Multi-timescale Microscopy Methods for the Characterization of Fluorescently-labeled Microbubbles for Ultrasound-Triggered Drug Release
Summary
The presented protocols can be used to characterize the response of fluorescently-labeled microbubbles designed for ultrasound-triggered drug delivery applications, including their activation mechanisms as well as their bioeffects. This paper covers a range of in vitro and in vivo microscopy techniques performed to capture the relevant length and timescales.
Abstract
Microbubble contrast agents hold great promise for drug delivery applications with ultrasound. Encapsulating drugs in nanoparticles reduces systemic toxicity and increases circulation time of the drugs. In a novel approach to microbubble-assisted drug delivery, nanoparticles are incorporated in or on microbubble shells, enabling local and triggered release of the nanoparticle payload with ultrasound. A thorough understanding of the release mechanisms within the vast ultrasound parameter space is crucial for efficient and controlled release. This set of presented protocols is applicable to microbubbles with a shell containing a fluorescent label. Here, the focus is on microbubbles loaded with poly(2-ethyl-butyl cyanoacrylate) polymeric nanoparticles, doped with a modified Nile Red dye. The particles are fixed within a denatured casein shell. The microbubbles are produced by vigorous stirring, forming a dispersion of perfluoropropane gas in the liquid phase containing casein and nanoparticles, after which the microbubble shell self-assembles. A variety of microscopy techniques are needed to characterize the nanoparticle-stabilized microbubbles at all relevant timescales of the nanoparticle release process. Fluorescence of the nanoparticles enables confocal imaging of single microbubbles, revealing the particle distribution within the shell. In vitro ultra-high-speed imaging using bright-field microscopy at 10 million frames per second provides insight into the bubble dynamics in response to ultrasound insonation. Finally, nanoparticle release from the bubble shell is best visualized by means of fluorescence microscopy, performed at 500,000 frames per second. To characterize drug delivery in vivo, the triggered release of nanoparticles within the vasculature and their extravasation beyond the endothelial layer is studied using intravital microscopy in tumors implanted in dorsal skinfold window chambers, over a timescale of several minutes. The combination of these complementary characterization techniques provides unique insight into the behavior of microbubbles and their payload release at a range of time and length scales, both in vitro and in vivo.
Introduction
Ultrasound is the most widely used medical imaging technique. It is non-invasive, fast, safe, cost-effective, and portable1,2,3. However, blood is a poor ultrasound scatterer, and the contrast of the blood pool can be enhanced by an intravenous injection of ultrasound contrast agents3. This enhanced blood-pool contrast enables the quantification of organ perfusion for diagnostic purposes, e.g., in the detection of coronary artery disease4 and metastatic liver disease5. Indeed, tumor vasculature was proven to be an important prognostic factor6. A major research effort is now directed towards microbubble-assisted, targeted molecular imaging and tailoring contrast agents for therapeutic use.
Commercially available ultrasound contrast agents typically consist of a suspension of coated microbubbles7,8 with diameters ranging from 1 µm to 10 µm9. Since ultrasound contrast agent microbubbles are slightly smaller than red blood cells7, the microbubbles can safely reach even the smallest capillaries without creating an occlusion3. Microbubbles have a dramatically increased ultrasound backscattering coefficient compared to tissue10, owing to their compressible gas core11. Furthermore, the microbubble echo is highly nonlinear, i.e., its spectrum contains harmonics and subharmonics of the driving frequency. In addition, the echo strength is strongly dependent on the resonant response of the bubble12. While tissue scatters only linearly, a small number of microbubbles is sufficient to achieve a high detection sensitivity in harmonic imaging13,14. This nonlinear contrast generation can even be strong enough to track single bubbles in the body15.
The shell of the ultrasound contrast agent stabilizes the bubbles against dissolution and coalescence, thereby increasing their circulation time in the blood pool16. The shell can consist of lipids, polymers, or denatured proteins3,8. It decreases the interfacial tension, thereby limiting the effect of Laplace pressure-driven dissolution17 and creates a resistive barrier against gas diffusion18. To further increase stability, the contrast microbubbles are typically filled with a high-molecular weight gas with low solubility in blood11. The microbubble shell dramatically changes the response of the microbubbles to ultrasound insonation11. Uncoated gas bubbles have a characteristic resonance frequency that is inversely proportional to their size and the addition of a lipid coating increases the resonance frequency with respect to that of an uncoated buble owing to the intrinsic stiffness of the shell3. Furthermore, the shell dissipates energy through dilatational viscosity, which constitutes the dominant source of damping for coated bubbles3. The stabilizing shell has the additional advantage that it can be functionalized, e.g., by binding targeting ligands to the surface of microbubbles. This targeting enables many applications for these bubbles and, in particular, molecular imaging with ultrasound14,19.
Microbubble contrast agents hold great promise for drug delivery applications with ultrasound. Microbubbles oscillating in the confinement of a blood vessel can cause microstreaming as well as local normal and shear stresses on the capillary wall3. At high acoustic pressures, large amplitude oscillations may lead to microbubble collapse in a violent process termed inertial cavitation, which, in turn, may lead to rupture or invagination of the blood vessel20. These violent phenomena can induce bioeffects such as sonopermeation21, enhancing the extravasation of therapeutic drugs into the interstitium across the endothelial wall, either paracellularly or transcellularly. It may also improve the penetration of therapeutic agents through the extracellular matrix of stroma-rich tumors21,22 and biofilms23,24, although this mechanism is still poorly understood26.
Ultrasound-mediated drug delivery has shown promising results both preclinically27,28 and in clinical trials22. Moreover, when used with relatively low-frequency ultrasound (~1 MHz), microbubbles have been reported to locally and transiently increase the blood-brain barrier permeability, thereby enabling drugs to enter the brain parenchyma, both in preclinical and clinical studies29,30,31,32,33,34.
There are generally two approaches to ultrasound-mediated drug delivery: the therapeutic material can be co-administered with the bubbles, or it can be attached to or loaded in the bubble shell28,35,36. The second approach has been shown to be more efficient in terms of drug delivery37. Microbubbles can be loaded with drugs or genetic material encapsulated in nanoparticles (liposomes or polymeric nanoconstructs) attached to the shell or incorporated directly in the microbubble shell35,36. Nanoparticle-loaded microbubbles can be activated by (focused) ultrasound to locally release the nanoparticle payload28,33,38,39,40. If such a microbubble is in direct contact with a cell, it has been shown in vitro that the payload can even be deposited onto the cell cytoplasmic membrane in a process called sonoprinting34,35.
The ultrasound parameter space for microbubble insonation is extensive, and the in vivo biological conditions further add complexity. Thus, the combination of focused ultrasound and nanoparticle-loaded microbubbles poses a challenge in the field of targeted therapeutics.
The aim of this work is to provide protocols that can be used to image, in detail, the response of microbubbles as a function of the ultrasound parameters and to study the mechanisms leading to shell rupture and subsequent release of the fluorescently-labeled shell material. This set of protocols is applicable to microbubbles with shells that contain a fluorescent dye. Figure 1 shows a schematic representation of the polymeric-nanoparticle-and-protein-stabilized microbubbles developed at SINTEF (Trondheim, Norway). These bubbles are filled with perfluoropropane gas (C3F8) and the nanoparticles that stabilize the shell contain NR668, which is a lipophilic derivative of Nile Red fluorescent dye38,43. The nanoparticles consist of poly(2-ethyl-butyl cyanoacrylate) (PEBCA) and are PEGylated. Functionalization with polyethylene glycol (PEG) reduces opsonization and phagocytosis by the mononuclear phagocyte system, thereby extending the circulation time14,44. As a result, PEGylation increases the amount of nanoparticles reaching the target site, thereby improving the efficacy of the treatment16. Figure 2 illustrates how the use of four microscopy methods allows researchers to cover all relevant time and length scales. It should be noted that the spatial resolution achievable in optical microscopy is determined by the diffraction limit, which depends on the wavelength of the light and numerical aperture (NA) of the objective and that of the object illumination source45. For the systems at hand, the optical resolution limit is typically 200 nm. Additionally, intravital microscopy can be used to image on the subcellular level46. For the nanoparticle-and-protein-stabilized microbubbles used in this work, the minimum length scale relevant for intravital microscopy is the size of small capillaries (≥10 µm). In vitro high-speed optical imaging (10 million frames per second) and high-speed fluorescence imaging (500,000 frames per second) experiments are described for single microbubbles. High-speed bright-field imaging at nanosecond timescales is suitable to study the time-resolved radial dynamics of the vibrating bubbles. In contrast, high-speed fluorescence microscopy allows for direct visualization of the release of the fluorescently-labeled nanoparticles. Furthermore, the structure of the microbubble shell can be investigated using Z-stack three-dimensional (3D) confocal microscopy, and scanning electron microscopy (the protocol for the latter is not included in the current work). Intravital microscopy consists in using multiphoton microscopy to image tumors growing in dorsal window chambers to provide real-time information on local blood flow and on the fate of fluorescently-labeled nanoparticles in vivo47. The combination of these microscopy methods ultimately provides detailed insight into the behavior of therapeutic microbubble agents in response to ultrasound, both in vitro and in vivo.
Protocol
NOTE: All experimental procedures were approved by the Norwegian Animal Research Authorities. Details of materials that were used in the protocol can be found in the Table of Materials.
1. Production of microbubbles
NOTE: In this work, the microbubbles of interest are protein-and-nanoparticle-stabilized microbubbles, for which the production protocol has been described previously28,33,48. Therefore, the fabrication protocol has been briefly summarized here.
- First, using a pipette, mix ultrapure water with 0.5 wt% of casein in phosphate-buffered saline (PBS) and 1 wt% of the nanoparticles labeled with 0.21 wt% of the fluorescent dye, NR668 (modified Nile Red), in a sterile glass crimp top vial (10 mL, diameter of 2 cm). The polymeric nanoparticles are prepared using the mini-emulsion polymerization method as described by Mørch et al.38.
NOTE: Here, the dye functions as a model drug to enable visualization of nanoparticle release. When working with the nanoparticle solution, wear a lab coat, goggles, and gloves. Wipe away any spills of the nanoparticle solution immediately with 100% acetone. - Cap the vial with the rubber cap, mix slightly, and place the vial in an ultrasonic bath for 10 min at room temperature to eliminate possible aggregates. Place a dispersion tool with the tip of the stirrer ~0.5 cm from the bottom of the glass vial. Using a glass pipette connected to the gas container, add the perfluoropropane gas to the head space of the vial containing the solution until the solution starts bubbling slightly.
NOTE: Wrap self-sealing film around the base of the dispersion tool to prevent slipping of the glass vial during stirring. - Stir the solution vigorously at 1935 × g (24,000 rpm with a radius of rotation of 3 mm) for 4 min using the dispersion tool. Close the vial with the rubber cap, and seal the vial for further use.
NOTE: The stirring entraps the gas in the liquid. The microbubble-shell subsequently self-assembles without requiring any active step. - Store the excess casein and nanoparticle solution at 4 °C, and clean the dispersion tool with 100% acetone.
2. Imaging single bubbles
- Confocal microscopy
- Sample preparation
- Dilute the bubble solution to image single microbubbles as follows. Place a venting needle (19 G-21 G) in a glass crimp top vial containing the microbubbles produced by following the procedure described in section 1. Turn the vial upside down to allow large bubbles to move away from the seal of the vial.
- Insert another needle tip (19 G) of a small (~1 mL) syringe into the vial, while the vial is still upside down. Remove a small amount of the bubble suspension, and transfer the contents of the syringe into a small tube for easier pipetting in the next step.
NOTE: The volume of the suspension to extract directly depends on the type and concentration of the bubble suspension. In this case, 0.2 mL was extracted. - Using a pipette, dilute the microbubble suspension (from section 1) in filtered PBS to achieve a concentration of approximately 2 × 105 to 6 × 105 microbubbles/mL to enable single-bubble imaging.
NOTE: Depending on the bubble type, it is recommended to wash the bubble suspension to remove free fluorescent dye. This is particularly important with bubbles for which the fluorescent dye is infused in the shell. To wash bubbles, dilute the bubble suspension (e.g., by taking 100 µL of the bubble solution in 10 mL of PBS), and centrifuge it (typically at speeds of the order of 100 × g). Finally, remove the supernatant containing the microbubbles with a pipette for further analysis. The remaining solution contains the free fluorescent particles and can be discarded. The washing step should be repeated as necessary. - Add glycerol to the mixture to increase the viscosity of the medium and eliminate the movement induced by Brownian motion that would otherwise interfere with the rather slow confocal Z-stack imaging.
NOTE: The amount of glycerol depends on the type of bubble that is imaged (here, ~50%). For some types of bubbles, glycerol might have an adverse effect on stability49. However, no noticeable change was observed in the bubbles over approximately 30 min under confocal imaging. Furthermore, glycerol may change the acoustic response of microbubbles and can therefore only be used with imaging methods where the microbubbles are not insonified. - Place the microbubble suspension in a chamber with thin walls for optimal imaging such as a channel slide.
- Imaging protocol
- Switch on the confocal microscope, and select a suitable objective and the desired laser and scanner to use during confocal microscopy.
NOTE: Here, use a 60x water immersion objective for a resolution of 0.08 µm/pixel and depending on the bubble size, image a region of 256 x 256 pixels or 128 x 128 pixels. In these specific experiments, use a 488 nm laser and a Galvano scanner. The emission wavelength depends on the fluorescent dye and is typically broadband. - Find a microbubble in bright-field, and switch to confocal microscopy. Set the desired top and bottom planes in between which the confocal microscope will scan. Acquire a Z-stack to observe the 3D structure; use a step size of 100 nm in the Z-direction.
- Switch on the confocal microscope, and select a suitable objective and the desired laser and scanner to use during confocal microscopy.
- Sample preparation
- Bright-field microscopy
- Assembly of the optical system
NOTE: A schematic representation of the bright-field microscopy setup is shown in Figure 3A. To ensure undisturbed ultrasound propagation, the water bath contains two openings: one for a light source and one for an ultrasound transducer. The optical system consists of a (modular) microscope, a high-speed camera, and matching optics. As the period of microbubble oscillations is typically of the order of 1 µs (using 1 MHz ultrasound), the camera should be set to record at a framerate of at least 5 million frames per second. Here, the camera is to be set to record at 10 million frames per second (256 x 400 pixels) for 256 frames (25.6 µs) to capture all details of the bubble dynamics including higher harmonics.- Attach a water-immersion objective with an appropriate magnification, working distance, and NA to the microscope.
NOTE: A water-immersion objective was used to provide a stable working distance despite gradual evaporation of the water. Here, a water-immersion objective with a magnification of 60x, a working distance of 2 mm, and an NA of 1 was selected. - Use a strobe light with a peak power output of at least 1 kW for illumination and a tube lens between the microscope and the camera to ensure that as little ambient light as possible reaches the sensor of the high-speed camera.
- Use a dimmable halogen light source for focusing on single microbubbles and alignment of the optical and acoustical system for real-time imaging.
- Attach a water-immersion objective with an appropriate magnification, working distance, and NA to the microscope.
- Assembly of the acoustical system
- Use a programmable arbitrary waveform generator and a power-amplifier (56 dB gain) to drive the transducer with a smooth envelop and waveform. Connect an oscilloscope to the arbitrary waveform generator to check the signal. Connect a personal computer to the arbitrary waveform generator to program the incoming acoustic pressure wave, using a script written in-house.
- Use a pulse/delay generator as master trigger to synchronize the optical and acoustical systems. Set the trigger delays on the pulse/delay generator and the camera software such that the recording starts 16 µs after ultrasound transmission to allow the ultrasound wave to reach the bubbles. Trigger the light source 1.5 µs before the start of the recording to ensure proper illumination during the bubble oscillations (see Figure 3B for the timing diagram).
- Choose a suitable transducer with an appropriate center frequency. Place it in an opening of the water bath, so that it is at an angle with respect to the optical axis to minimize reflections from the sample holder membranes and to reduce standing wave formation.
NOTE: Here, a single-element focused immersion transducer with a center frequency of 2.25 MHz, a focal distance of 1" and element diameter of 0.75" was placed at an angle of 35° with respect to the optical axis. The calibration of the transfer function needs to be performed using the same amplifier that is used in the acoustical system. Calibrate the transfer function from voltage amplitude to pressure amplitude of the transducer using a fiber optic hydrophone as a function of the ultrasound transmit frequency.
- Choosing the sample holder
- Use a sample holder with optically and acoustically transparent membranes and a volume that is large enough to allow for imaging of several single microbubbles within the same sample.
NOTE: Here, a cell culture cassette with a volume of 10 mL, membrane areas of 25 cm2, and membrane thickness of 175 µm was used. Due to acoustic reflections on the lower membrane, and interference from waves reflected by the microscope objective and the upper membrane, the in situ acoustic pressure might differ from that programmed on the arbitrary waveform generator. Placing the transducer at an angle with respect to the sample holder membranes reduces standing wave formation, but can increase reflections from the membranes. - Ensure that the sample can be fully submerged and brought within the focus of both the transducer and the microscope objective. Use an aluminum support attached to a 3D micropositioning stage to move the sample holder independently.
- Use a sample holder with optically and acoustically transparent membranes and a volume that is large enough to allow for imaging of several single microbubbles within the same sample.
- Alignment of the optical and acoustical systems
- For 3D translation to align the setup, attach the water bath to an XY-translation stage, and attach the stage to an optical table to ensure it does not move during experiments. Then, fill the water bath with water, and switch on the dimmable halogen light source. During alignment, move the microscope objective to the side to prevent ultrasound reflections.
- Attach a needle hydrophone (0.2 mm) to the sample holder arm, and place the needle hydrophone in the water bath, with the tip in the field of view of the objective. Turn on the amplifier and the arbitrary waveform generator; use single pulses of 5 to 10 ultrasound cycles and a pulse repetition frequency of 15 Hz. Make sure that the hydrophone tip is centered and in focus on the microscope image. Move the tank in the XY-direction and the needle in the Z-direction until the maximum pressure amplitude is reached.
- Adjust the focus of the microscope to refocus on the tip of the hydrophone.
NOTE: This protocol ensures the alignment between the microscope focus and the transducer focus. Do not change the position of the microscope and the transducer after alignment.
- Sample preparation
- Repeat steps 2.1.1.1 through 2.1.1.3 to prepare the sample solution. Dilute the bubble solution to enable single-bubble imaging and to rule out acoustic interactions of neighboring bubbles.
- Open the outlet of the sample holder. Using a syringe, inject the sample solution into the other opening of the sample holder until it is completely filled. Ensure that there are no air bubbles inside the sample holder to prevent unwanted interactions with the ultrasound field.
- Close both valves of the sample holder, and place the sample holder perpendicular to the optical axis.
NOTE: Keep the filled sample holder level to prevent shifting of the bubbles to one side of the sample holder during moving.
- Imaging protocol
- Program the desired ultrasound driving frequency and acoustic pressure in the arbitrary waveform generator through the aforementioned in-house written script.
NOTE: Here, the acoustic pressure wave was a single burst of 40 cycles, with an 8-cycle Gaussian-tapered pulse. Ultrasound frequencies used in these experiments were 1 MHz, 2 MHz, or 3 MHz, with acoustic pressure amplitudes ranging from 81 kPa to 1200 kPa. - Move the sample holder containing the sample solution using the XYZ-stage to locate single microbubbles in the focus of the microscope. Start with a field of view at a corner of the sample holder, and ensure that the edge of the microbubbles is clearly visible and in focus (see Figure 3C for an ideal camera view).
- Attach the end of an optical fiber that was previously connected to the halogen light to a strobe light, so that the other end is still connected to the water bath. Trigger the recording.
- Repeat steps 2.2.6.2 through 2.2.6.3 as many times as desired per ultrasound setting (frequency and acoustic pressure), moving the cell culture cassette containing the microbubbles at least 2 mm (in the focal plane) from the previous location to ensure that the microbubbles in the field of view are not insonified in previous experiments.
NOTE: Here, each experiment was repeated ~20 times. When the whole sample holder is insonified, empty the sample holder, and refill it with fresh sample solution for subsequent experiments.
- Program the desired ultrasound driving frequency and acoustic pressure in the arbitrary waveform generator through the aforementioned in-house written script.
- Data analysis
- Adopt a programming environment to perform data analysis according to the research question, and perform edge detection after processing the images. Using a function that measures properties of image regions, find the centroid of a bubble and the derivative of the intensity profile around each bubble to detect the contour of the bubble (and thus, the bubble radius R). Extract relevant parameters from the radius over time for single microbubbles.
NOTE: In the present study, a programming environment was used for image processing to binarize and filter recordings of single microbubbles. An in-house script was used to find the derivative of the intensity profile around each bubble.
- Adopt a programming environment to perform data analysis according to the research question, and perform edge detection after processing the images. Using a function that measures properties of image regions, find the centroid of a bubble and the derivative of the intensity profile around each bubble to detect the contour of the bubble (and thus, the bubble radius R). Extract relevant parameters from the radius over time for single microbubbles.
- Assembly of the optical system
- Fluorescence microscopy
- Assembly of the optical system
- Build the setup for fluorescence microscopy (Figure 4A), with the same base used in the bright-field microscopy described in section 2.2.
NOTE: The setup described in section 2.3 can be combined with that described for bright-field microscopy in section 2.2. Combining both fluorescence microscopy and bright-field microscopy enables visualization of the microbubble gas core while imaging the nanoparticle release. - Set the high-speed camera to record at 500,000 frames per second (400 x 250 pixels) for 128 frames (256 µs).
NOTE: The imaging time is longer than in the bright-field experiments as the light intensity is limited in fluorescence, and because the timescale over which particle delivery occurs is longer than that of the bubble dynamics. - Select a laser with a power high enough to supply sufficient light and that has a suitable excitation wavelength, and ensure that it is coupled with an acousto-optic modulator to avoid bleaching the sample.
NOTE: In this study, a 5 W continuous wave laser with an excitation wavelength of 532 nm was used to excite the fluorescence of the nanoparticles. - Place a beam splitter, dichroic mirror and notch filter between the laser and the microscope objective to direct the excitation light towards the sample while allowing the fluorescence emission to reach the camera.
- Build the setup for fluorescence microscopy (Figure 4A), with the same base used in the bright-field microscopy described in section 2.2.
- Assembly of the acoustical system
- To insonify the microbubbles, use the same acoustical setup as in section 2.2.2. Change the transducer in these specific experiments to a single-element, focused immersion transducer with a center frequency of 2.25 MHz, a focal distance of 1.88", and element diameter of 1". Place it at an angle of 35° with respect to the optical axis to minimize reflections from the sample holder membranes and reduce standing wave formations.
- Alignment of the optical and acoustical systems
- Repeat steps described in section 2.2.4.
- Sample preparation
- Prepare the sample solution as described in section 2.2.5.
- Imaging protocol
- Set the desired ultrasound driving frequency and acoustic pressure amplitude on the arbitrary waveform generator through the aforementioned in-house written script.
NOTE: Here, the acoustic pressure wave was programmed to be a single burst of ultrasound of 140 cycles, with a 10-cycle Gaussian-tapered pulse. Longer pulse durations are generally required to induce bio-effects compared to those required to study bubble dynamics. Ultrasound frequencies used in these experiments were 1 MHz, 2 MHz, or 3 MHz, with acoustic pressure amplitudes ranging from 81 kPa to 1200 kPa. - On the pulse/delay generator, set the trigger delay for the laser for fluorescent excitation of the nanoparticles from the microbubbles during the recording.
NOTE: For these specific experiments, the trigger delay was between 20 µs and170 µs for a total duration of 150 µs. The timing diagram is shown in Figure 4B. - Move the sample holder containing the sample solution using the XYZ-stage to locate single microbubbles in the focus of the microscope. Start with a field of view of a corner of the sample holder; see Figure 4C for an ideal camera view in which the interface of the microbubbles is clearly visible and in focus. Trigger the recording.
- Repeat step 2.3.5.3 as many times as desired per ultrasound setting (frequency and acoustic pressure), moving the cell culture cassette containing the microbubbles at least 2 mm (in the optical plane) from the previous location to ensure microbubbles in the field of view are not sonicated in previous experiments.
NOTE: In this study, each experiment was repeated ~10-20x. When the whole sample holder is insonified, empty the sample holder, and refill it with fresh sample solution for subsequent experiments. Which distance to move the sample holder between experiments depends on the acoustic beam size.
- Set the desired ultrasound driving frequency and acoustic pressure amplitude on the arbitrary waveform generator through the aforementioned in-house written script.
- Data analysis
- Analyze the fluorescence microscopy recordings according to the research question. For every microbubble, visually determine whether delivery of the nanoparticles in the fluorescence microscopy experiments occurred. If detachment and deposition of the nanoparticles from the gas core onto the sample holder membrane is observed for a single microbubble, manually enter that delivery occured in the programming environment.
- Assembly of the optical system
3. Intravital microscopy
- Dorsal skinfold window chamber surgery (described previously26,47,50)
- Acclimate the animals for one week before placing the window chambers. Although both female and male mice can be used, and age is unimportant, ensure that the weight of the mice is at least 22-24 g so that the skin is sufficiently flexible.
- Perform the surgery under general anesthesia with intraoperative and postoperative analgesic treatment. Anesthetize the animal by a subcutaneous injection of fentanyl (0.05 mg/kg)/medetomidine (0.5 mg/kg)/midazolam (5 mg/kg)/water (2:1:2:5) at a dose of 0.1 mL per 10 g weight. Use a heating pad or a heating lamp to maintain the body temperature of the animal.
- Pull gently on the double layer of skin on the back of the animal so that the skin is sandwiched between two symmetric polyoxymethylene frames of the window chamber. Fix the chamber by placing two screws extending through the double skin layer and suturing along the upper edge of the chamber.
- Remove the skin within the circular frame of the chamber on one side of the skin fold. Place a cover glass with a diameter of 11.8 mm within the frame where the skin is removed to form a window into the tissue.
- Use a subcutaneous injection of atipemazole (2.5 mg/kg), flumazenil (0.5 mg/kg), and water (1:1:8) at a dose of 0.1 mL per 10 g as antidote to terminate the anesthesia. Place the animal in a heated recovery rack overnight. Supplement the water for the animals with 25 mg/mL of enrofloxacin to prevent infection in the surgical site.
- Tumor model creation
- Maintain cancer cells at 37 °C and in a 5% CO2 atmosphere in appropriate culture medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin and 100 mg/mL streptomycin.
NOTE: The human osteosarcoma (OHS) cell line was used in this protocol, but other cell lines can also be used. - On the day after step 3.1.5, anesthetize the animal by isoflurane (5% during induction and 1-2% during maintenance) for a couple of minutes. Remove the cover glass, apply 5 × 106 cancer cells in 30 µL of cell culture medium, and replace the cover glass.
- Allow the tumors to grow for 2 weeks before imaging, and monitor the weight and health status of the animals at least 3 times per week during this period.
- Maintain cancer cells at 37 °C and in a 5% CO2 atmosphere in appropriate culture medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin and 100 mg/mL streptomycin.
- Assembly of the optical system
- Perform intravital imaging during ultrasound treatment (as described in previous work26) with a suitable microscope and objective depending on the research question at stake. See Figure 5A for a schematic representation of the experimental setup.
NOTE: For this specific experiment, a multiphoton microscope was used, equipped with a 20x water dipping objective (NA of 1.0 and working distance of 2 mm) and a pulsed laser. Images were acquired in resonant scanning mode at 31 frames per second (512 x 512 pixels) with a field of view of 400 x 400 µm2. The excitation wavelength was 790 nm. The filters in front of the two gallium arsenide phosphide detectors were long-pass 590 nm and band-pass 525/50 nm for the detection of fluorescence.
- Perform intravital imaging during ultrasound treatment (as described in previous work26) with a suitable microscope and objective depending on the research question at stake. See Figure 5A for a schematic representation of the experimental setup.
- Assembly of the acoustical system
- Mount a suitable ultrasound transducer in a waveguide (custom made) positioned below the objective at an angle of 45° with respect to the optical axis to minimize reflections from the cover glass of the dorsal skinfold window chamber and to reduce standing wave formations. Fill the waveguide with distilled and degassed water. Apply ultrasonic coupling gel on top of the waveguide.
- Alignment of the optical and acoustical systems
- Align the optical axis with the focus of the ultrasound. Position a fiber-optic hydrophone in the focus of the objective. Then, turn on the amplifier and the arbitrary waveform generator to excite the transducer with short bursts (5-10 cycles) with a pulse repetition frequency of 100 Hz, and move the ultrasound transducer to the position where the highest pressure is detected with the hydrophone signal on the oscilloscope.
NOTE: Do not change the position of the transducer after alignment.
- Align the optical axis with the focus of the ultrasound. Position a fiber-optic hydrophone in the focus of the objective. Then, turn on the amplifier and the arbitrary waveform generator to excite the transducer with short bursts (5-10 cycles) with a pulse repetition frequency of 100 Hz, and move the ultrasound transducer to the position where the highest pressure is detected with the hydrophone signal on the oscilloscope.
- Imaging protocol
- Position the heated animal holder (custom designed) connected to an XY-positioning stage between the waveguide and the objective, and add more coupling gel. Anesthetize the animal, and place a tail vein catheter. Place the mouse in the heated holder, and fix the window chamber in the holder. Add a water droplet on top of the cover slip in the window chamber, and move the objective in place to image the tumor tissue.
- Figure 5B shows the timing diagram of the experiments depicting the order of events. Inject fluorescently-labelled 2 MDa dextran intravenously (30 µL, 4 mg/mL diluted in saline) to visualize the vasculature, and move the mouse using the XY-translation stage to find a position with suitable blood vessels. Record baseline images before the ultrasound treatment. Adjust frame rate, field of view, and length of recording depending on the research question and the specifics of the microscope and dyes to be imaged.
NOTE: In these experiments, 31 frames per second were recorded with a field of view of 400 x 400 µm2, and imaging was done continuously for 5 min. - Set the desired ultrasound driving frequency, pulse length, and acoustic pressure amplitude on the arbitrary waveform generator.
NOTE: For these experiments, a frequency of 1 MHz was used with a pulse length of 10 ms and peak negative pressure amplitudes between 0.2 MPa and 0.8 MPa. A pulse repetition frequency of 0.5 Hz or 0.1 Hz was used to allow new microbubbles to reperfuse into the treated area between ultrasound pulses. - Inject 50 µL microbubbles (2 × 108 to 5 × 108 microbubbles/mL) intravenously, and apply ultrasound while imaging, as described in26.
- Data analysis
- Depending on the research question, analyze images with (open source) image processing software and a programming environment, as described in26, to determine blood vessel parameters (diameter, branching, flow speed, and direction), accumulation of nanoparticles in the vessels, and kinetics and penetration depth of extravasation of dextran and nanoparticles into tumor tissue.
Representative Results
The microbubbles, produced as described in the protocol, were analyzed using various microscopy methods and at various timescales.
The fluorescence of the nanoparticles in confocal microscopy (Figure 6A) indicates that the shell has a non-uniform particle distribution. Other microscopy methods can be used for bubble characterization. For example, Figure 6B shows the overall structure of the microbubble using scanning electron microscopy, as presented in previous work34.
Radial dynamics and phenomenological bubble behavior can be studied using the described in vitro bright-field microscopy method wherein microbubbles were imaged at 10 million frames per second. The radius of single microbubbles was extracted over time using a script written in-house. An example of such a radial response is shown in Figure 7.
An image sequence of typical successful nanoparticle delivery, as described in section 2.3.6, is shown in Figure 8A. The nanoparticles embedded in the microbubble shell can be seen to light up due to fluorescence when the laser light reaches the bubble. Driven by ultrasound insonation, the fluorescent nanoparticles detach from the gas core of the microbubbles and are deposited on the membrane of the sample holder. Finally, the laser is turned off, and the fluorescent nanoparticles are no longer excited. Unsuccessful delivery of the fluorescently-labeled payload of the microbubbles typically looks like the image sequence shown in Figure 8B, where the fluorescent nanoparticles light up on the shell of the microbubble that stays intact during ultrasound exposure.
Real-time intravital multiphoton microscopy during ultrasound was used to investigate the effects of ultrasound and microbubbles on nanoparticle behavior in the blood, enhancement of the permeability of tumor blood vessels, and improvement of the delivery of nanoparticles. The extent and kinetics of penetration into the extracellular matrix as a function of acoustic pressure, frequency, and pulse lengths can be characterized. The effect of the ultrasound treatment may vary with respect to the size and morphology of the vessels and resulting confinement of the bubble. How the ultrasound treatment affects the blood flow and direction can be determined. An example experiment showing the extravasation of nanoparticles over time is shown in Figure 9 at a mechanical index (MI) of 0.826. Results of intravital multiphoton microscopy elucidate the spatial and temporal extravasation of nanoparticles during ultrasound exposure, which is highly beneficial for the complete understanding of the mechanisms underlying ultrasound-mediated delivery of nanoparticles and to optimize such technologies26.

Figure 1: Schematic representation of a microbubble with a shell of fluorescently-labeled polymeric nanoparticles in denatured casein. The microbubbles are typically between 1 µm and 10 µm in diameter. The nanoparticles have a diameter mostly between 100 nm and 200 nm38. Abbreviation: C3F8 = perfluoropropane gas. Please click here to view a larger version of this figure.

Figure 2: Schematic overview showing the relevant time and length scales for bright-field, fluorescence, confocal, and intravital microscopy. Please click here to view a larger version of this figure.

Figure 3: Schematic representation of bright-field microscopy experiments. (A) Experimental setup, (B) the timing diagram, and (C) a typical recorded frame. Scale bar in (C) = 10 µm. Abbreviation: fps = frames per second. Please click here to view a larger version of this figure.
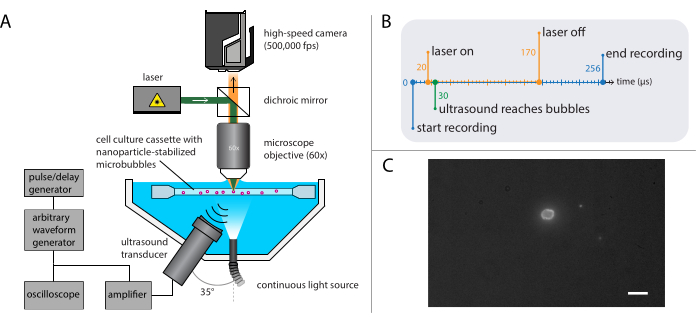
Figure 4: Schematic representation of fluorescence microscopy experiments. (A) Experimental setup, (B) the timing diagram, and (C) a typical recorded frame. Scale bar in (C) = 10 µm. Abbreviation: fps = frames per second. Please click here to view a larger version of this figure.

Figure 5: Schematic representation of intravital microscopy experiments. (A) Experimental setup, (B) the timing diagram, and (C) a typical recorded frame. Scale bar in (C) = 50 µm. Green corresponds to dextran-FITC and red to nanoparticles. Abbreviation: GaAsP = gallium arsenide phosphide. Please click here to view a larger version of this figure.

Figure 6: 3D structure of a single nanoparticle-and-protein-stabilized microbubble. (A) Using confocal microscopy to show the nanoparticles, and (B) using a scanning electron microscope to show the 3D structure. (B) has been reproduced with permission from34. Scale bar in (A) = 5 µm; scale bar in (B) = 2 µm. Please click here to view a larger version of this figure.

Figure 7: Typical spherical oscillations of a 2.89 µm radius nanoparticle-and-protein-stabilized microbubble insonified at an ultrasound frequency of 1 MHz and an acoustic pressure amplitude of 142 kPa. (A–D) Images from the high-speed recording and the corresponding bubble radius over time curve (bottom). Scale bars = 5 µm, and the red line indicates the initial radius. The illumination profile (arbitrary units) is indicated by yellow. The magnification is 120x. Please click here to view a larger version of this figure.

Figure 8: Image sequence from high-speed fluorescence microscopy. (A) Successful delivery of fluorescently-labeled nanoparticles of a nanoparticle-and-protein-stabilized microbubble insonified at an ultrasound frequency of 2 MHz and an acoustic pressure amplitude of 600 kPa. (B) Unsuccessful delivery of fluorescently-labeled nanoparticles of a nanoparticle-and-protein-stabilized microbubble insonified at an ultrasound frequency of 2 MHz and an acoustic pressure amplitude of 210 kPa. Scale bars = 10 µm. The magnification is 120x. Please click here to view a larger version of this figure.

Figure 9: Intravital microscopy after insonation of nanoparticle-and-protein-stabilized microbubbles at an ultrasound frequency of 1 MHz and an acoustic pressure amplitude of 800 kPa. (A) Nanoparticles within the vessel, and (B) an image sequence of the area indicated by the white dashed square in (A) depicting the extravasation of dextran (green) and nanoparticles (red). Scale bars = 50 µm. The magnification is 20x. Please click here to view a larger version of this figure.
Discussion
Different optical microscopy methods were combined to obtain information on the various steps in the delivery of nanoparticles from the surface of microbubbles to the surrounding medium. Imaging of the bubble oscillations was performed, as well as imaging of the release of the nanoparticles from the bubble shell, the extravasation, and the penetration through the extracellular matrix of tumors in vivo. In vitro imaging enables screening of many ultrasound parameters compared to the more complex in vivo setups. The benefit of combining this range of imaging modalities is the complementary information that can be obtained at different timescales – a feature that is crucial to characterize and optimize the microbubbles for successful delivery and to obtain therapeutic efficacy. This approach is useful to understand the delivery mechanisms for all microbubbles alike, including constructs with fluorescently-labeled nanoparticles and drugs.
The most critical steps in the microscopy methods used to study single microbubbles are as follows. For fluorescence microscopy, the nanoparticles should be fluorescently-labeled to enable visualization of the particle release. Furthermore, the sample solution should be diluted enough to isolate single microbubbles for analysis in confocal, bright-field, and fluorescence microscopy methods. In addition, it is important to choose an ultrasound driving frequency and acoustic pressure to excite the bubbles most efficiently, namely at their resonance. If the research question concerns delivery of the nanoparticle payload, the appropriate ultrasound parameters should be part of the investigation. Next to resonance, these bubbles should also be driven at or beyond their threshold for nanoparticle release, typically at relatively high acoustic pressure amplitudes (MI > 0.3)51. For bright-field microscopy imaging, it is critical to choose a high-speed camera with a sufficiently high framerate to minimize motion blur and to avoid aliasing.
Bright-field microscopy is mainly limited by the imaging framerate and intensity of light sources available, as a higher framerate would give a more detailed time-resolved insight into the bubble dynamics, but requires more intense illumination due to shorter exposure times. To study particle release in more detail, the framerate for fluorescence imaging can, in principle, be increased by increasing the intensity of the laser light. However, absorption of the high-intensity laser light by the fluorescently-labeled microbubbles generates heat, even with high quantum yield dyes. This heat can interfere with the experiments at stake, and in extreme cases, induce photo-thermal cavitation52. Thus, in practice, there is a limit to the applied laser fluence. However, intense laser illumination can also be deliberately used to induce particle release from liposomes53. Temperature influences bubble dynamics and ultrasound response, depending on bubble type54. Therefore, if in vitro and intravital methods are to be compared objectively, the in vitro methods discussed in the protocol should be performed at 37 °C. Another limitation of the in vitro methods discussed in the current paper is that the bubbles are not in a free-field environment, as microbubbles will float below the sample holder membrane. Furthermore, there is a selection bias when imaging single microbubbles. However, performing repeated experiments on single bubbles allows for the investigation of the effect of size and removal of the confounding factor-the size distribution. If the bubble response as a function of size can be understood while the concentration is not too high to prevent bubble-bubble interactions, the response of any arbitrary bubble population can be calculated. Finally, both bright-field and fluorescence microscopy methods provide insight into microbubbles convoluted in a two-dimensional (2D) image. If the research question requires more than 2D imaging, the 3D behavior of the bubbles can be resolved by combining the setup described in the protocol with a sideview setup for multiplane imaging55.
An alternative method to study microbubbles is acoustic characterization56. However, measuring the echo of a single microbubble requires locating and isolating a single microbubble within the ultrasound beam56, which poses a challenge typically tackled by the use of a narrow tube or optical or acoustical tweezers57,58. To size bubbles acoustically, the microbubbles can be insonified in the geometrical scattering regime at frequencies much higher than their resonance frequency, which does not induce volumetric microbubble oscillations59. The use of an "acoustical camera" is such a method to image the radial dynamics of single microbubbles in response to ultrasound, wherein a high frequency ultrasound probe is used to determine the radial response of the bubble to a low-frequency driving wave60. The disadvantage of this method is that it can only be used to determine the relative change of the microbubble radius; hence, another method is needed to determine the absolute bubble radius, e.g., through optical imaging61,62. The disadvantage of methods wherein microbubbles are exposed to ultrasound at frequencies higher than their resonance frequency is that at such high frequencies, the penetration depth is decreased59, limiting the usability for in vivo applications. Other forms of microscopy may also be used to study microbubbles such as scanning electron microscopy, atomic force microscopy, and transmission electron microscopy63. The achievable spatio-temporal resolution of these alternative microscopy techniques, however, is generally more limited, and these techniques have the disadvantage that imaging is performed either before or after ultrasound exposure by off-line analysis and typically present a low throughput63. Another alternative is to use a light scattering method, which can be used to study radial dynamics of single microbubbles in real-time, but has a low signal to noise ratio as compared to acoustic scattering methods64.
Real-time intravital microscopy during ultrasound exposure is a powerful method to acquire new insight on the vasculature, behavior of microbubbles, nanoparticles, or other molecules (such as dextran in this case) during ultrasound exposure. A general limitation when performing real-time intravital microscopy is that only a small area of the tissue is imaged, and the penetration depth of the light into tissue is limited. If the imaged vessels contain very few microbubbles and/or nanoparticles within the field of view, little or no information on the nanoparticle behavior and extravasation can be obtained. In addition, because of the limited field of view, a proper alignment between the light and ultrasound paths is crucial. If the ultrasound pressure is high enough to induce bubble destruction, it is also important to choose a pulse repetition frequency that allows fresh bubbles to reperfuse into the field of view between ultrasound pulses. Moreover, as the ultrasound will be reflected from the cover glass in the window chamber and the objective, placing the transducer at an angle is important to reduce reflections as to prevent the formation of standing waves, which distort the calibrated pressure field. Another practical issue is that the setup needs to have sufficient space to mount the ultrasound transducer and waveguide above or below the objective in the microscope setup. The tumors in the dorsal window chamber will have a limited thickness due to the confining chamber and the cover slip; however, if needed, other models could be used. Examples are skinfold tumors, for instance, in the mammary fat pad65 or abdominal intravital imaging of tumors in the various organs66. Such tumors can be grown orthotopically in the appropriate microenvironment, and as such, present a more clinically relevant case.
The methods described in this work enlighten the potential of fluorescently-labeled microbubbles to study the fundamentals of drug delivery applications using bubbles and ultrasound. This combination of microscopy methods provides valuable insight into the microbubble response to ultrasound insonation and its associated acoustic parameter space and presents a clear view of the microbubble and payload behavior over a relevant range of time and length scales.
Disclosures
The authors have nothing to disclose.
Materials
| 100 MS/s Dual-Channel Arbitrary Waveform Generator model 8026 | Tabor Electronics | Arbitrary waveform generator (programmable) | |
| 2100 L | ENI | Amplifier, used in window chamber setup | |
| 2 MDa dextran | Sigma-Aldrich | ||
| 33522 A | Agilent Technologies | Arbitrary wave form generator, used in window chamber setup | |
| A1R | Nikon Instruments | Confocal microscope | |
| ACE I | SCHOTT | Dimmable AC halogen light source | |
| Atipemazol | Orion Pharma | Antidote to wake animal | |
| Baytril | Bayer | Enrofloxacin | |
| BD Neoflon 24 G | Becton Dickinson & Company | Tail vein catheter | |
| BNC model 575 | Berkely Nucleonics Corporation | Pulse/delay generator | |
| Branson 2510 Ultrasonic Cleaner | Branson | Ultrasonic bath | |
| Channel slide | Ibidi | ||
| CLINIcell 25 | Laboratoires Mabio International | Cell culture casette (volume 10 mL, membrane area 25 cm2, membrane thickness 175 µm) | |
| Cohlibri | Lightline | Laser (5 W, excitation wavelength 532 nm) | |
| DP03014 Digital Phosphor Oscilloscope | Tektronix | Oscilloscope | |
| Fentanyl | Actavis Group HF | Anaesthesia of mouse | |
| Fetal Bovine Serum | Sigma-Aldrich | Supplement for cell culture medium | |
| Fiber-optic hydrophone | Precision Acoustics | Used for alignment | |
| Flumanezil | Fresenius Kabi | Antidote to wake animal | |
| Heated animal holder | Custom design | A steel holder where the mouse is positioned on its side in a cavity fitting the size of a mouse, with the window chamber lying flat and immobilized with screws on each side. Below the chamber there is a hole in the holder to secure acoustic contact between the transducer and the skin. The holder is heated to a maximum temperature of 37°C, and the temperature is controlled by feedback from a rectal temperature probe in the mouse. The holder is mounted to an XY positioning stage so the animal can be moved independently to image different areas of the window chamber | |
| Hyper Vision HPV-X2 | Shimadzu | High-speed camera | |
| ImageJ | National Institutes of Health and the Laboratory for Optical and Computational Instrumentation, University of Wisconsin | open source image processing program | |
| In vivo SliceScope | Scientifica | Multiphoton microscope | |
| Isoflurane | Baxter | ||
| ISOTON | Beckman Coulter | Filtered, phosphate-buffered saline solution | |
| LUMPLFLN60XW | Olympus | Water immersion objective (magnification 60x, working distance 2 mm) | |
| MaiTai DeepSee | Spectra-Physics | Pulsed laser | |
| MATLAB | Mathworks | Programming environment | |
| Medetomidine | Orion Pharma | Anesthesia of mouse | |
| Midazolam | Accord Healthcare Limited | Anesthesia of mouse | |
| Milli-Q | Merck | Ultrapure water | |
| MVS 7010 High Intensity Xenon Strobe | PerkinElmer | Strobe light | |
| Panametrics-NDT C305 | Olympus | Single-element focused immersion transducer (center frequency 2.25 MHz, focal distance 1", diameter 1") | |
| Panametrics-NDT V304 | Olympus | Single-element focused immersion transducer (center frequency 2.25 MHz, focal distance 1.88", diameter 1.25") | |
| Penicillin | Sigma-Aldrich | Addition to cell culture medium before implantation of tumor in animals | |
| Perfluoropropane gas | F2 Chemicals | ||
| Roswell Park Memorial Institute 1640 | Gibco Thermo-Fisher | Cell culture medium | |
| Safe-Lock tube | Eppendorf | ||
| Streptomycin | Sigma-Aldrich | Addition to cell culture medium before implantation of tumor in animals | |
| T 25 basic ULTRA-TURRAX | IKA laboratory technology | Dispersion tool | |
| TDS 210 | Tektronix | Oscilloscope, used in window chamber setup | |
| Transducer | Precision Acoustics Ltd | Used in window chamber setup | |
| U-TLU | Olympus | Tube lens | |
| VBA100-200 | Vectawave | Amplifier | |
| Window chambers | Custom made | Used in window chamber setup | |
| XLUMPLFLN20 XW | Olympus | 20x water dipping objective | |
| XY(Z) translation stages | Thorlabs |
References
- Szabo, T. L. . Diagnostic ultrasound imaging: inside out. , (2004).
- Paefgen, V., Doleschel, D., Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Frontiers in Pharmacology. 6, 197 (2015).
- Versluis, M., Stride, E., Lajoinie, G., Dollet, B., Segers, T. Ultrasound contrast agents modeling. Ultrasound in Medicine and Biology. 46 (9), 2117-2144 (2020).
- Coelho-Filho, O. R., Rickers, C., Kwong, R. Y., Jerosch-Herold, M. MR myocardial perfusion imaging. Radiology. 266 (3), 701-715 (2013).
- Pandharipande, P. V., Krinsky, G. A., Rusinek, H., Lee, V. S. Perfusion imaging of the liver: current challenges and future goals. Radiology. 234 (3), 661-673 (2005).
- Weidner, N., Carroll, P. R., Flax, J., Blumenfeld, W., Folkman, J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. The American Journal of Pathology. 143 (2), 401-409 (1993).
- Quaia, E., Quaia, E. Classification and safety of microbubble-based contrast agents. Contrast Media in Ultrasonography. Medical Radiology (Diagnostic Imaging). , 3-14 (2005).
- Unger, E. C., Porter, T., Culp, W., Labell, R., Matsunaga, T., Zutshi, R. Therapeutic applications of lipid-coated microbubbles. Advanced Drug Delivery Reviews. 56 (9), 1291-1314 (2004).
- Blomley, M. J. K., Cooke, J. C., Unger, E. C., Monaghan, M. J., Cosgrove, D. O. Microbubble contrast agents: a new era in ultrasound. BMJ. 322 (7296), 1222-1225 (2001).
- Faez, T., et al. 20 years of ultrasound contrast agent modeling. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 60 (1), 7-20 (2012).
- De Jong, N., Emmer, M., Van Wamel, A., Versluis, M. Ultrasonic characterization of ultrasound contrast agents. Medical & Biological Engineering & Computing. 47 (8), 861-873 (2009).
- De Jong, N. . Acoustic properties of ultrasound contrast agents. , (1993).
- Schneider, M. Characteristics of sonovueTM. Echocardiography. 16, 743-746 (1999).
- Klibanov, A. L. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Investigative Radiology. 41 (3), 354-362 (2006).
- Averkiou, M. A., Powers, J., Skyba, D., Bruce, M., Jensen, S. Ultrasound contrast imaging research. Ultrasound Quarterly. 19 (1), 27-37 (2003).
- Snipstad, S., et al. Contact-mediated intracellular delivery of hydrophobic drugs from polymeric nanoparticles. Cancer Nanotechnology. 5 (1), 8 (2014).
- Epstein, P. S., Plesset, M. S. On the stability of gas bubbles in liquid-gas solutions. The Journal of Chemical Physics. 18 (11), 1505-1509 (1950).
- Borden, M. A., Longo, M. L. Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: effect of lipid hydrophobic chain length. Langmuir. 18 (24), 9225-9233 (2002).
- Deshpande, N., Needles, A., Willmann, J. K. Molecular ultrasound imaging: current status and future directions. Clinical Radiology. 65 (7), 567-581 (2010).
- Miller, M. W., Miller, D. L., Brayman, A. A. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound in Medicine & Biology. 22 (9), 1131-1154 (1996).
- Snipstad, S., et al. Sonopermeation to improve drug delivery to tumors: from fundamental understanding to clinical translation. Expert Opinion on Drug Delivery. 15 (12), 1249-1261 (2018).
- Dimcevski, G., et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. Journal of Controlled Release. 243, 172-181 (2016).
- May, J. -. N., et al. Multimodal and multiscale optical imaging of nanomedicine delivery across the blood-brain barrier upon sonopermeation. Theranostics. 10 (4), 1948-1959 (2020).
- Carmen, J. C., et al. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. Journal of Infection and Chemotherapy. 10 (4), 193-199 (2004).
- Runyan, C. M., et al. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. The Journal of General and Applied Microbiology. 52 (5), 295-301 (2006).
- Yemane, P. T., et al. Effect of ultrasound on the vasculature and extravasation of nanoscale particles imaged in real time. Ultrasound in Medicine & Biology. 45 (11), 3028-3041 (2019).
- van Wamel, A., et al. Acoustic Cluster Therapy (ACT) enhances the therapeutic efficacy of paclitaxel and Abraxane® for treatment of human prostate adenocarcinoma in mice. Journal of Controlled Release. 236, 15-21 (2016).
- Snipstad, S., et al. Ultrasound improves the delivery and therapeutic effect of nanoparticle-stabilized microbubbles in breast cancer xenografts. Ultrasound in Medicine & Biology. 43 (11), 2651-2669 (2017).
- Sheikov, N., McDannold, N., Vykhodtseva, N., Jolesz, F., Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound in Medicine & Biology. 30 (7), 979-989 (2004).
- Hynynen, K., McDannold, N., Sheikov, N. A., Jolesz, F. A., Vykhodtseva, N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 24 (1), 12-20 (2005).
- Aslund, A. K. O., et al. Nanoparticle delivery to the brain-By focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. Journal of Controlled Release. 220, 287-294 (2015).
- Downs, M. E., Buch, A., Karakatsani, M., Konofagou, E. E., Ferrera, V. P. Blood-brain barrier opening in behaving non-human primates via focused ultrasound with systemically administered microbubbles. Scientific Reports. 5, 15076 (2015).
- Baghirov, H., et al. Ultrasound-mediated delivery and distribution of polymeric nanoparticles in the normal brain parenchyma of a metastatic brain tumour model. PloS One. 13 (1), 0191102 (2018).
- Sulheim, E., et al. Therapeutic effect of cabazitaxel and blood-brain barrier opening in a patient-derived glioblastoma model. Nanotheranostics. 3 (1), 103 (2019).
- Lentacker, I., et al. Lipoplex-loaded microbubbles for gene delivery: a Trojan Horse controlled by ultrasound. Advanced Functional Materials. 17 (12), 1910-1916 (2007).
- De Temmerman, M., et al. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials. 32 (34), 9128-9135 (2011).
- Burke, C. W., Alexander, E., Timbie, K., Kilbanov, A. L., Price, R. J. Ultrasound-activated agents comprised of 5FU-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Molecular Therapy. 22 (2), 321-328 (2014).
- Mørch, &. #. 2. 2. 1. ;., et al. Nanoparticle-stabilized microbubbles for multimodal imaging and drug delivery. Contrast Media & Molecular Imaging. 10 (5), 356-366 (2015).
- Jamburidze, A., et al. Nanoparticle-coated microbubbles for combined ultrasound imaging and drug delivery. Langmuir. 35 (31), 10087-10096 (2019).
- Snipstad, S., et al. Sonopermeation enhances uptake and therapeutic effect of free and encapsulated cabazitaxel. Ultrasound in Medicine and Biology. , (2021).
- De Cock, I., Lajoinie, G., Versluis, M., De Smedt, S. C., Lentacker, I. Sonoprinting and the importance of microbubble loading for the ultrasound mediated cellular delivery of nanoparticles. Biomaterials. 83, 294-307 (2016).
- Roovers, S., et al. Sonoprinting of nanoparticle-loaded microbubbles: Unraveling the multi-timescale mechanism. Biomaterials. 217, 119250 (2019).
- Klymchenko, A. S., et al. Highly lipophilic fluorescent dyes in nano-emulsions: towards bright non-leaking nano-droplets. RSC Advances. 2 (31), 11876 (2012).
- Aslund, A. K. O., et al. Quantification and qualitative effects of different PEGylations on Poly (butyl cyanoacrylate) Nanoparticles. Molecular Pharmaceutics. 14 (8), 2560-2569 (2017).
- Born, M., Wolf, E. . Principles of optics: electromagnetic theory of propagation, interference and diffraction of light. , (1999).
- Pittet, M. J., Weissleder, R. Intravital imaging. Cell. 147 (5), 983-991 (2011).
- Hak, S., Reitan, N. K., Haraldseth, O., de Lange Davies, C. Intravital microscopy in window chambers: a unique tool to study tumor angiogenesis and delivery of nanoparticles. Angiogenesis. 13 (2), 113-130 (2010).
- Fusser, M., et al. Cabazitaxel-loaded Poly (2-ethylbutyl cyanoacrylate) nanoparticles improve treatment efficacy in a patient derived breast cancer xenograft. Journal of Controlled Release. 293, 183-192 (2019).
- Abou-Saleh, R. H., et al. Molecular effects of glycerol on lipid monolayers at the gas-liquid interface: impact on microbubble physical and mechanical properties. Langmuir. 35 (31), 10097-10105 (2019).
- Seynhaeve, A. L. B., ten Hagen, T. L. M. Intravital microscopy of tumor-associated vasculature using advanced dorsal skinfold window chambers on transgenic fluorescent mice. Journal of Visualized Experiments. (131), e55115 (2018).
- Luan, Y., et al. Lipid shedding from single oscillating microbubbles. Ultrasound in Medicine & Biology. 40 (8), 1834-1846 (2014).
- Lajoinie, G., et al. Ultrafast vapourization dynamics of laser-activated polymeric microcapsules. Nature Communications. 5 (1), 1-8 (2014).
- Mathiyazhakan, M., et al. Non-invasive controlled release from gold nanoparticle integrated photo-responsive liposomes through pulse laser induced microbubble cavitation. Colloids and Surfaces B: Biointerfaces. 126, 569-574 (2015).
- Vos, H. J., Emmer, M., de Jong, N. Oscillation of single microbubbles at room versus body temperature. 2008 IEEE Ultrasonics Symposium. , 982-984 (2008).
- Vos, H. J., Dollet, B., Bosch, J. G., Versluis, M., de Jong, N. Nonspherical vibrations of microbubbles in contact with a wall-a pilot study at low mechanical index. Ultrasound in Medicine & Biology. 34 (4), 685-688 (2008).
- Sijl, J., et al. Acoustic characterization of single ultrasound contrast agent microbubbles. The Journal of the Acoustical Society of America. 124 (6), 4091-4097 (2008).
- Garbin, V., et al. Changes in microbubble dynamics near a boundary revealed by combined optical micromanipulation and high-speed imaging. Applied Physics Letters. 90 (11), 114103 (2007).
- Baresch, D., Garbin, V. Acoustic trapping of microbubbles in complex environments and controlled payload release. Proceedings of the National Academy of Sciences of the United States of America. 117 (27), 15490-15496 (2020).
- Maresca, D., et al. Acoustic sizing of an ultrasound contrast agent. Ultrasound in Medicine & Biology. 36 (10), 1713-1721 (2010).
- Renaud, G., Bosch, J. G., vander Steen, A. F. W., de Jong, N. An “acoustical camera” for in vitro characterization of contrast agent microbubble vibrations. Applied Physics Letters. 100 (10), 101911 (2012).
- Renaud, G., Bosch, J. G., Van Der Steen, A. F. W., De Jong, N. Low-amplitude non-linear volume vibrations of single microbubbles measured with an “acoustical camera.”. Ultrasound in Medicine & Biology. 40 (6), 1282-1295 (2014).
- Luan, Y., et al. Combined optical sizing and acoustical characterization of single freely-floating microbubbles. Applied Physics Letters. 109 (23), (2016).
- Lajoinie, G., et al. In vitro methods to study bubble-cell interactions: Fundamentals and therapeutic applications. Biomicrofluidics. 10 (1), 011501 (2016).
- Guan, J., Matula, T. J. Using light scattering to measure the response of individual ultrasound contrast microbubbles subjected to pulsed ultrasound in vitro. The Journal of the Acoustical Society of America. 116 (5), 2832-2842 (2004).
- Sofias, A. M., Åslund, A. K. O., Hagen, N., Grendstad, K., Hak, S. Simple and robust intravital microscopy procedures in hybrid TIE2GFP-BALB/c transgenic mice. Molecular Imaging and Biology. 22 (3), 486-493 (2020).
- Ritsma, L., Steller, E. J. A., Ellenbroek, S. I. J., Kranenburg, O., Borel Rinkes, I. H. M., van Rheenen, J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nature Protocols. 8 (3), 583-594 (2013).

