactive surface, capacitance measurements are done at different strain values. Based on Randles-Sevcik expression33, the plots of scan rates vs currents are linear and the slopes correspond to capacitance of the double layer. If an increase in the electroactive surface from capacitance data is significantly smaller than increases in HER or OER electrocatalytic activities, an assumption can be made that simple surface fissuring due to grain rearrangement is not the only (if any) contributor to the strain effects on electrocatalytic activities. Representative capacitance results and analysis are given in Figure 7.
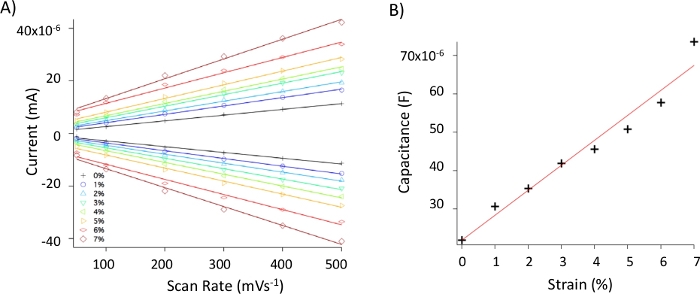
Figure 7: Capacitance measurements. (A) Plot of current vs scan rate from cyclic voltammograms collected within 50 mV of the OCV for TiNi/TiO2 electrodes strained from 0 to 7%, where the slope represents the capacitance of the double-layer. (B) Plot shows changes in capacitance with strain. Please click here to view a larger version of this figure.
To further determine if the changes in electroactivities with strain are due to elastic or inelastic deformation under applied tensile stress, experiments are conducted with pristine and purposely cracked TiO2 films. When 7% strain is imposed on NiTi/TiO2 films, surface fissures are clearly visible on SEM images (Figure 8). Films that were intentionally cracked did not show appreciable changes in electrochemical activity with increasing strain, likely due to the loss in elastic properties (Figure 6D). Samples that were purposely cracked show only small increases in HER activities within the 0-3% strain range, and these increases are irreversible, while pristine samples show significantly larger and reversible effects within the 0-3% strain range.
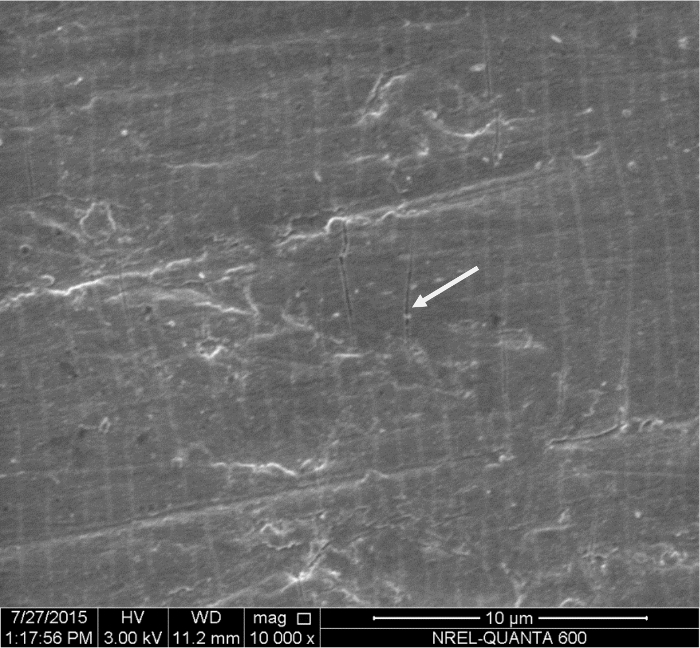
Figure 8: SEM image of the purposely cracked TiNi/TiO2 foils. Please click here to view a larger version of this figure.
When surface characterization experiments are done with instrumentation that require sample enclosure (i.e., vacuum is required), the tensile stretcher cannot be directly connected to the sample in order to keep it under a defined strain. In these cases, custom-made sample holders are used, where the size and geometry are adapted for different instrumentation (Figure 9).
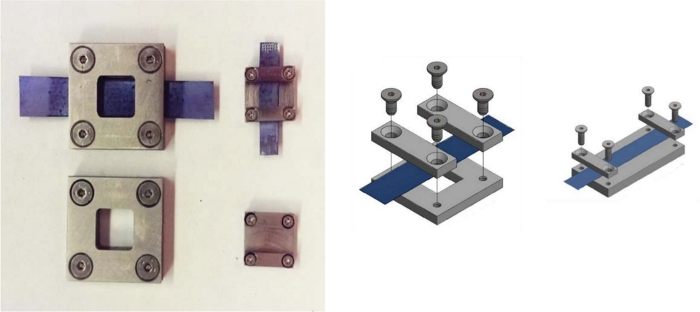
Figure 9: Sample holders used to “lock” NiTi/TiO2 foil under strain for surface characterization experiments. Figure shows different sizes and geometries. Please click here to view a larger version of this figure.
Thermal treating on nitinol typically leads to rutile TiO2 structure. Raman and XPS spectroscopy show characteristic signals for rutile TiO2 thin films34,35 as shown in Figure 10. Specifically, for the highly n-type doped TiO2 films, 0-5% strain primarily effects distribution of oxygen vacancies rather than TiO2 crystal structure, which does not lead to significant changes in XPS spectra21.
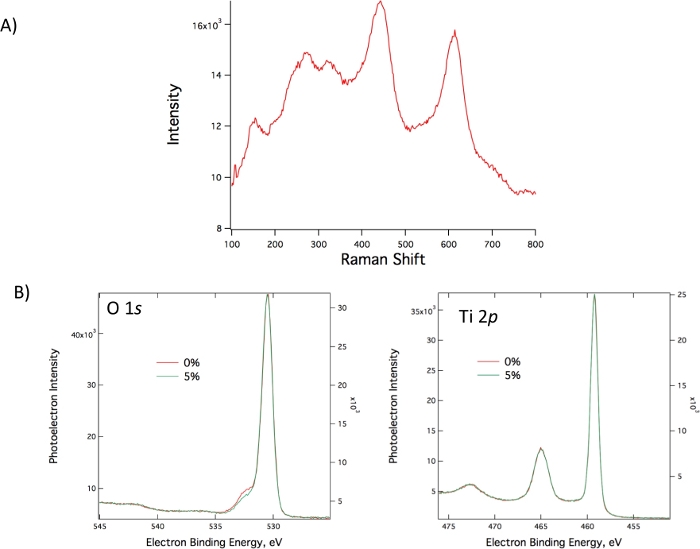
Figure 10: Surface characterization of TiO2 thin films. (A) Raman peaks characteristic for Rutile TiO2. (B) XPS measurements showing spectra for oxygen and titanium surface atoms. This figure has been modified from Benson et al.21. Please click here to view a larger version of this figure.
Discussion
Nitinol is a suitable elastic substrate for applying mechanical stress on thin films. It is commercially available, highly conductive and can be easily functionalized. Preparation of rutile TiO2 thin films by thermal treatment of nitinol, results in highly n-type doped TiO2. It is important to emphasize that NiTi/TiO2 is a unique system where TiO2 films are prepared by thermal treatment of NiTi rather than a deposition method. Our previous publications have shown that strain applied on NiTi/TiO2 primarily effects distribution, diffusion and energy of oxygen vacancies rather than TiO2 crystal structure itself21. Currently reported studies on strained NiTi/TiO2 are incomplete17,21 as they include only effects of tensile and not compressive strain. Compressive and tensile strain imposed on a catalyst structure often have opposite effects on electroactivities and therefore analyzing both is especially interesting for mechanistic studies. Instrumentation and methods presented here have not be tested for compression studies, as it can be challenging to prevent the foil wrinkling upon compression. Compression-tension studies with Nitinol substrate can be conducted using its shape-memory properties, where changes in sample geometry are induced through applied heat as demonstrated previously19.
The described methods can be used to study the effects of dynamic strain on electroactivities of thin films made from different materials and deposited by various methods (e.g., physical or chemical vapor deposition, atomic layer deposition, electrodeposition). For example, dynamic strain applied on copper films deposited on NiTi could be used to tune product selectivity for CO2 electroreduction, as previously demonstrated with Cu films under static strain imposed either by alloying4 or through epitaxial growth36. For each system, the characteristic elastic limit for a deposited film should be determined to achieve reproducible results and high effects of strain. The film elasticity will likely depend on multiple factors: deposited material, deposition method and film thickness as well as film crystallinity and grain structure. Determining an elastic limit can be challenging. For example, surface analysis using SEM does not have high enough resolution to detect nano-scale cracks and/or grain rearrangements; therefore, electrochemical or gas adsorption measurements are more appropriate. Purposely cracked films can be used as a control. Previous studies showed that increases in activities with strain for cracked films were not as significant as with pristine films and effects of strain were irreversible, suggesting that true elastic deformation causes high electroactivities16,17. Interaction between the elastic substrate and a film (adhesiveness) and chemical compatibility are also important. The film deposition method can have significant effect on interaction between the elastic substrate, the adhesion promoter (if any) and thin films. As an alternative to Nitinol, stainless steel could be used as an elastic substrate, where large elasticity range is not required. Stainless steel is chemically compatible with metallic films that can allow good adhesion, especially due to high (~20%) chromium content.
A relatively simple electrochemical cell can be constructed to study effects on strain on various electrochemical systems. Photoelectrochemical experiments with a light-harvesting material deposited on an elastic substrate can also be conducted using the same system when an optically transparent material is placed as the cell window. Effects of strain on photoelectrochemical activities of organic dyes or polymers covalently attached to elastic substrates could also be investigated.
We show that tuning a dynamic strain with a relatively simple experimental setup can be used to find an optimal material structure with improved target activities as well as for tuning electrocatalytic properties in situ. For example, we demonstrate that the low HER activity of TiO2 can reversibly approach those of the state-of-the-art, non-precious metal catalysts when the TiO2 is strained by 3%17. By applying an external mechanical stress, it could be possible do create a precisely controlled, multifunctional catalysts or electro-strain sensors for a range of applications.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was conducted by all co-authors, employees of the Alliance for Sustainable Energy, LLC, the manager and operator of the National Renewable Energy Laboratory for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by the U.S. DOE, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, Solar Photochemistry Program.
Materials
| 2-Propanol | Sigma Aldrich | 109634 | |
| Ag/AgCl (3M NaCl) Reference Electrode | BASi | MF-2052 | |
| Alkaline Reference Electrode | Basi | EF-1369 | |
| Ethyl alcohol, Pure, 200 proof, anhydrous, =99.5% | Sigma Aldrich | 459836 | |
| MT I I / F u l l am SEMTester Series | MTI Instruments | ||
| Nitinol foil, 0.05mm (0.002in) thick, superelastic, flat annealed, pickled surface | Alfa Aesar | 45492 | |
| PK-4 Electrode Polishing Kit | BASi | MF-2060 | |
| Potentiostat 600D | CHI instruments | 600D | |
| Pt wire | Sigma Aldrich | 267228-1G | |
| Sodium hydroxide | Sigma Aldrich | 221465 | |
| Sulfuric acid | Sigma Aldrich | 30743 |
References
- Li, J., Shan, Z., Ma, E. Elastic strain engineering for unprecedented materials properties. MRS Bulletin. 39, 108-114 (2014).
- Luo, M., Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nature Reviews Materials. 2, 17059 (2017).
- Yang, S., Liu, F., Wu, C., Yang, S. Tuning Surface Properties of Low Dimensional Materials via Strain Engineering. Small. 2016, 4028-4047 (2016).
- Clark, E. L., Hahn, C., Jaramillo, T. F., Bell, A. T. Electrochemical CO2 Reduction over Compressively Strained CuAg Surface Alloys with Enhanced Multi-Carbon Oxygenate Selectivity. Journal of the American Chemical Society. 139, 15848-15857 (2017).
- Lu, Z., et al. Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nature Communications. 5, 4345 (2014).
- Sethuraman, V. A., et al. Role of Elastic Strain on Electrocatalysis of Oxygen Reduction Reaction on Pt. The Journal of Physical Chemistry C. 119, 19042-19052 (2015).
- Gu, J., et al. A graded catalytic-protective layer for an efficient and stable water-splitting photocathode. Nature Energy. 2, 16192 (2017).
- Mariano, R. G., McKelvey, K., White, H. S., Kanan, M. W. <a target="_blank" href="http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=PubMed&cmd=Search&doptcmdl=Citation&defaultField=Title+Word&term=Selective+increase+in+CO2+electroreduction+activity+at+grain-boundary+surface+terminations.”>Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science. 358, 1187-1192 (2017).
- Liu, F., Wu, C., Yang, S. Strain and Ligand Effects on CO2 Reduction Reactions over Cu-Metal Heterostructure Catalysts. The Journal of Physical Chemistry C. 121, 22139-22146 (2017).
- Wang, X., et al. Strain Effect in Bimetallic Electrocatalysts in the Hydrogen Evolution Reaction. ACS Energy Letters. 3, 1198-1204 (2018).
- Deng, Q., Smetanin, M., Weissmüller, J. Mechanical modulation of reaction rates in electrocatalysis. Journal of Catalysis. 309, 351-361 (2014).
- Yang, Y., Kumar, S. Elastic Strain Effects on the Catalytic Response of Pt and Pd Thin Films Deposited on Pd-Zr Metallic Glass. Journal of Materials Research. 32, 2690-2699 (2017).
- Yan, K., et al. The Influence of Elastic Strain on Catalytic Activity in the Hydrogen Evolution Reaction. Angewandte Chemie International Edition. 55, 6175-6181 (2016).
- Lee, J. H., Jang, W. S., Han, S. W., Baik, H. K. Efficient Hydrogen Evolution by Mechanically Strained MoS2 Nanosheets. Langmuir. 30, 9866-9873 (2014).
- Yang, Y., Adit Maark, T., Peterson, A., Kumar, S. Elastic strain effects on catalysis of a PdCuSi metallic glass thin film. Physical Chemistry Chemical Physics. 17, 1746-1754 (2015).
- Svedruzic, D., Gregg, B. A. Mechano-Electrochemistry and Fuel-Forming Mechano-Electrocatalysis on Spring Electrodes. The Journal of Physical Chemistry C. 118, 19246-19251 (2014).
- Benson, E. E., et al. Dynamic Tuning of a Thin Film Electrocatalyst by Tensile Strain. Scientific Reports. 9, 15906 (2019).
- Wang, A., et al. Tuning the oxygen evolution reaction on a nickel-iron alloy via active straining. Nanoscale. 11, 426-430 (2019).
- Du, M., Cui, L., Cao, Y., Bard, A. J. Mechanoelectrochemical Catalysis of the Effect of Elastic Strain on a Platinum Nanofilm for the ORR Exerted by a Shape Memory Alloy Substrate. Journal of the American Chemical Society. 137, 7397-7403 (2015).
- Wang, H., et al. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science. 354, 1031-1036 (2016).
- Benson, E. E., et al. Semiconductor-to-Metal Transition in Rutile TiO2 Induced by Tensile Strain. Chemistry of Materials. 29, 2173-2179 (2017).
- Muralidharan, N., et al. Tunable Mechanochemistry of Lithium Battery Electrodes. ACS Nano. 11, 6243-6251 (2017).
- Muralidharan, N., Carter, R., Oakes, L., Cohn, A. P., Pint, C. L. Strain Engineering to Modify the Electrochemistry of Energy Storage Electrodes. Scientific Reports. 6, 27542 (2016).
- Buehler, W. J., Gilfrich, J. V., Wiley, R. C. Effect of Low-Temperature Phase Changes on the Mechanical Properties of Alloys near Composition TiNi. Journal of Applied Physics. 34, 1475-1477 (1963).
- Wang, F. E., Buehler, W. J., Pickart, S. J. Crystal Structure and a Unique “Martensitic” Transition of TiNi. Journal of Applied Physics. 36, 3232-3239 (1965).
- Mavrikakis, M., Hammer, B., Nørskov, J. K. Effect of Strain on the Reactivity of Metal Surfaces. Physical Review Letters. 81, 2819-2822 (1998).
- Hwang, J., et al. Tuning perovskite oxides by strain: Electronic structure, properties, and functions in (electro)catalysis and ferroelectricity. Materials Today. 31, 100-118 (2019).
- Kushima, A., Yip, S., Yildiz, B. Competing strain effects in reactivity of LaCoO3 with oxygen. Physical Review B. 82, 115435 (2010).
- Li, Z., Potapenko, D. V., Osgood, R. M. Controlling Surface Reactions with Nanopatterned Surface Elastic Strain. ACS Nano. 9, 82-87 (2015).
- Petrie, J. R., Jeen, H., Barron, S. C., Meyer, T. L., Lee, H. N. Enhancing Perovskite Electrocatalysis through Strain Tuning of the Oxygen Deficiency. Journal of the American Chemical Society. 138, 7252-7255 (2016).
- Ling, T., et al. Activating cobalt(II) oxide nanorods for efficient electrocatalysis by strain engineering. Nature Communications. 8, 1509 (2017).
- Tavares, C. J., et al. Strain analysis of photocatalytic TiO2 thin films on polymer substrates. Thin Solid Films. 516, 1434-1438 (2008).
- Bard, A. J., Faulkner, L. R. . Electrochemical Methods: Fundamentals and Applications. , (2001).
- Frank, O., et al. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Physical Chemistry Chemical Physics. 16, 14567-14572 (2012).
- Metikoš-Huković, M., Katić, J., Milošev, I. Kinetics of passivity of NiTi in an acidic solution and the spectroscopic characterization of passive films. Journal of Solid State Electrochemistry. 16, 2503-2513 (2012).
- Reske, R., et al. Controlling Catalytic Selectivities during CO2 Electroreduction on Thin Cu Metal Overlayers. The Journal of Physical Chemistry Letters. 4, 2410-2413 (2013).

