Extraction and Dissection of the Domesticated Pig Brain
Summary
This protocol details the technique for removal of the pig brain in its entirety and dissection of several brain regions commonly studied in neuroscience.
Abstract
Use of the pig as a preclinical and translatable animal model has been well-documented and accepted by research fields investigating cardiovascular systems, gastrointestinal systems, and nutrition, and the pig is increasingly being used as a large animal model in neuroscience. Furthermore, the pig is an accepted model to study neurodevelopment as it displays brain growth and development patterns similar to what occurs in humans. As a less common animal model in neuroscience, surgical and dissection procedures on pigs may not be as familiar or well-practiced among researchers. Therefore, a standardized visual protocol detailing consistent extraction and dissection methods may prove valuable for researchers working with the pig. The following video showcases a technique to remove the pig brain while keeping the cortex and brainstem intact and reviews methods to dissect several commonly investigated brain regions including the brainstem, cerebellum, midbrain, hippocampus, striatum, thalamus, and medial prefrontal cortex. The purpose of this video is to provide researchers with the tools and knowledge necessary to consistently perform a brain extraction and dissection on the four-week-old pig.
Introduction
The pig has been well documented and accepted as a translatable animal model for research in cardiovascular systems1, gastrointestinal systems2, nutrition3,4, diabetes5, toxicology6, and surgical techniques7. Use of the pig in neuroscience is beginning to increase, as PubMed searches for the keywords "swine brain animal model" result in four-times more results from 1996-2005 than the preceding 10 year period8, and even more results at present. A primary reason that the popularity of the pig model is expanding is due to its similarities in growth, structure, and function of the brain when compared with humans. In comparison to the human brain, the pig brain exhibits similar gyral patterning, vascularization and distribution of gray and white matter9. Moreover, the pig brain has been used in neuroimaging procedures, evoked potential recording, and in establishing neurosurgery techniques8,9. Unlike other animal models, however, the pig and human experience perinatal brain growth spurts, as opposed to pre- or post-natal growth spurts. At birth, the human and pig brain weigh approximately 27 and 25 percent of their adult brain weight, respectively, compared to the rat brain that weighs 12 percent of its adult brain weight and the rhesus monkey brain at 76 percent of adult weight10.
One reason the pig has been only slowly adopted as an animal model for neuroscience is because many researchers are unfamiliar with the animal in this context. Researchers may not be aware of its potential uses in the field or may not know the proper techniques required to use such a model. As use of the pig as a biomedical and preclinical model gains attention and use in neuroscience, it is necessary to establish standardized procedures of tissue removal to ensure accurate comparison of data across studies. Although dissection and surgical techniques involving the pig brain have been published elsewhere11,12,13, there is a need for simple and standardized protocols to collect pig brain tissue, especially for use in biochemical assays. As such, the aim of this video is to provide the knowledge necessary to allow researchers to perform a standardized brain extraction and dissection. This video illustrates one proper technique to remove the pig brain while keeping the cortex and brainstem intact, and subsequently review methods to dissect several key brain regions.
Protocol
Procedures involving animal subjects have been approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign
NOTE: Prior to euthanasia, the pig was anesthetized via intramuscular injection with a combination of telazol:ketamine:xylazine (50.0 mg of tiletamine HCl plus 50.0 mg of zolazepam HCl reconstituted with 2.50 mL of ketamine HCl (100 g/L) and 2.50 mL of xylazine (100 g/L) and administered at 0.06 mg/kg BW). Once anesthetized, the pig was euthanized via intracardiac administration of sodium pentobarbital (390 mg/mL administered at 1 mL/5 kg BW). For brain dissection, it is recommended that the method of euthanasia be chosen based on the desired analytical procedure of the tissue. The method of euthanasia should cause as little damage to the brain as possible.
1. Extraction of the pig brain
- Following humane euthanasia, decapitate the pig by cutting above the nape of the neck, between the first and second vertebra (atlas and axis, respectively).
- Secure the head in an immobilized bench vise modified to include spikes. Ensure that the head is completely immobilized before proceeding.
- Using a scalpel, make a sagittal cut along the midline of the skull that continues to the posterior of the head.
- Make a second (transverse) cut on the posterior of the head.
- Make a third (transverse) cut on the posterior end of the snout and in-line with the eyes. Cut the skin as far away from the skull as possible to ensure easy access to the skull.
- Using a bone saw, make two anterior-to-posterior cuts lateral to the midline, extending from the eyes to the apex of the skull curvature. Bevel the saw towards the midline and cut just deep enough to penetrate the skull.
- Repeat the above process on the perpendicular sides, creating a rectangular "window" in the skull.
- Using a meat hook, pry the rectangular section of the skull off. Begin by placing the hook into one of the corners of the section and apply upward pressure to loosen the skull piece. Take care to place the meat hook only at the level of the skull to prevent inadvertently penetrating the brain tissue.
- If any meningeal layers remain on the brain, use forceps and blunt scissors (or alternatively, a scalpel) to gently remove the layers without cutting into the brain.
- Use a scalpel to cut away muscle and fat at the posterior portion of the head, exposing the posterior portion of the skull.
- Make two transverse cuts along the posterior of the skull. Be sure not to cut into brain tissue.
- Secure the head by placing a firm hand on the snout and apply a backwards pressure to pull the posterior portion of the skull off, exposing the cerebellum.
- Remove the head from the bench vice and place on a surgical mat.
- Use a long slender tool, such as the blunt end of a scalpel, to remove the brain. Invert the head and use a gentle scooping motion to coax the brain out of the skull cavity without damaging the surface of the brain.
NOTE: It will be necessary to sever cranial nerves to remove the brain. Do so gently and do not attempt to forcefully pull the brain out. The brain will fall out on its own when it is properly and completely detached from the skull and spine.
2. Dissection of the pig brain
NOTE: It may be helpful to use a brain atlas or fiber dissection guide14 as a visual representation during dissection procedures. Make sure dissected tissue samples are stored properly according to project-specific needs upon removal of each sample (described in more detail below). Additionally, please note that for the purposes of this video, all brain regions shown were dissected from the right hemisphere, but this may differ per laboratory based on experimental objectives.
- To remove the brainstem (predominantly the medulla), make a coronal cut caudal to the cerebellum.
- To remove the cerebellum, make a coronal cut posterior to the cortex. Isolate desired regions (e.g., vermis, flocculus, etc.) of the cerebellum from this sample. Be sure not to include any portions of the brain stem in this sample.
- Separate the two hemispheres of the brain by making a mid-sagittal cut along the longitudinal fissure. Make this cut as a continuous motion to prevent causing damage to the cortex.
- To remove the midbrain, dissect a desired amount of tissue just ventral to the superior and inferior colliculi.
- To remove the hippocampus, place the blunt end of a scalpel in the posterior portion of the corpus callosum and gently roll the hippocampus out, using a "J" motion starting from the posterior portion of the corpus callosum; an entire hippocampal horn is 'bean-shaped'.
- The striatum (caudate nucleus is primarily shown) is a grey and white matter-striped region just below the corpus callosum and anterior to the hippocampus. Bevel the scalpel and remove this region, revealing striated tissue upon removal.
- To remove the medial prefrontal cortex, dissect tissue from the frontal gyrus to the corpus callosum, removing the most medial portion of that section. The right cortex should remain after removal of the medial prefrontal cortex.
- To remove the thalamus, remove the bulb-like structure at the center of the midsagittal surface of the brain and rostral to the midbrain. The thalamus has a spherical shape and will look slightly darker than the surrounding tissue.
3. Post-dissection
- Upon completion of the dissection, preserve tissue samples properly for subsequent analyses. Omission of this step will result in significant autolysis and degradation within as little as 20 minutes.
- Leave each brain region intact at the time of dissection if structural analyses will be performed, or mince the tissues to create a homogenous tissue sample prior to preservation. Common sample preservation methods for brain tissues include cryopreservation and chemical fixation using crosslinking agents15.
- For standard lab assays (e.g., gene or protein expression), immerse in liquid nitrogen or solutions that stabilize genetic material prior to long-term storage at -80 °C to provide convenient and cost-effective preservation methods.
- If maintaining tissue structure is a priority, preserve brain tissues through traditional fixation methods (e.g., using aldehyde-based fixatives to crosslink proteins), either without or with perfusion of the animal or organ of interest prior to tissue dissection.
Representative Results
This section describes examples of results obtained after correct extraction and dissection of a 4-week-old pig brain. Figure 1 outlines the shape of each brain region for use as a guide during dissection. Part of the brainstem may remain in the skull after removal of the cerebellum (Figure 1B). This can be removed while isolating the desired region of the cerebellum. Table 1 displays the average weight (mean ± standard error of the mean) for each of the dissected brain regions (n=5).
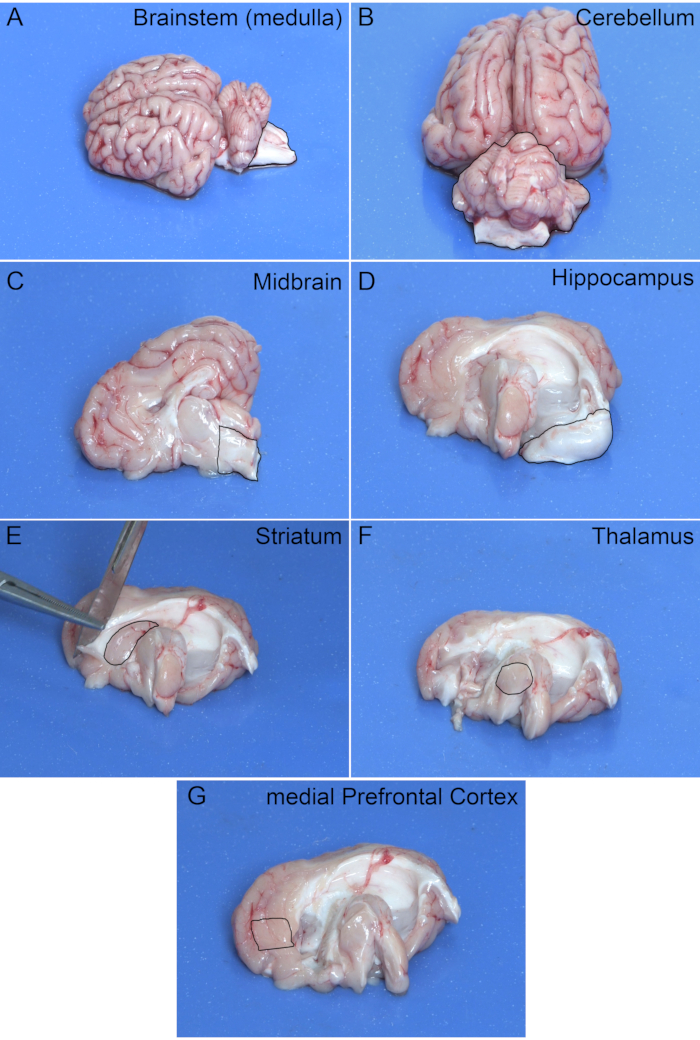
Figure 1: Extracted Pig Brain. Outlines of brain regions for use as a guide during dissection. Regions shown are from the right hemisphere. Please click here to view a larger version of this figure.
| Region | Weight (g) | SEM |
| Whole Pig* | 8.006 | 0.545 |
| Brainstem | 0.829 | 0.132 |
| Cerebellum | 5.929 | 0.137 |
| Midbrain | 0.376 | 0.047 |
| Hippocampus | 0.500 | 0.051 |
| Striatum | 0.410 | 0.115 |
| Thalamus | 0.476 | 0.120 |
| medial Prefrontal Cortex | 0.459 | 0.122 |
| *Weight presented as kg |
Table 1: Brain Regions Weights. Average weight of the 4-week-old pig brain and each dissected brain region (n=5).
Discussion
The techniques described herein were designed for pigs approximately 4 weeks of age. It is critical to perform these steps immediately after the pig has been humanely euthanized to ensure the integrity of brain tissue structure is maintained, especially when considering subsequent biochemical assays. It is helpful to use an atlas or fiber16 dissection guides when first learning the techniques. It is recommended that the experimenter practice several brain extractions and dissections prior to obtaining samples for data collection. The most difficult step is removal of the skull. This step will become easier with experience as it largely requires firsthand practice to know where on the skull to saw and when the skull has been cut through. This procedure is similar to that described by Bassi et al.12, though it does not require the need to create a hexagonal cranial window and provides a visual tutorial of how to perform the technique.
A limitation of this technique it that when working with older pigs, it may take more time to remove the skull as it becomes significantly thicker with age. If using a saw is too laborious or ineffective for thicker skulls, it may be necessary to either use a hammer and chisel, like that shown by Bjarkam et al.13, or powered surgical equipment (e.g., bone saw). Furthermore, this technique does not always ensure the capture of the olfactory bulbs.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Jim Knoblauch and Martin-Booth Hodges of the College of Agricultural, Consumer and Environmental Sciences Information Technology and Communication Services for their expertise in shooting, recording, and editing audio and video.
Materials
| #22 Scalpel Blades for #4 Handles | Ted Pella, inc. | 549-4S-22 | |
| 11 1/2" Satterlee Bone Saw | Leica Biosystems | 38DI13425 | |
| 5 1/2" Skull Breaker with Chisel End (Meat Hook) | Leica Biosystems | 38DI37636 | |
| 5-inch Heavy Duty Workshop Bench Vise | Pony | 29050 | |
| Butcher Knife 25cm | Victorinox | 5.7403.25 | Sharpen before use |
| CM40 Light Duty Drop Forged C Clamps | Bessey | 00655BC3120 | |
| Diamond Hone Knife Shaper | Chef’s Choice | 436-3 | |
| Shandon Stainless-Steel Scalpel Blade Handle #4 | ThermoScientific | 5334 | |
| Tissue Forceps | Henry Schein | 101-5132 | |
| Vinyl Dissecting pad | Carolina | 629006 |
References
- Hughes, H. C. Swine in cardiovascular research. Laboratory Animal Science. 36 (4), 348-350 (1986).
- Yen, J. Anatomy of the Digestive System and Nutritional Physiology. Biology of the Domestic Pig. , 31-63 (2001).
- Pond, W. G. Of Pigs and People. Swine Nutrition. , 3-24 (2001).
- Odle, J., Lin, X., Jacobi, S. K., Kim, S. W., Stahl, C. H. The Suckling Piglet as an Agrimedical Model for the Study of Pediatric Nutrition and Metabolism. Annual Review of Animal Biosciences. 2 (1), 419-444 (2014).
- Larsen, M. O., Rolin, B. Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR Journal. 45 (3), 303-313 (2004).
- Lehmann, H. The minipig in general toxicology. Scandinavian Journal of Laboratory Animal Science. 25, 59-62 (1998).
- Richer, J., et al. Sacrococcygeal and transsacral epidural anesthesia in the laboratory pig. Surgical Radiologic Anatomy. 20, 431-435 (1998).
- Lind, N. M., et al. The use of pigs in neuroscience: Modeling brain disorders. Neuroscience and Biobehavioral Reviews. 31 (5), 728-751 (2007).
- Sauleau, P., Lapouble, E., Val-Laillet, D., Malbert, C. -. H. The pig model in brain imaging and neurosurgery. Animal. 3 (8), 1138-1151 (2009).
- Dobbing, J., Sands, J. Comparative aspects of the brain growth spurt. Early Human Development. 311, 79-83 (1979).
- Aurich, L. A., et al. Microsurgical training model with nonliving swine head. Alternative for neurosurgical education. Acta Cirurgica Brasileira. 29 (6), 405-409 (2014).
- Bassi, T., Rohrs, E., Fernandez, K., Ornowska, M., Reynolds, C. S. Direct brain excision: An easier method to harvest the pig’s brain. Interdisciplinary Neurosurgery. 14, 37-38 (2018).
- Bjarkam, C. R., et al. Exposure of the pig CNS for histological analysis: A manual for decapitation, skull opening, and brain removal. Journal of Visualized Experiments. 122, e55511 (2017).
- Pascalau, R., Szabo, B. Fibre dissection and sectional study of the major porcine cerebral white matter tracts. Anatomia, Histologia, Embryologia. 46, 378-390 (2017).
- McFadden, W. C., et al. Perfusion fixation in brain banking: a systematic review. Acta Neuropathologica Communications. 7, 146 (2019).
- Félix, B., et al. Stereotaxic atlas of the pig brain. Brain Research Bulletin. 49, 1 (1999).

