Investigating Target Gene Function in a CD40 Agonistic Antibody-induced Colitis Model using CRISPR/Cas9-based Technologies
Summary
Here, we describe the methodology to knock out a gene of interest in the immune system using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated endonuclease (Cas9)-based technologies and the evaluation of these mice in a cluster of differentiation 40 (CD40) agonistic antibody-induced colitis model.
Abstract
The immune system functions to defend humans against foreign invaders such as bacteria and viruses. However, disorders of the immune system may lead to autoimmunity, inflammatory disease, and cancer. The inflammatory bowel diseases (IBD)-Crohn’s disease (CD) and ulcerative colitis (UC)-are chronic diseases marked by relapsing intestinal inflammation. Although IBD is most prevalent in Western countries (1 in 1,000), incident rates are increasing around the world. Through association studies, researchers have linked hundreds of genes to the pathology of IBD. However, the elaborate pathology behind IBD and the high number of potential genes pose significant challenges in finding the best therapeutic targets. Additionally, the tools needed to functionally characterize each genetic association introduce many rate-limiting factors such as the generation of genetically modified mice for each gene. To investigate the therapeutic potential of target genes, a model system has been developed using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated endonuclease (Cas9)-based technologies and a cluster of differentiation 40 (CD40) agonistic antibody. The present study shows that CRISPR/Cas9-mediated editing in the immune system can be used to investigate the impact of genes in vivo. Limited to the hematopoietic compartment, this approach reliably edits the resulting reconstituted immune system. CRISPR/Cas9-edited mice are generated faster and are far less expensive than traditional genetically modified mice. Furthermore, CRISPR/Cas9 editing of mice has significant scientific advantages compared to generating and breeding genetically modified mice such as the ability to evaluate targets that are embryonic lethal. Using CD40 as a model target in the CD40 agonistic antibody-induced colitis model, this study demonstrates the feasibility of this approach.
Introduction
Autoimmune diseases refer to conditions in which a patient's immune system attacks their own cells and organs, resulting in chronic inflammation and tissue damage. Nearly 100 different types of autoimmune conditions have been described to date, affecting 3-5% of the human population1. Many of the autoimmune conditions, including systemic lupus erythematosus and IBD, lack effective treatments and present significant unmet medical needs. Currently affecting around 1.5 million people in the USA alone, IBD is a devastating disease marked by progressive, persistent, and relapsing intestinal inflammation with no available cure. Unraveling the underlying pathogenesis and pathophysiology is needed to deliver the novel treatment and prevention strategies that IBD patients require2,3.
Over 230 different IBD loci have been identified through genome-wide association analyses (GWAS)4. Although these associations have elucidated new genes that are potentially important players in the key mechanisms and pathways of IBD, only a few genes from these loci have been studied. Some genes have been implicated in specific pathways. For example, the microbe-sensing pathway has been linked to nucleotide-binding oligomerization domain-containing protein 2 (NOD2); the autophagy pathway has been linked to autophagy-related 16 like 1 (ATG16L1), immunity-related GTPase family M (IRGM), and caspase recruitment domain family member 9 (CARD9); and the pro-inflammatory pathway has been linked to interleukin (IL)-23-driven T-cell responses4. Various in vivo mouse models have been used to functionally characterize genes identified through GWAS5,6.
One of the key models used to study IBD pathogenesis7,8 is the CD40 model of colitis, which induces innate immune intestinal inflammation following the injection of a CD40 agonistic antibody into immunodeficient (T and B-cell) mice. Primarily used to examine the contribution of innate immunity to IBD development, mostly macrophages and dendritic cells9, it is unclear if disease can be induced in fully immune-competent wild-type (WT) mice. In addition to animal models, gene-specific tools are also required for the functional characterization of a gene, including chemical compounds and biologics. More importantly, genetically modified animals are essential in revealing the function of a specific gene. However, the strategies typically used to make genetically modified mice-embryo injection and breeding-often take over a year and incur a significant financial cost. This rate-limiting process presents a significant challenge in the quest to elucidate the functions of the IBD-related genes identified by GWAS.
The protocol presented here provides a viable alternative to breeding genetically modified mice. First, as shown in the Figure 1 schematic, lineage-negative, stem cell antigen1-positive, receptor tyrosine kinase Kit-positive (lineage-Sca1+c-Kit+ or LSK) cells are isolated from the bone marrow of Cas9 knockin (KI) mice bearing a specific allele (CD45.2) to allow donor immune cell tracking. Next, these cells are exposed to lentiviruses bearing different guide RNAs (gRNAs) and a fluorescent marker, violet-excited green fluorescent protein (VexGFP), to allow tracking of transduced cells. Two days later, VexGFP+ cells are sorted and injected into lethally irradiated recipient Ly5.1 Pep Boy mice, which are C57Bl/6 mice bearing the CD45.1 allele to allow for recipient immune cell tracking. Twelve weeks later, the immune system is fully reconstituted, and the mice can be enrolled into in vivo models.
In addition to the benefit of cost savings and faster time-to-generation compared to the generation and breeding of genetically modified animals, this methodology is ideal for targets that are embryonic lethal, as it specifically targets the hematopoietic compartment. Furthermore, for targets where there are no tools available, such as an antibody, this system provides a feasible approach. In summary, to address the challenges described thus far, an in vivo CRISPR/Cas9-based genome editing platform was developed to expeditiously generate genetically modified animal models10,11,12,13,14. This study demonstrates that intestinal inflammation in WT C57Bl/6 mice can be induced by a CD40 agonistic antibody. CD40 is a key regulator of disease in this model and was therefore used as a model target to validate the CRISPR/Cas9-based knockout and loss of gene function.
Protocol
All animal experiments performed following this protocol must be approved by the respective Institutional Animal Care and Use Committee (IACUC). All procedures described here were approved by the AbbVie IACUC.
1. Generation of required lentiviruses and procurement of donor and recipient animals
NOTE: The Table of Materials includes source and order number details for all animals, instruments, and reagents used in this protocol.
- Construct the plasmids using a lentiGuide-puro vector modified to VexGFP or mCherry.
- Clone the scrambled non-targeting gRNA (SgNone) or CD40-targeting gRNA into the modified vector.
NOTE: In this study, the sequences for each gRNA were as follows: SgNone, CTATGATTGCAACTGTGCAG; SgCD40.1, AGCGAATCTCCCTGTTCCAC; SgCD40.2, GACAAACAGTACCTCCACGA; and SgCD40.3, ACGTAACACACTGCCCTAGA. - Produce the lentiviral particles as described previously15.
- Co-transfect the gRNA-encoding plasmids into 293T cells with VSV-G and pLEX packaging plasmids.
- Change the culture medium after 18 h of transfection, and collect the supernatants containing the virus after 24-48 h following the medium change.
- To evaluate CD40 gRNA efficiency, generate a CD40 stable 293T cell line by transfection of a pcDNA3.1 plasmid that encodes for CD40.
- Transfect the cells using the described lentiviruses, and evaluate them by fluorescence-activated cell sorting (FACS) two weeks later.
NOTE: Cas9 KI mice were used as donor mice. Ensure that the Cas9 KI mice are on a C57Bl/6 background (and are expressing the CD45.2 allele) when using C57Bl/6-Ly5.1 Pep Boy mice (expressing the CD45.1 allele) as recipients, so GvHD is avoided and to track donor and recipient cells.
- Clone the scrambled non-targeting gRNA (SgNone) or CD40-targeting gRNA into the modified vector.
- Design the experiment. For gene knockout (KO), use 3-6 gRNAs per gene. For each gRNA, prepare 20% extra recipient mice to account for animal loss during and after transplantation.
NOTE: The CD40 agonistic antibody-induced colitis model requires a minimum of 8 mice per group for statistical powering. Hence, 10 mice per gRNA were prepared in this study. The number of mice needed for other in vivo models will vary based on the model used. - Use same sex donor and recipient mice that are 8-12 weeks of age.
NOTE: Mice were euthanized under IACUC approved guidelines using a minimum 5% isoflurane and confirmed with cervical dislocation as a secondary method.
2. Bone marrow harvest and preparation for cell sorting
- Using forceps and scissors, harvest bones from each donor mouse (femur, tibia, humerus, and ulna), carefully removing as much muscle/tissue as possible.
- Puncture the bottom of a 0.6 mL microcentrifuge tube with a 23 G needle, and place the tube inside a 1.5 mL centrifuge tube. Make 2 sets per animal.
- Cut one end of the bones open, and place 4-8 bones inside the microcentrifuge tube with open ends facing toward the 23 G needle hole.
NOTE: The number of bones per tube depends on the bone, e.g., 8 tibiae, humeri, and ulnae or 4 femurs will fit. - Centrifuge the tubes (with the 0.6 mL microcentrifuge tube containing the bones inside the 1.5 mL centrifuge tube) at 300 × g for 2 min at room temperature (RT).
- Discard the 0.6 mL microcentrifuge tube, now containing bones with no marrow, leaving just the 1.5 mL centrifuge tube now full of marrow. Add 1 mL of red blood cell lysis buffer (containing ammonium chloride) to each centrifuge tube, and resuspend the cell pellet by pipetting. Incubate at RT for 1 min. Repeat the lysis step if needed.
- Transfer the cell suspension into a 50 mL conical tube, and add Dulbecco's phosphate buffered saline (DPBS) without calcium and magnesium (at least double the volume of the cell suspension) to neutralize the lysis buffer. Pellet cells at 300 × g for 5 min. Aspirate the supernatant completely.
- Load a 70 µm filter on a 50 mL conical tube, and filter the cells through, using DPBS to wash the tube and filter. Discard the filter, and count the cells.
NOTE: Using 30 donor mice (12 weeks old) will yield 200-300 × 106 cells on average.
3. Cell sorting to isolate LSK cells for transduction
- Re-suspend the cells counted in step 2.7 in magnetic cell sorting (MACS) buffer (DPBS, 2 mM ethylenediamine tetraacetic acid, 0.5% bovine serum albumin, 10 µg/mL penicillin-streptomycin) in the desired volume (90 µL per 107 cells).
- Add 10 µL of CD117+ beads per 107 cells. Mix well, protect from light, and incubate at 4 °C for 15 min.
- Prepare the MACS LS column by placing the column in the magnetic field and rinsing it with MACS buffer.
- Wash the cells from step 3.2 with an appropriate volume of MACS buffer (at least double your volume), and centrifuge at 300 × g for 10 min.
- Aspirate the supernatant completely. Re-suspend the pellet so that the final concentration is 108 cells in 500 µL of MACS buffer.
- Apply the cell suspension onto the LS column. Wash with 3 times with 3 mL of MACS buffer.
NOTE: One LS column can take up to 108 labeled cells and 2 × 109 total cells. - Remove the column from the magnetic separator, and place it in a suitable collection tube. Add 5 mL of MACS buffer, and immediately flush out the magnetically labeled cells by firmly pushing the plunger into the column.
- Pellet the cells at 300 × g for 10 min. Aspirate the supernatant completely.
- Re-suspend the cells in 100 µL of MACS buffer per 107 cells, and add the staining antibodies for lineage (CD3, B220, Ter119, Gr-1, CD11b) and Sca-1.
NOTE: Using 30 donor mice (12 weeks old) will yield 100-180 × 106 cells on average. - Mix well, protect from light, and incubate at 4 °C for 20 min.
- Wash the cells by adding 5-10 mL of MACS buffer, and centrifuge at 300 × g for 10 min. Aspirate the supernatant completely.
- Re-suspendup to 108 cells in 500 µL of MACS buffer
- Filter the cells with a 70 µm cell strainer, and perform FACS for lineage-Sca1+ population.
NOTE: Approximately 1.8-2.6% of the population should be lineage-Sca1+. - Collect the cells in LSK culture medium (100 µL per 10,000 cells): serum-free expansion medium, 100 ng/mL thrombopoietin, mouse stem cell factor, Fms-related tyrosine kinase 3 ligand, and IL-7 with 100 µg/mL penicillin-streptomycin.
- Pellet cells at 300 × g for 10 min. Aspirate the supernatant completely.
4. LSK transduction and culture to generate control and knockout cells
- Resuspend the cells in LSK culture medium.
- Seed 10,000 cells per well in 96-well flat-bottom tissue culture (TC) plates in LSK culture medium.
- Incubate overnight at 37 °C with 5% CO2 and 95% humidity under aseptic conditions.
- Prepare 50 µg/mL retronectin solution in DPBS, and add 300 µL to each well of a non-TC-treated 24-well polystyrene plate. Incubate overnight at 4 °C.
- The next day, discard the retronectin solution, rinse the coated plate with 300 µL of DPBS, and repeat.
- Transfer 50,000 LSK cells in 500 µL of medium (5 wells from the 96-well plate in step 4.2) to each retronectin-coated well of the 24-well polystyrene plate from step 4.4.Use an additional 100 µL of LSK medium per 5 wells to rinse and collect any extra cells remaining in the 96-well plate. Ensure that 600 µL is the volume per well in the 24-well plate following this step (5 × 100 µL wells from the 96-well plate + 100 µL used to wash those 5 wells).
NOTE: After transferring, look under a microscope at the now empty 24 well plate to confirm that all cells were collected. Use LSK medium to collect cells that were not transferred to minimize cell loss from this small population. - Add 300 µL of virus supernatant to each well, and shake the plate at a setting value of 500 for 5 min. Spin the plate at 600 × g and 37 °C for 20 min. Use at least 5 × 106 viral particles per mL.
NOTE: In this study, a vector modified from pLentiPuro was used, in which the puromycin resistance element was swapped to VexGFP. The virus was added at a multiplicity of infection of 50-100. - Incubate for 1 h. Add 500 µL of pre-warmed LSK medium.
- Incubate for 2 days at 37 °C with 5% CO2 and 95% humidity under aseptic conditions.
5. Animal irradiation to prepare for donor stem cell engraftment
- After 2 days of incubation, irradiate the recipient animals with 475 cGy twice at an interval of 4 h. Following the second round of irradiation, place the animals in autoclaved cages, and treat them as immunodeficient animals for 12 weeks. Mice received enrichment, hydrogel, and cage side monitoring to provide supportive care and intervention as needed. Mice may receive antibiotic food or water in addition to supportive care and intervention methods.
NOTE. The irradiation dosages may differ with different irradiators. Perform a dose titration of the irradiator to identify the best dosage. Doses between 700 and 1300 cGy for C57Bl/6 mice are found to be effective in various literature examples17. Select 3-4 doses in this range, and evaluate 5 mice per dose for survival and engraftment. If engraftment is unsuccessful or the radiation dose is too high, mice will not live past 3 weeks post-engraftment. At 4 weeks post-engraftment, bleed the surviving mice to evaluate engraftment by FACS. We perform retro-orbital (RO) blood collections of ~50-100 µL per mouse to evaluate engraftment. Mice were under anesthesia (2-3% isoflurane) during collection and received eye lubricant and 1.0 mL subcutaneous saline as supportive care following RO blood collections.
6. Cell preparation and injection into irradiated recipient animals
- Pipet the cells out of each well from step 4.9, keeping the groups separate now that the cells have been transduced with different gRNAs, and pellet them at 300 × g for 10 min.
- Resuspend the cells in MACS buffer, filter with a 70 µm strainer, and sort the VexGFP+ population.
NOTE: Approximately 10-15% of the cells should be VexGFP+. - Pellet the cells at 300 × g for 10 min. Resuspend them in Hank's balanced salt solution: 5,000 or more cells per mouse in 200 µL.
NOTE: HBSS is non-pharmaceutical grade. You may also consider using a pharmaceutical grade sterile saline for injection. - Inject the cells intravenously 3 h after the last dose of irradiation.
NOTE: Twelve weeks later, the mice will have a fully engrafted immune system and can be enrolled into in vivo models.
7. CD40 agonistic antibody-induced colitis model in wild-type mice
NOTE: Weigh and assess the animals daily. Provide supportive care as needed: 1.0 mL of subcutaneous sodium chloride solution at 10% weight loss or if they are dehydrated. The positive control for this model is anti-p40 dosed intraperitoneally at 25 mg/kg twice per week beginning on day -1.
NOTE: In this study, experimental groups included naïve control, vehicle (negative) control, and anti-p40 (positive) control groups. Together, these groups control for the normal behavior of the CD40 agonistic antibody-induced colitis model. Vector, SgNone, and SgRNA groups: The vector controls for the common lentiviral vector, SgNone is a scrambled non-targeting guide control, and the SgRNA groups are the "treatment" groups bearing reduced expression of the target gRNA.
- Day 0: Inject CD40 agonistic antibody at 10 mg/kg intraperitoneally in DPBS into all animals except for naïve control.
- Days 3 and 6: Perform video endoscopy to evaluate disease progression as previously described16.
- Anesthetize the mice with 2-3% isoflurane. Provide thermal support via internal anesthesia machine mechanism and/or external heat pad during recovery.
- Once the mice are under anesthesia, administer a DPBS (without calcium and magnesium) enema to prepare the colon for endoscopy.
NOTE: Approximately 1-2 mL is required to perform an enema. - After an enema and while under anesthesia, move the mouse to the nose cone of the anesthesia machine.
- Gently insert the endoscope into the colon, slowly advance to the proximal colon while keeping the camera centered and the colon inflated with air (using either the included air pump or attach a tube/syringe to manually inflate), then slowly withdraw the endoscope and collect a video and images at 3 cm, 2 cm, and 1 cm from the anus.
NOTE: To minimize irritation of the colon, sterile lubricant may be added to the tip of the endoscope. - Score each image individually based on the vascular pattern and thickening of the mucosa as described in Table 1. Combine the scores from the 3 images per mouse to assign a sum score to each animal.
- Day 7: Euthanize all mice by isoflurane overdose or CO2 chamber, and collect as much blood as possible by cardiac puncture for serum. Weigh the spleen to measure splenomegaly and collect for flow cytometry, and collect the colon for histopathology.
NOTE: Formalin-fixed and paraffin-embedded tissue sections of mouse colon were prepared and used for immunohistochemistry (IHC) as previously described by Wang et al19.
Representative Results
Following the procedure described above, mice expressing CD40-targeted gRNA were generated. By week 2, B-cells, CD11b+ macrophages, and CD11c+ dendritic cells (DCs) were engrafted (Figure 2). T-cells however, as expected based on previous literature18, took longer to fully engraft and required 12 weeks post-engraftment to reach ~90% (Figure 2). Immune organs, such as the spleen and lymph nodes, had the most notable population of donor-derived cells; however, other organs including the liver, lung, and intestine also showed the presence of donor cells (Figure 3). A strong reduction in CD40 expression was observed only in mice expressing CD40-targeting gRNA (Figure 4).
Similar to immunodeficient mice, following the CD40 agonistic antibody injection, WT C57Bl/6J mice exhibited body weight loss, vascular loss, and mucosal thickening as observed by colonoscopy, and myeloid cell infiltration determined by IHC with ionized calcium-binding adaptor molecule 1 (IBA1) (Figure 5). In addition to the typical readouts for the CD40 agonistic antibody-induced colitis model, an adaptive immune response was also observed, as shown by CD3 IHC revealing T-cell infiltration in the colon and by FACS analyses revealing T and B cell activation through CD86 upregulation on splenocytes (Figure 5). Importantly, an anti-p40 monoclonal antibody inhibited disease induction (Figure 5), which was consistent with the findings with the model using immunodeficient strains9.
A reduction in CD40 expression by targeted gRNA protected the mice from CD40 agonistic antibody-induced colitis. The degree of protection correlated with the editing efficiency of each gRNA (Figure 6). More specifically, SgCD40.1 was the most efficient gRNA (Figure 4), which led to the most powerful disease inhibition, as indicated by CD86 upregulation and intestinal infiltrate of immune cells (Figure 6D,E). Conversely, SgCD40.2 had the least editing efficiency of all three gRNAs used in vitro, which resulted in the least protection in vivo; SgCD40.3 exhibited intermediate editing and protection (Figure 6D,E). Taken together, the data shown here reveal the feasibility of using CRISPR/Cas9 to reduce the expression of a target, which can successfully protect from colitis induction. Most importantly, these results demonstrate that this in vivo CRISPR/Cas9-based platform can be used to investigate gene function in the pathogenesis of intestinal inflammation.
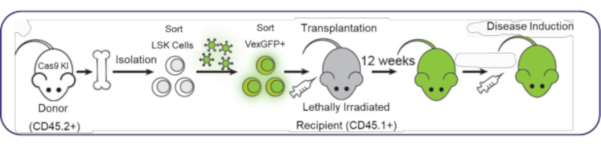
Figure 1: Stem cell harvest, transduction, and transplantation strategy used to generate CRISPR/Cas9-edited mice. Bones are harvested from Cas9 KI mice, bearing the CD45.2 allele as a donor cell marker. Bone marrow is isolated, and the cells are sorted for Lin-Sca1+c-Kit+ (LSK) stem cells. These cells are then exposed to various lentiviruses (vector control, SgNone Control, SgCD40.1), all bearing VexGFP as a fluorescent marker to indicate transduced. Stem cells are then sorted for VexGFP+ cells to inject a pure population of edited stem cells into lethally irradiated recipient mice. The recipient mice are Ly5.1 Pep Boy mice, which are C57Bl/6 WT mice bearing the CD45.1 allele as a recipient cell marker. Abbreviations: KI = knockin; VexGFP = violet-excited green fluorescent protein; WT = wild-type. Please click here to view a larger version of this figure.
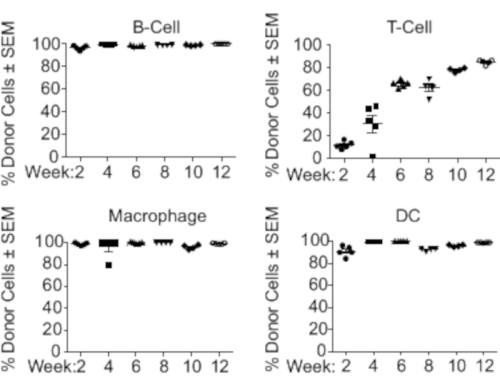
Figure 2: Differentiation of immune cell subsets after LSK transplantation. CD45.2+ donor mice were euthanized, and their LSK cells were isolated and transplanted into lethally irradiated CD45.1 congenic C57Bl/6 animals. Every other week following transplantation, a small cohort of mice (n=5) was euthanized, and the engraftment rates in the spleen, bone marrow, and blood were evaluated by FACS. The percentage of donor cell subsets at different timepoints post-transplantation is shown. Each dot represents a data point from a single animal. Abbreviations: LSK = Lin-Sca1+c-Kit+; FACS = fluorescence-activated cell sorting; DC = dendritic cell; SEM = standard error of the mean. Please click here to view a larger version of this figure.
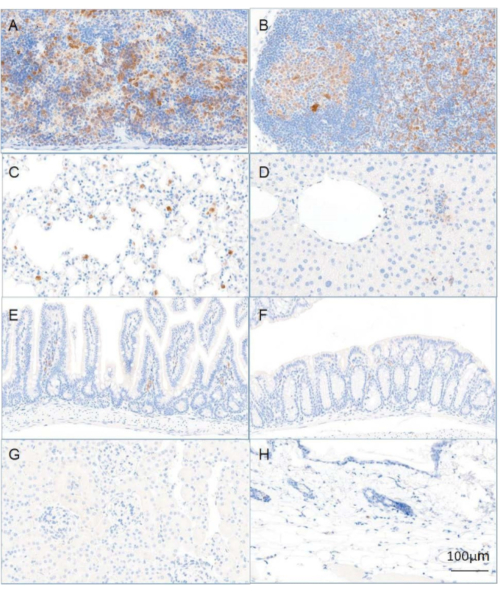
Figure 3: Distribution of donor LSK-differentiated cells in reconstituted animals. Recipient animals were reconstituted using LSK cells infected with mCherry-expressing virus. Tissues were harvested at week 12 post-transplantation and mCherry expression (brown) was evaluated by IHC: (A) spleen, (B) mesenteric lymph nodes, (C) lung, (D) liver, (E) small intestine, (F) large intestine, (G) kidney, (H) skin. This figure has been modified from Wang et al.19. Scale bar = 100 µm. Abbreviations: LSK = Lin-Sca1+c-Kit+; IHC = immunohistochemistry. Please click here to view a larger version of this figure.
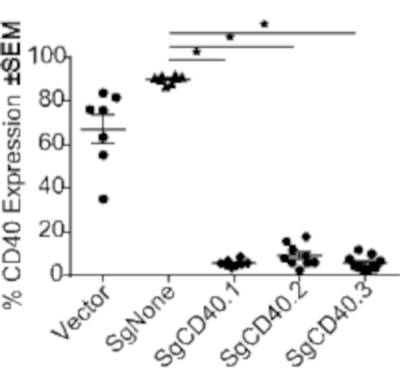
Figure 4: CRISPR/Cas9-mediated reduction in CD40 expression on B-cells. CD40 expression reduction in reconstituted mice, modulated using a CRISPR/Cas9-based platform. Splenocytes were evaluated 8 weeks post-transplantation by FACS for CD40 expression on B-cells. Each dot represents an individual animal. *p<0.005 Data shown are representative of two independent experiments. Abbreviations: FACS = fluorescence-activated cell sorting; SEM = standard error of the mean; Sg = single guide RNA. Please click here to view a larger version of this figure.
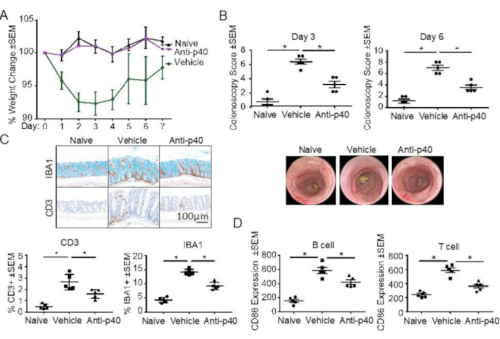
Figure 5: CD40 agonistic antibody-induced intestinal inflammation in C57Bl/6 mice. CD40 agonistic antibody was injected into C57Bl/6 mice to induce inflammation, and disease induction was evaluated based on (A) body weight change, (B) colonoscopy at day 3 and day 6 post-CD40 agonistic antibody injection, (C) percentage of IBA1+ and CD3+ areas of total mucosal area, mucosal thickness, as well as percentage of goblet cell area of total mucosal area, and (D) upregulation of CD86 expression in splenic B and T cells. In (B), representative images for day 6 colonoscopy are shown. In (C), representative images for day 7 histology are shown. Scale bar = 100 µm. *p<0.001 Data are representative results from two independent experiments. This figure has been modified from Wang et al19. Abbreviations: IBA1 = ionized calcium-binding adaptor molecule 1; SEM = standard error of the mean. Please click here to view a larger version of this figure.
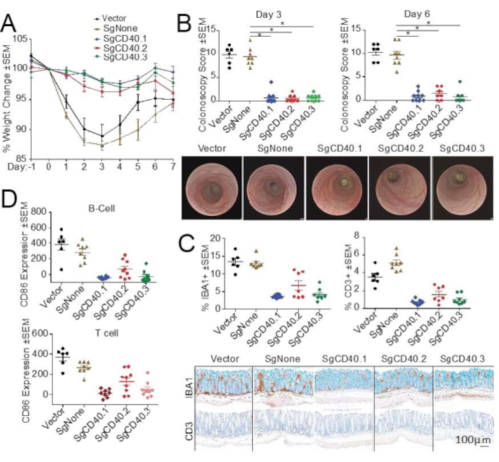
Figure 6: CRISPR/Cas9-based knockout of CD40 ameliorates disease pathogenesis in a CD40 agonistic antibody-induced colitis model. LSK cells were infected with lentivirus-expressing control or SgCD40 and sorted for VexGFP. VexGFP+ cells were used to transplant lethally irradiated CD45.1+ recipient mice (n=10). Twelve weeks post-transplantation, colitis was induced by injecting CD40 agonistic antibody. (A–D) Disease induction was assessed by (A) body weight change, (B) colonoscopy at day 3 and day 6 post-CD40 agonistic antibody injection, (C) percentage of IBA1+ and CD3+ cell areas of total mucosal area, mucosal thickness as well as percent of goblet cell area of total mucosal area, and (D) upregulation of CD86 expression in splenic B and T cells. Representative (B) day 6 colonoscopy images and (C) day 7 histology images are included. Each dot represents an individual animal. Scale bar = 100 µm. *p<0.001. Data shown are representative of two experiments. This figure has been modified from Wang et al19. Abbreviations: IBA1 = ionized calcium-binding adaptor molecule 1; LSK = Lin-Sca1+c-Kit+; VexGFP = violet-excited green fluorescent protein; Sg = single guide RNA; SEM = standard error of the mean. Please click here to view a larger version of this figure.
| Score | Vasculature | Thickening |
| 0 | The small and large blood vessels are bright, sharp, and have a continuous pattern, | The surface of the colon is smooth and shiny. |
| 1 | The small and large blood vessels are visible, but not connecting and out of focus. | The mucosal wall is less transparent and slightly bumpy with a shiny mucous layer. |
| 2 | The large blood vessels are still visible, but discontinuous, and a number of small vessels appear to have burst. | There is a clear white, shiny, bumpy layer covering most of the circumference. |
| 3 | No blood vessels are visible, and the surface of the colon is very bumpy. | There is an opaque, white, bumpy surface covering the circumference. |
Table 1: CD40 agonistic antibody-induced colitis endoscopy scoring scale. Images of the colon were collected at 3 cm, 2 cm, and 1 cm from the anus. Each image was then evaluated for vascularity and thickening, scoring each parameter from 0-3 as indicated. The total score from all 3 images per animal was then combined to assign a sum endoscopy score to each animal.
Discussion
The results shown here introduce a novel CRISPR/Cas9-based genome editing platform capable of investigating gene function in this CD40 agonistic antibody-induced colitis model. Cell sorting enriched the pool of genetically modified LSK cells, resulting in over 90% reduction in CD40 expression within the reconstituted animals-in just 4 months. Furthermore, the reduced expression of CD40 within the immune system had a profound effect within the CD40 agonistic antibody-induced colitis model, significantly reducing disease endpoints. Based on these results, an in vivo CRISPR/Cas9-based platform was established, which provides researchers with a powerful tool to study a gene's function within the immune system. This technological advancement will expedite the process of validating new target biology, and ultimately, the ability to deliver transformative therapies to IBD and autoimmune patients in need.
The CRISPR/Cas9-based platform presented here enables efficient and efficacious gene modulation in WT C57Bl/6 mice. As the platform utilizes lethal irradiation and bone marrow transplants, it is expected that animals will be lost prior to complete engraftment. Therefore, powering the groups by 20% extra mice will help to account for these losses. As LSK cells isolated after 5-fluorouracil treatment had reduced c-kit expression, cell sorting is recommended to isolate LSKs. Additionally, high-titer virus should be used after concentration via ultracentrifugation. However, despite the high titer, the viruses did not efficiently infect LSK cells in this study. Perhaps ultracentrifugation concentrated the inhibitory factors, necessitating the use of a sucrose gradient to improve efficiency.
Usually the CD40 agonistic antibody-induced colitis model is used with immunodeficient recombination-activating gene (RAG) and severe combined immunodeficient (SCID) mice, as the primary value in the model is to evaluate innate immunity rather than adaptive immunity. Induced mice exhibit body weight loss, splenomegaly, intestinal inflammation, and myeloid cell infiltration in the colon. Demonstrated here, wild-type C57Bl/6 mice respond similarly to the CD40 agonistic antibody and to the positive control, anti-p40, which inhibited disease. The two main differences between the WT C57Bl/6 mice and immunodeficient mice are 1) WT C57Bl/6 mice require twice the dose of the CD40 agonistic antibody and 2) WT C57Bl/6 mice can have reduced disease levels on day 6 compared to day 3, as measured by endoscopy. Generally, immunodeficient mice maintain a similar level of disease from day 3 to day 6, sometimes showing exacerbation. More experiments are needed to definitively elucidate the cause of this difference, but the hypothesis points to regulatory T-cells, which are not present in immunodeficient mice, limiting and/or reversing disease a week after CD40 agonism.
In conclusion, this in vivo CRISPR/Cas9-based platform utilizes and combines LSK transplantation with CRISPR gene editing to efficiently reduce the expression of target genes within the immune system. With the potential to edit multiple genes in LSK cells via CRISPR/Cas9 in concert, this platform may provide the opportunity to evaluate digenic or polygenic phenotypes seen in IBD patients. Critical to efficiently and effectively assessing genes linked to IBD patients, this platform expedites the ability to accurately evaluate the function of genes within the immune system, which will reduce the time from discovery to the development of life-changing therapeutics.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Thank you to Ruoqi Peng, Donna McCarthy, Jamie Erikson, Liz O'Connor, Robert Dunstan, Susan Westmoreland, and Tariq Ghayur for your efforts supporting this work. Thank you to Pharmacology leaders including Rajesh Kamath and others for their leadership in establishing the CD40 agonistic antibody-induced colitis model in WT C57Bl/6 mice. Additionally, thank you to all those at AbbVie Bioresearch Center and Cambridge Research Center in the Comparative Medicine East Department supporting in vivo experiments.
We would like to thank the Zhang lab from the Broad Institute and McGovern Institute of Brain Research at the Massachusetts Institute of Technology for providing CRISPR reagents [multiplex Genome Engineering Using CRISPR/Cas Systems. Cong, L, Ran, FA, Cox, D, Lin S, Barretto, R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F Science. 2013 Jan 3].
Materials
| 6-well tissue culture plates | Corning/Costar | #3506 | |
| TransIT-LT1 | Mirus Bio | MIR 2300/5/6 | |
| MACS Buffer (autoMACS Running Buffer) | Miltenyi Biotec | 130-091-221 | |
| 0.45 µm filter unit | Millipore | #SLHV013SL | |
| 0.6 mL microcentrifuge Tube | Axygen | MCT-060-C-S | |
| 1.5 mL Eppendorf Tube | Axygen | MCT-150-C-S | |
| 15mL Conical | VWR | 21008-918 | |
| 23 G Needle | VWR | #305145 | |
| 24 Well Non-TC Plates | Falcon | #351147 | |
| 24-Well TC Plates | Falcon | #353047 | |
| 50 mL Conical tube | VWR | 21008-951 | |
| 5 mL Syringe | BD Biosciences | #309647 | |
| 70 µm Filter | Miltenyi | #130-098-462 | |
| 96-Well Flat Bottom Plates | Corning | #3599 | |
| 96-Well U-Bottom Plates | Corning/Costar | #3365 | |
| Anesthesia Machine | VetEquip – COMPAC5 | #901812 | |
| Anti-CD40 Agonist monoclonal antibody | BioXcell | BE-0016 | |
| Anti-p40 monoclonal antibody | BioXcell | BE-0051 | |
| B220 PE Antibody | BioLegend | #103208 | |
| Bovine serum albumin | Sigma Aldrich | A7906-100G | |
| Cas9 Knock-in Mice | Jackson Labs | #026179 | C57Bl/6 background |
| CD117+ Beads | Miltenyi | #130-091-224 | |
| CD11b PE Antibody | BioLegend | #101208 | |
| CD3 PE Antibody | BD Biosciences | #553240 | |
| Centrifuge | Beckman Coulter | Allegra 6KR Centrifuge | |
| Countertop Centrifuge | Eppendorf | Centrifuge 5424 | |
| DPBS | ThermoFisher | #14190136 | |
| Dulbecco’s Modified Eagle Medium | Mediatech | #10-013-CV | |
| Ethylenediamine tetraacetic acid (EDTA) | Invitrogen | AM9260G | |
| Endoscope | Karl Storz | N/A | Custom Coloview Tower |
| Flow cytometer | BD Biosciences | FACS Aria II | |
| Fms-related tyrosine kinase 3 ligand (Flt-L) | PeproTech | #250-31L | |
| Gr-1 PE Antibody | BD Biosciences | #553128 | |
| Hank's balanced salt solution (HBSS) | ThermoFisher | #14170120 | |
| Heat-Inactivated Fetal Bovine Serum | HyClone | #SH30071.03 | |
| IL-7 | PeproTech | #217-17 | |
| Incubator | Binder | #9040-0116 | |
| Isoflurane | HenrySchein | #6679401710 | |
| LS Column | Miltenyi | #130-042-041 | |
| Ly5.1 Pepboy Mice | Jackson Labs | #002014 | C57Bl/6 background |
| mouse stem cell factor (mSCF) | PeproTech | #250-03 | |
| Sodium chloride (NaCl) | Hospira | #00409488850 | |
| OPTI-MEM serum-free media | Invitrogen | #31985-070 | |
| Penicillin-streptomycin (PenStrep) | ThermoFisher | #15140-122 | |
| Plate Shaker | ThermoFisher | #88880023 | |
| pLentiPuro | Addgene | #52963 | |
| Polybrene (10 µg/µL) | Sigma Aldrich | #TR-1003-G | |
| Red Blood Cell Lysis Buffer | eBioscience | #00-4333 | |
| Retronectin | Takarbio | #T100B | |
| Sca-1 APC Antibody | BioLegend | #108112 | |
| StemSpan | StemCell Technologies | #09600 | |
| Ter119 PE Antibody | eBioscience | #12-5921 | |
| Thrombopoietin (TPO) | PeproTech | #315-14 | |
| X-ray Irradiator | Precision X-Ray | X-Rad 320 |
References
- Wang, L., Wang, F. S., Gershwin, M. E. Human autoimmune diseases: a comprehensive update. Journal of Internal Medicine. 278 (4), 369-395 (2015).
- Uhlig, H. H., Powrie, F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annual Review of Immunology. 36, 755-781 (2018).
- Gajendran, M., Loganathan, P., Catinella, A. P., Hashash, J. G. A comprehensive review and update on Crohn’s disease. Disease-a-Month. 64 (2), 20-57 (2018).
- Uhlig, H. H., Muise, A. M. Clinical genomics in inflammatory bowel disease. Trends in Genetics. 33 (9), 629-641 (2017).
- Sollid, L. M., Johansen, F. E. Animal models of inflammatory bowel disease at the dawn of the new genetics era. PLoS Medicine. 5 (9), 198 (2008).
- Jiminez, J. A., Uwiera, T. C., Douglas Inglis, G., Uwiera, R. R. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathogens. 7, 29 (2015).
- Kiesler, P., Fuss, I. J., Strober, W. Experimental models of inflammatory bowel diseases. Cell Molecular Gastroenterology and Hepatology. 1 (2), 154-170 (2015).
- Mizoguchi, A. Animal models of inflammatory bowel disease. Progress in Molecular Biology and Translational Science. 105, 263-320 (2012).
- Uhlig, H. H., et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 25 (2), 309-318 (2006).
- Dow, L. E. Modeling disease in vivo With CRISPR/Cas9. Trends in Molecular Medicine. 21 (10), 609-621 (2015).
- Hochheiser, K., Kueh, A. J., Gebhardt, T., Herold, M. J. CRISPR/Cas9: A tool for immunological research. European Journal of Immunology. 48 (4), 576-583 (2018).
- Ran, F. A., et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 8 (11), 2281-2308 (2013).
- Fellmann, C., Gowen, B. G., Lin, P. C., Doudna, J. A., Corn, J. E. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nature Reviews Drug Discovery. 16 (2), 89-100 (2017).
- Yin, H., et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nature Biotechnology. 32 (6), 551-553 (2014).
- Bagley, J., Tian, C., Iacomini, J. Prevention of type 1 diabetes in NOD mice by genetic engineering of hematopoietic stem cells. Methods in Molecular Biology. 2008 (433), 277-285 (2008).
- Becker, C., Fantini, M. C., Neurath, M. F. High resolution colonoscopy in live mice. Nature Protocols. 1 (6), 2900-2904 (2006).
- Duran-Struuck, R., Dysko, R. C. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. Journal of the American Association for Laboratory Animal Science. 48 (1), 11-22 (2009).
- Haynes, B. F., Martin, M. E., Kay, H. H., Kurtzberg, J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. Journal of Experimental Medicine. 168 (3), 1061-1080 (1988).
- Wang, R., et al. CRISPR/Cas9-targeting of CD40 in hematopoietic stem cells limits immune activation mediated by anti-CD40. PLoS One. 15 (3), 0228221 (2020).

.
